ABSTRACT
Capsicum fructescens is a variety of Capsicum with much nutritional and therapeutic usefulness. This study is aimed at investigating the antimicrobial, chemical and biochemical properties of C. fructescens. The biochemical, mineral and phytochemical analyses of the extract were carried out using standard methods. The organic contents of the extracts were determined by GCMS to identify its bioactive constituents which were tested for antibacterial and antifungal activities against 7 bacterial and 3 fungal isolates using disc-diffusion method. The biochemical analysis showed that the leaves were high in protein, fibre, carbohydrate and fat. Different mineral elements were detected in the leaves and they include Magnesium, Calcium, Iron, Sodium, Copper, Potassium, Zinc and Manganese. GC-MS revealed 13 different organic compounds belonging to four groups of chemicals namely alkanol, alkanoic acid, alkanoate and ester. The methanolic extracts of C. fructescens leaves at a dose range of 5 and 25 mg/ml showed significant antibacterial and antifungal activity on some test organisms. The presence of great quantity of dodecanoic acid among other compounds in the extract of bawa suggested the reason for its profound anti-staphylococcal and anti-candidal activities. This study concluded that C. fructescens foliar extract is rich in important chemical and biochemical metabolites which have shown some therapeutic properties. An advocacy in the higher consumption of these peppers among folks is hereby recommended.
Key words: Capsicum fructescens extracts, chemicals and biochemical composition, antimicrobial activity.
Plants including cryptogams have shown to be rich in bioactive constituents (Femi-Adepoju et al., 2018). Capsicum species with several bioactive compounds are increasingly gaining interest due to their effectiveness in improving nutrition and human health (Unuofin et al., 2017).
The origin of the name Capsicum is most likely from the Latin word, “capsa” meaning to bite, in allusion to the hot pungent properties of the fruit and seeds with several universal English names, include chili, sweet pepper, hot pepper and bell pepper, belongs to the family Solanaceae (Adepoju et al., 2019). Their fruit becomes brightly colored once its seeds are mature enough to germinate thereby attracting the attention of birds that then distribute the seeds. In West Africa, the genus Capsicum is represented by two cultivated species, namely, Capsicum annuum and Capsicum frutescens, with numerous varieties. They are the third in Nigeria among the cultivated vegetables being utilized in the dry state as spice due to their capsaicin content. Peppers generally have been found to contain essential vitamins, minerals, and nutrients in various quantity which serves great usefulness for human health. They also contain a number of phytochemicals such as carotenoids, capsaicinoids, flavonoids, ascorbic acid, and tocopherols which have been reported to prevent inflammatory diseases associated with oxidative damages and maintain optimum health (Kim et al., 2019). Many reports have been given and published on the comparative analysis of metabolite compositions in Capsicum spp. and C. frutescens has not been left out (Kantar et al., 2016; Sarpras et al., 2016).
Capsicum belongs to a group of crops that are widely cultivated for its spicy nature and nutritional value. Five species (C. annuum var. annuum, Capsicum chinense, Capsicum frutescens, Capsicum baccatum varieties pendulum and umbilicatum, and Capsicum pubescens) were domesticated by American natives. After Columbus, they became widely exploited in tropical to temperate regions because of their fruits, which have high nutritional contents, especially in vitamins. Peppers are constituents of the human diet, the pungent cultivars are used as spice ("ajies," "paprika," "chilies," "hot peppers") and the sweet types as vegetables ("sweet pepper," "bell pepper," "pimiento"). Hot red peppers consist of spicy compounds called capsaicinoids which include capsaicin, dihydrocapsaicin, nordihydrocapsaicin and other compounds (Ludy et al., 2012). Capsaicin, water-insoluble derivative of homovanillic acid and the main active ingredient in capsicum fruits, is responsible for hot sensation to the tongue (Papoiu and Yosipovitch, 2010). C. chinense species have been reported traditionally to contain the hottest cultivars (Canton-Flick et al., 2008). According to González et al. (2004), the seed and placenta tissues of C. chinense are reported to contain most of the capsaicin with 37 and 62%, respectively.
The crop is employed both as condiment and food; the thick sweet fleshy or non-pungent varieties are used in salads or stuffed with meat and cooked (Adepoju et al., 2019). In addition to the use of capsicum fruits in traditional medicine and food additives, it has been reported to be useful in the treatment of sore throat, cough, healing wound, toothache, parasitic infections and rheumatism (Singletary, 2011), it has also been utilized as an antiseptic (Pawar et al., 2011), immunomodulator and antioxidant (Otunola et al., 2017; Maji and Banerji, 2016), to protect against gastrointestinal ailments (Low Dog, 2006) including dyspepsia, loss of appetite, gastroesophageal reflux disease and gastric ulcer (Maji and Banerji, 2016). Other useful effects of Capsicums include antibacterial (Neelam et al., 2016) and anticancer (Pawar et al., 2011).
The fruits of pepper contain a range of bioactive phytochemicals including flavonoids, carotenoids, phenolics, and other antioxidant compounds (Alvarez-Parrilla et al., 2011). Several classes of plant chemicals including phenolic compounds and antioxidants are sufficiently available in high quantity in vegetables and fruits; thereby forming an important part of human consumption. Since numerous studies have suggested that eating foods rich in phytochemicals reduces risk of certain forms of cancer, cardiovascular diseases and stroke, much attention has been drawn to natural foods especially spices and vegetables rich in these compounds (Kaur and Kapoor, 2001; Prior and Cao, 2000). Takahashi et al. (2018) reported high antioxidant properties of the fruit extracts of C. frutescens from green to red stages based on the oxygen radical absorbance capacity (ORAC) and DPPH tests (Takahashi et al., 2018). This work focused on investigating the antibacterial and antifungal efficacies vis-avis biochemical, mineral, phytochemical and organic chemical contents of three varieties of C. fructescens, with a view to establish any relationships between them.
Collection and identification of plant samples
Fresh leaves of three different C. fructescens varieties ijosi, bawa and sombo were collected into a sterile polythene bag. The leaves were identified at the Biology Laboratory Complex, Ladoke Akintola University of Technology, Ogbomoso, Nigeria. The harvested leaves were cleaned using clean drinkable water and air-dried for four weeks in the general biology laboratory complex, Ladoke Akintola University of Technology, Ogbomoso, Nigeria. The dried leaves were crushed using porcelain mortar and pestle and the resulting powders were kept in air-tight containers placed in cool, dry environment.
Preparation of extracts
Extractions were carried out by soaking 20 g of each of the powders in 200 ml of absolute methanol in well-labelled clean conical flasks and corked. After seven days of extraction, the soaked leaf powder were removed and the decanted solutions were concentrated on a rotary evaporator. The dried extracts were kept in a refrigerator in readiness for analyses. Enough quantity of powder was kept for various analyses for which they were needed. The analyses carried out on the dried leaf samples included those of biochemical and mineral compositions, while phytochemicals and organic chemical compositions by GC-MS analysis were carried out on the leaf extracts, which were also used for the antimicrobial testing (Sukhdev et al., 2008).
Collection, growth and maintenance of test organism
Test organisms used for this study were obtained from the microbial gene bank of Pure and Applied Biology Department, Ladoke Akintola University of Technology, Ogbomoso, Nigeria. These include bacteria cultures of different strains, such as Bacillus cereus, Bacillus subtilis, Escherichia coli, Pseudomonas aeruginosa, Pseudomonas putida, Staphylococcus aureus and Klebsiella pneumoniae maintained at 37°C; then, fungal culture of Aspergillus niger, Aspergillus flavus and Candida albicans maintained at 28°C.
Inoculum preparation
For bacteria inoculums, two loopful of overnight grown and morphologically similar colony of bacteria was inoculated into 5 ml of sterile nutrient broth and incubated for 2 h at 37°C when the turbidity was equivalent to a 0.5 BaSO4 standard. For fungi inoculums, 0.2 g of yeast extract was mixed with 1 g of sucrose in 100 ml of distilled water. 5 ml was pipette in test tubes and sterilized for 15 min at 121°C. After cooling, two loopful of test fungi was inoculated into the medium and incubated for 2 h after which the suspensions were maintained at 4°C for further use.
Biochemical analysis
The biochemical compositions of the dried leaf samples were determined using standard analytical methods. All measurements were done in duplicates and their values are presented in percentages. Moisture, ash, crude fibre, crude protein, fat and carbohydrate in the leaves of all the species studied were determined in accordance with the following procedures: moisture content (AOAC, 1995); crude fibre (James, 1995); protein (Pearson, 1976); fat (Onwuka, 2005); carbohydrate (Arithmetic Difference Method, that is, %CHO = 100 - (% fat + % ash + % fiber + % protein).
Mineral content analysis
Estimation of mineral substances in dried grinded leaves was performed by using a NOVA400 atomic absorption spectrometer (ANALYTIK JENA AG, Jena, Germany) hollow cathode lamps and acetylene/air flame to measure absorbance. By using slits, wavelengths and lamp current; sodium (Na), potassium (K), manganese (Mn), magnesium (Mg), zinc (Zn), phosphorus (P), copper (Cu) and iron (Fe) were calculated. The analyzed results for Na, Mg, Ca, K, Zn, Cu and Fe contents were expressed in ppm.
Qualitative and quantitative phytochemical analysis of plant extracts
Chemotaxonomic studies on C. fructescens varieties ijosi, bawa and sombo focused on qualitative and quantitative analyses for phlobatannins, alkaloids, tannins, cardiac glycosides, saponins, flavonoids, terpenoids and phenols. The qualitative analysis of the bio-constituents present in the plant extracts was performed using the methods of Trease and Evans (1989).
Quantitative phytochemical screening
Determination of saponin content was done by the spectrophotometric method described by Brunner (1984), flavonoids was determined according to the method outlined by Harborne (1984). The quantity of alkaloids, tannins, total phenols and cardiac glycosides were determined by the alkaline precipitation gravimetric method described by Harborne (1984), spectrophotometric method of Makkar et al. (1993), the method described by Mahadevan and Sridhar (1982) and the use of Buljet’s reagent as described by El-Olemy et al. (1994), respectively.
Analysis and identification of organic compounds in the plant extracts
The GC-MS analysis of the leaf extracts was carried out at the department of Chemical Engineering, University of Ilorin on Agilent 19091S Gas chromatograph (GC) interfaced to a mass spectrometer 433HP-5MS instrument employing the following conditions: silica capillary column fused with 100% phenyl methyl silox, (length; 30 m × 250 μm; film thickness 0.25 μm). For GC-MS detection, an electron ionization system with ionization energy of 70 eV was used. Helium gas (99.999%) was used as the carrier gas at constant flow rate 1.5 ml/min and an injection volume of 1 μl was employed (Split ratio of 50:1) injector temperature-300°C; average velocity of 45.67 cm/s. The oven temperature was programmed from 100°C (Isothermal for 4 min) with an increase of 4°C min-1 to 240°C. Total GC running time was 49 min. The relative percentage amount of each component was calculated, by comparing its average peak area to the total areas. The software adopted to handle mass spectra and chromatogram was a turbomass. The detection employed the NIST Ver. 2.0 year 2009 library29. After the performance of the GCMS, was the identification of the components detected using their spectra.
Identification of components
Interpretation on mass spectrum of GC-MS was done using the database of National Institute of Standard and Technology (NIST) which contains more than 62,000 patterns. The mass spectra of the unknown components were compared with the spectrum of the known components contained in the NIST library. The name, molecular weight and structure of the components of the test materials were also ascertained using the fragmentation patterns they exhibited and the information available in the library.
Antimicrobial assay of plant extracts on test organisms
The antibacterial and antifungal activities of the plant extracts were studied using the disc-diffusion method. Petri plates were prepared with 20 ml of sterile nutrient agar for bacteria and potato dextrose broth for fungi. The bottom of each plate was divided into segment 5 and 25 mgL-1, respectively with the control at the centre which each name of the organisms boldly written on each plate. The test organisms were swabbed on the solidified sterile media and the perforated filter paper (4 mm) each was put into the extract at varied concentration and was put into the marked area that match the disc. Methanol was used as control. Plates were incubated at 37°C for 24 h and 25°C for 48 h for bacteria and fungi, respectively. Positive control was prepared using broad spectrum antibiotic entamicin and augmentin as the case may be. The diameters of zone of inhibition (clearance) were recorded in millimeter.
Statistical analysis
Data obtained were analyzed with IBM SPSS version 20 software and subjected to one-way ANOVA to assess significant difference between groups followed by Tukey post-hoc test at 95% significance level and expressed in mean ± standard deviation (SDEV) using Microsoft office Excel version 2007.
The result of the biochemical composition of the leaves studied is shown in Table 1 while that of the mineral elements is presented in Table 2. The leaves of all the plants were significantly rich in important nutritional factors such as crude fibre, carbohydrate, fat and protein. Of all the nutrients detected, the leaves were particularly high (30-33%) in protein, but low (2.1-2.6%) in dry matter content (Table 1). The quantities of the proximate contents (%) of the Capsicum spp. studied can be listed from the highest to the lowest as moisture>protein> crude-fibre>total ash> crude fat>carbohydrate>dry matter.
The mineral elements detected in the leaves analyzed were Calcium, Magnesium, Iron, Sodium, Potassium, Copper, Zinc and Manganese. The leaves were particularly high in Calcium (26.0-27.3%), Potassium (27-27.7%) and Magnesium (13%), but low as expected of trace elements, in Manganese, Zinc and Copper (Table 2). On the whole, the quantities of the mineral elements in the plants studied can be enumerated from the highest to the lowest as Potassium>Calcium>Magnesium>Sodium>Zinc>Copper>Phosphorus>Iron and Manganese.
Crude fibre reportedly increases stool mass and hastens digestion. It does not get digested in humans and animals but it aids the normal functioning of the intestinal tract. According to Bello et al. (2008), fibre assists in maintaining human health and it has been reported to function in the reduction of cholesterol levels of the body (Bello et al., 2008). Diets with low fibre contents have been associated with increased risks of heart malfunctions, cancer of the colon and rectum cells, varicose veins, phlebitis, obesity, appendicitis, diabetes and even constipation (Ibanga and Okon, 2009; Lajide et al., 2008; Saldanha, 1995) established that minerals are essential in human nutrition while O'Dell (1979) stated that minerals from plants are less readily available than those from animals. From a recent research conducted by Kim et al. (2019), the results of percentage variability for proximate analysis of some Capsicum spp. extract, it was reported that, the quantity of ash, crude fiber, moisture, and crude fat were significantly affected by year and location (Kim et al., 2019). The levels of each mineral across four environments showed a high variation, which indicates that these compounds are strongly influenced by environmental factors. However, previous studies have shown that mineral contents in peppers depend on a variety of factors (Sarpras et al., 2016). Four varieties belonging to the Capsicum spp. were characterized by their nutritional constituents, antioxidant vitamins and capsaicin contents in another research conducted by Olatunji and Afolayan (2020). They found out that, there was variability in nutritional, vitamins and capsaicin contents among varieties and that higher levels of vitamins could be found in fresh than in dry samples. Reports have also shown that varieties of Capsicum spp. contain important micro- and macro-elements with antioxidative vitamins, in varying quantities which can provide significant proportions of the recommended daily intake and help improve overall health of humans (Olatunji and Afolayan, 2020)
Minerals are vital for the overall physical and mental healthiness of man as they are important constituent of bones, muscles, teeth, tissues, nerve and blood cells (Soetan et al., 2010). Calcium and phosphorus are jointly essential for growth and maintenance of muscles, bones and teeth (Okaka et al., 2006; Ladan et al., 1996). Magnesium, according to Borgert and Briggs (1975) is a component of chlorophyll and it is important for calcium metabolism in bones whose deficiency can lead to ischemic heart disease (Elegbede, 1998). Zinc is involved in normal functioning of immune system and is associated with protein metabolism. Iron is an essential trace element for the formation of haemoglobin and also for the normal functioning of central nervous system (Asaolu et al., 1997). The deficiency of these nutrients and minerals are known to adversely affect health in animals.
Phytochemicals in the leaves of Nigerian cultivars of Capsicum
Total of eight secondary metabolites were detected in the leaves of the three Nigerian species of Capsicum studied. These included alkaloids, saponin, phenols, tannins, flavonoids, terpenoids, cardiac glycosides and phlobatannins (Table 3). Of these eight, the quantities of only two, that is, cardiac glycosides and phlobatannins did not show significant differences among the taxa studied.
The presence of phytochemicals in the foliar extracts of the Capsicum cultivars studied, suggests possible medicinal properties in them (AOAC, 1995). Saponins, phlobatannins and steroidal glycosides were detected in all the cultivars. Saponins have also been reported in Senna alata and Cajanus cajan by Lawal et al. (2014) and also in Lophira lanceolata seeds by Lohlum et al. (2010). According to Harborne (1984), saponins possess anti-hypercholesterol, anti-inflammatory, cardiac health enhancement properties and also appear to mortalise or inhibit growth of cancer cells without adversely affecting the normal body cells (Okwu, 2001). Phlobatannins were reported by Asquith and Butter (1986) as inhibitors of growth in many microorganisms like bacteria, fungi and viruses. According to De-Bruyne et al. (1997), tannins are plant polyphenols which can form complexes with metals ions and with macro-molecules such as polysaccharides and proteins. Enujiugha and Agbede (2000) established that tannins usually form insoluble complexes with proteins, thereby interfering with their bioavailability.
The phytochemicals
UAMME=Undecanoic acid, 10-methyl, methyl ester; HEAME=Hexadecanoic acid methyl ester; 811- OCME=8,11- octadecanoic acid methyl ester; 710-OCME=7,10- octadecanoic acid methyl ester; 912-OCME=9,12- octadecanoic acid methyl ester (E,E); 912-OCPE=9,12,15-octadecatienoic acid, 2,3-dihydroxy propyl ester (Z,Z,Z); (Z,Z,Z); 912-OCIME=9,12-Octadecadienoic acid, methyl ester; 13-OCME=Cis-13-octadecenoic acid methyl ester; 9-OCME=9-octadecenoic acid methyl ester; HH-12-EE=Hexadecanoic acid, 1-(hydroxymethyl)- 1,2-ethanediyl ester; METED=Methyl tetradecanoate; DODEA=Dodecanoic acid; and ET-2-OCD=Ethanol, 2-(9-octadecenyloxy)- Z.
The foliar organic compounds in Nigerian species of Capsicum via Gas Chromatography Mass Spectroscopy (GCMS)
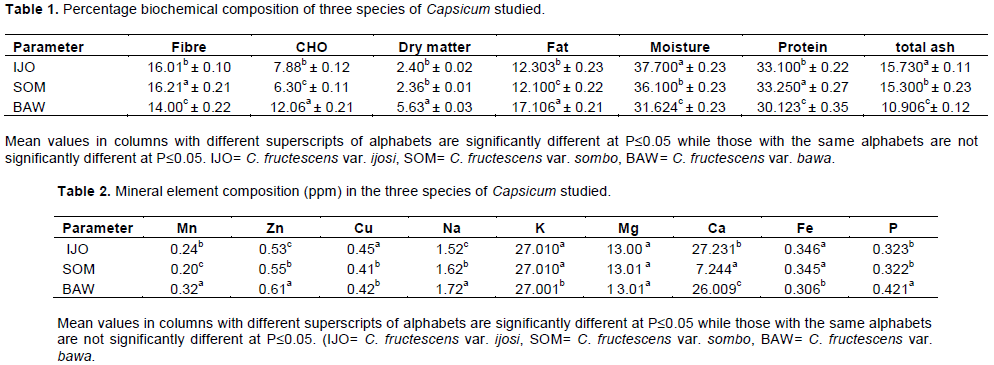
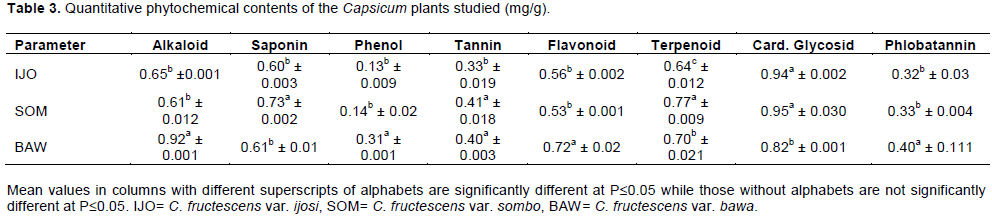
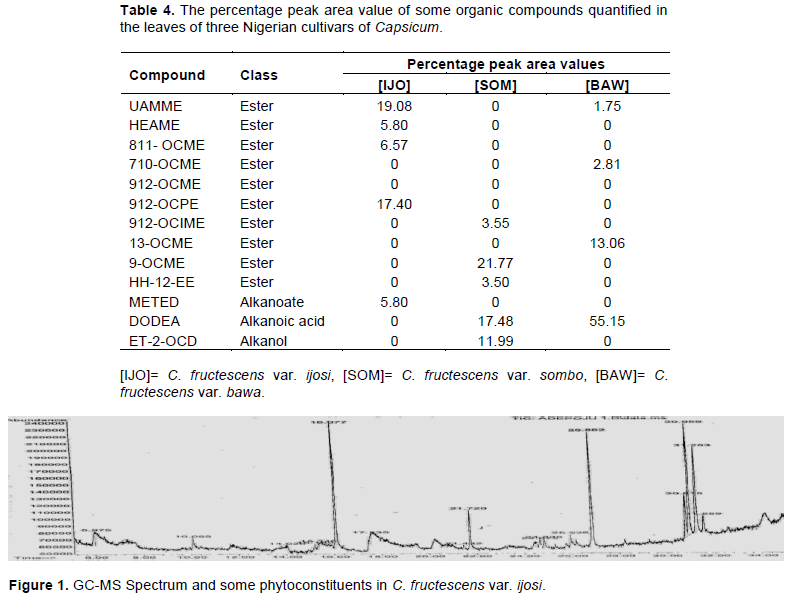
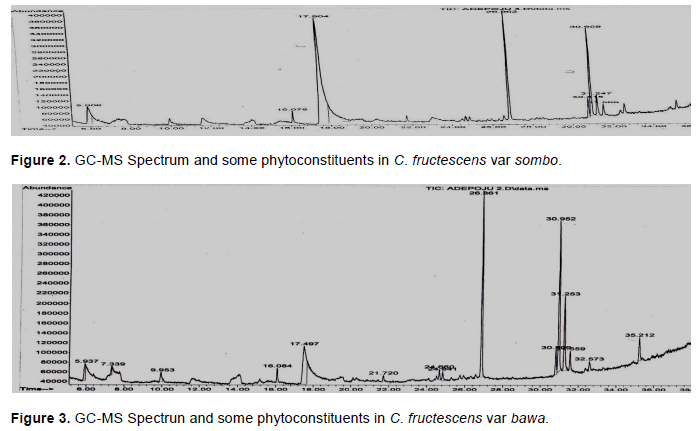
The entries in Table 4 are the results of GC-MS analysis conducted on the leaves of the cultivars of Capsicum studied. A total of 13 different organic compounds were detected and these belong to four different groups of chemicals namely ester, alkanoate, alkanoic acid and alkanol. While 10 of the organic compounds were esters, only one belonged to each of the other groups (Table 4). Each of C. fructescens var sombo and C. fructescens var ijosi had five, the highest number of organic compounds detected by GCMS analysis, while C. fructescens var. bawa had four. Figures 1 to 3 present the GC-MS spectra and phytoconstituents in C. fructescens varieties. Neelam et al. (2016) reported that the GC-MS analysis of n-hexane and chloroform extracts of the seeds of C. frutescens revealed the presence of a total of 29 compounds from different classes. The major components found include Octadecadienal (Z), 3-Carene, Hexadecanoic acid, Tetracosane, Heptadec-8-ene-2,4-dione, 2(3H)-Furanone,dihydro-5-(2-octenyl)-, (Z) and Hexadec-8-ene-2,4-dione, in the n-hexane extract, while in the chloroform extract, Hexadecanoic acid, 9,12-Octadeca dienoic acid, 1-Hexadecene and 5-Eicosene, (E) were found. Octadecane, Eicosane, Docosane, 9,12-Octadecadienoic acid, methyl ester and Hexadecanoic acid, were found to be common in both the extracts (Neelam et al., 2016). Some of the compounds reported by Neelam et al. (2016) are also discovered by this study.
Antimicrobial activities of methanolic extracts of C. fructescens varieties on test organisms
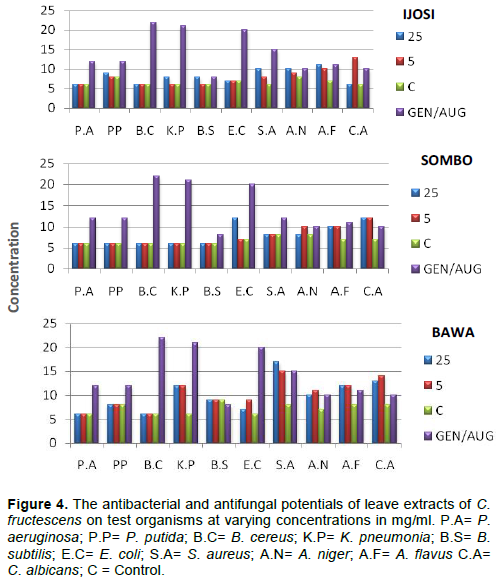
The antimicrobial activity of methanolic extracts of Capsicum leaf were investigated against the selected pathogens P. aeruginosa, P. putida, B. cereus, K. pneumonia, B. subtilis, E. coli, S. aureus, A. niger, A. flavus and C. albicans by disc diffusion method. The antimicrobial effects of leaves extracts of C. fructescens as shown by zone of inhibition in millimeters (mm) on selected bacteria and fungi are presented in Figure 4. The antibacterial activity of the extracts was found to be the highest at 5 mg/ml against C. albicans which produced an inhibition zone of 13 mm for ijosi; 5 and 25 mg/ml produced the highest zone of inhibition of 12 mm for sombo and 17/15 mm for bawa against C. albicans. S. aureus was found to be susceptible most to the foliar extract of bawa.
Significance of organic, proximate, mineral and phytochemical contents on antimicrobial efficacy of the extracts
In the present study, most of the identified volatile compounds belong to the class ester, alkanol, alkanoic acid and alkanoate. These compounds have been reported to be pharmacologically active. For example, hexadecanoic acid is known to have potential antibacterial and antifungal activities (McGraw et al., 2002); unsaturated fatty acids are also suggested to be responsible for the anti-inflammatory activity (Li et al., 2004); long-chain unsaturated fatty acids, such as linoleic acid, also show antibacterial activity and are the key ingredients of antimicrobial food additives and some antibacterial herbs (Chang et al., 2005), and hexadecanoic acid, methyl ester and 9,12-octadecadienoic acid (Z,Z)-, methyl ester have shown antioxidant and anticancer properties, respectively (Wei et al., 2011).
According to Ishida et al. (2000), crude fibers in the diet are necessary for digestion and effective to eliminate the risk of coronary heart disease, constipation, hypertension, colon, and diabetes and breast cancer (Ishida et al., 2000). Thus, these medicinal plants are regarded as a valuable source of dietary fiber in human nutrition. There is a strong correlation between fibre and moisture contents, as the fibre are easily digested and disintegrated which could be of interest to human health (Hussain et al., 2010). Minerals are required for normal growth, activities of muscles and skeletal development, copper and iron are responsible for cellular activity and oxygen transport, respectively, magnesium for chemical reaction in the body and intestinal absorption, sodium and potassium used in fluid balance and nerve transmission. Manganese plays a major role in production of energy and in supporting the immune system (Muhammad et al., 2011). Deficiency of these nutrients and minerals are known to affect the performance and health in both humans (MERCK, 2005).
In earlier studies, the antimicrobial activity of ethanolic, methanolic and aqueous extracts of C. frutescens has already been reported against a number of microorganisms (KoffiNevry et al., 2012; Shariati et al., 2010). In a study by Vinayaka et al. (2010), even the foliar methanolic extract of C. frutescens showed dose-dependent antibacterial activity against S. aureus, K. pneumoniae and P. aeruginosa. Aqueous extracts of the leaf and fruit of C. frutescens have also exhibited potentials to prevent growth of seed-borne fungi (Soumya and Nair, 2012). In the study carried out by Neelam et al. (2016), broad spectrum activity was shown to be exhibited by low polar n-hexane and chloroform extracts of C. frutescens. This significant antimicrobial activity could be attributed to the compounds identified in the GC-MS spectrum. Isolation and proper identification of these antimicrobial agents from capsicum can lead to an important improvement in the area of food safety and can be used in the prevention of certain human diseases (Neelam et al., 2016). Also, the antimicrobial property of silver nanoparticles fabricated from the extract of C. frutescens was higher than that of the other two spices (Otunola et al., 2017).
Flavonoids have been studied to be very useful as an antimicrobial agent, inhibitor of mitochondrial adhesion, as an anticancer agent and antiulcer agent (Biju et al., 2014). It has been confirmed that consumption of food and beverages rich in phenol prevent diseases, such as cancer (Chalise et al., 2010). In this study, the presence of flavonoid and phenolic compounds in C fructescens var. leaf extracts confer health benefits associated with it. The significant antimicrobial activity of methanolic extracts of C. fructescens var. might be due to the synergistic effects of compounds identified in the proximate, organic, mineral and phytochemical analyses of the plant part. Something striking to note is that bawa extract was found to be most active against S. aureus and C. albicans with a zone of inhibition that suggests a better performance than the test drugs.
The leaves of the Nigerian species of Capsicum studied are rich in important mineral elements such as calcium, magnesium, iron, sodium, potassium, copper, zinc and manganese; and are also sufficiently rich in some notable nutritional factors such as protein, crude fibre, total ash, crude fat and carbohydrates. The data obtained from the antimicrobial tests, as well as those of phytochemical constituents and organic compounds in the leaf extracts have revealed that the extracts contain important bioactive compounds. The significance of this is that an advocacy for more consumption of these peppers among folks can deal with infections associated with these pathogens. They can also be useful in the formulation of disinfectants and antiseptics as well as in chemotherapy. Relative abundance of the foliar nutrients in the species of the genus studied has far reaching implications in an effort to diagnose the species as potential leafy vegetables and as components of herbal formulations.
The authors have not declared any conflict of interests.
REFERENCES
|
Adepoju AO, Ogunkunle ATJ, Azeez MA, Femi-Adepoju AG (2019). Value of Seed Protein Profile in the Taxonomy of cultivars of Capsicum in Nigeria. Nigerian Journal of Biotechnology 36(2):1-8.
|
|
|
|
Alvarez-Parrilla E, De La Rosa LA, Amarowicz R, Shahidi F (2011). Antioxidant activity of fresh and processed Jalapeño and Serrano peppers. Journal of Agricultural and Food Chemistry 59(1):163-173.
Crossref
|
|
|
|
|
AOAC (1995). Official Methods of Analysis. 16th Edition, Association of Official Analytical Chemist, Arlington. 1995.
|
|
|
|
|
Asaolu SS, Ipinmoroti KO, Adeyinwo CE, Olaofe O (1997). Seasonal variation in heavy metals concentration distribution in sediments of Ondo State Coastal Region. Ghana Journal of Chemistry 31:11-16.
|
|
|
|
|
Asquith TN, Butter LG (1986). Interaction of condensed tannins with selected proteins. Phytochemistry Journal 25:1591-1593.
Crossref
|
|
|
|
|
Bello MO, Falade OS, Adewusi SR, Olawore NO (2008). Studies on the Chemical Compositions and Anti-nutrients of Some Lesser Known Nigeria Fruits. African Journal of Biotechnology 7(21):3972-3979.
|
|
|
|
|
Biju J, Sulaiman CT, Satheesh G, Reddy VRK (2014). Total phenolics an flavonoids in selected medicinal plants from Kerala. International Journal of Pharmacy and Pharmaceutical Sciences 6:406-408.
|
|
|
|
|
Borgert GM, Briggs CH (1975). Nutritional and physical fitness. W.B Saunder and cp, Philadephia USA. pp. 34-50.
|
|
|
|
|
Brunner JH (1984). Direct spectrophotometric determination of saponin. Analytical Chemistry 34:1314-1326.
|
|
|
|
|
Canton-Flick A, Balam-Uc E, Jabın Bello-Bello J, Lecona-Guzman C, Solıs-Marroquın D, Aviles-Vinas S, Gomez-Uc E, Lopez-Puc G, Santana-Buzzy N (2008). Capsaicinoids content in Habanero pepper (Capsicum chinense Jacq.): Hottest known cultivars. HortScience 43(5):1344-349.
Crossref
|
|
|
|
|
Chalise JP, Acharya K, Gurung N, Bhusal RP, Gurung R, Skalko-Basnet N, Basnet P (2010). Antioxidant activity and polyphenolcontent in edible wild fruits from Nepal. International Journal of Food Sciences and Nutrition 61:425-432.
Crossref
|
|
|
|
|
Chang JZ, Jung-Sung Y, Tae-Gyu L, Hee-Young C (2005). Fatty acid synthesis is a target for antibacterial activity of unsaturated fatty acids. FEBS Letters 579:5157-5162.
Crossref
|
|
|
|
|
De-Bruyne T, Cimanga K, Pieters L, Claeys M, Domnusse R and vlietinck A (1997). Galloctechim (4-0-7) Epigallocatechin. A new Biflavonoid isolated from B. ferruginea. Natural Product Letters 11:47-52.
Crossref
|
|
|
|
|
Enujiugha VN, Agbede JO (2000). Nutritional and anti-nutritional characteristics of African oil bean (Pentaclethra macrophylla Benth) seeds. Applied Tropical Agriculture 5:11-14.
|
|
|
|
|
El- Olemy MM, Farid JA, Abdel- Fattah AA (1994). Ethanol Extract of P. stratiotes. NISEB Journal 1(1):51-59.
|
|
|
|
|
Elegbede JA (1998). Legumes. In: Nutritional quality of plant foods. Osagie AU, Eka OU (Eds). Post-Harvest Research Unit, University of Benin pp. 53-83.
|
|
|
|
|
Femi-Adepoju AG, Adepoju AO, Durodola FA, Akanbi-Gada MA (2018). Phytochemical, Antimicrobial and Bio-Active Component Analysis of Platycerium Superbum (L.) Methanolic Extract. International Journal of Sciences: Basic and Applied Research 40(1):98-107.
|
|
|
|
|
González T, Villanueva L, Cisneros O, Gutiérrez L, Contreras F, Peraza S, Trujillo J, Espadas G (2004). Analysis of fruit morphology of Habanero pepper (Capsicum chinense Jacq.). Book of Abstracts of the 17th International Pepper Conference, November 14-17, Naples, Florida, USA. P. 6.
|
|
|
|
|
Harborne JB (1984). Phytochemical methods, London. Chapman and Hall, Ltd. 1984. pp. 100-101.
Crossref
|
|
|
|
|
Hussain J, Ullah R, Rehman N, Khan AL, Muhammad Z, Khan FU (2010). Endogenous transitional metal and proximate analysis of selected medicinal plants from Pakistan. Journal of Medicinal Plants Resouces 4:267-270.
|
|
|
|
|
Ibanga OI, Okon DE (2009). Mineral and anti-nutrients in two varieties of African pear (Dacryodes edulis). Journal of food technology 7(4):106-110.
|
|
|
|
|
Ishida H, Suzuno H, Sugiyama N, Innami S, Todokoro T, Maekawa A (2000). Nutritional evaluation of chemical component of leaves stalks and stems of sweet potatoes (Ipomoea batatas poir). Food Chemistry 68:359-367.
Crossref
|
|
|
|
|
James CS (1995). Analytical Chemistry of Foods, Chapman and Hall, New York.
Crossref
|
|
|
|
|
Kantar MB, Anderson JE, Lucht SA, Mercer K, Bernau V, Case KA, Le NC, Frederiksen MK, DeKeyser HC, Wong Z-Z, Hastings JC, Baumler DJ (2016). Vitamin variation in Capsicum spp. Provides opportunities to improve nutritional value of human diets. PLoS ONE 7:1-12.
Crossref
|
|
|
|
|
Kaur C, Kapoor HC (2001). Antioxidants in fruits and vegetables-the millennium's health. International Journal of Food Science & Technology 36(7):703-725.
Crossref
|
|
|
|
|
Kim EH, Lee SY, Baek DY, Park SY, Lee SG, Ryu TH, Lee SK, Kang HJ, Kwon OH, Kil M, Oh SW (2019). A comparison of the nutrient composition and statistical profile in red pepper fruits (Capsicums annuum L.) based on genetic and environmental factors. Applied Biological Chemistry 62:48.
Crossref
|
|
|
|
|
KoffiNevry R, Kouassi CK, Zinzerdof YN, Marina K, Guillaume YL (2012). Antibacterial activity of two bell pepper extracts: Capsicum annuum L. and Capsicum frutescens. International Journal of Food Properties 15:961-971.
Crossref
|
|
|
|
|
Ladan MJ, Bilbils LS, Lawal M (1996). Nutrient composition of some green leafy vegetable consumed in Sokoto. Nigerian Journal of Basic and Applied Sciences 5:39-44.
|
|
|
|
|
Lajide L, Oseke MO, Olaoye OO (2008). Vitamin c, fibre, lignin and mineral contents of some edible legume seedlings. Journals of food technology 6(6):237-241.
|
|
|
|
|
Lawal IO, Grierson DS, Afolayan AJ (2014). Phytotherapeutic Information on Plants Used for the Treatment of Tuberculosis in Eastern Cape Province, South Africa. Evidence-Based Complementary and Alternative Medicine 11 p.
Crossref
|
|
|
|
|
Li RW, Leach DN, Myers P, Leach GJ, Lin GD, Brushett DJ, Waterman PG (2004). Anti-inflammatory activity, cytotox-icity and active compounds of Tinospora Smilacina Benth. Phytotherapy Research 18:78-83.
Crossref
|
|
|
|
|
Lohlum SA, MaikidiGH, Solomon M (2010). Proximate Composition, Amino acid Profile and Phytochemical Screening of Lophira lanceolata Seeds, African Journal of Food, Agriculture, Nutrition and Development 10(1):2012-2023.
Crossref
|
|
|
|
|
Low Dog T (2006). A reason to season: the therapeutic benefits of spices and culinary herbs. Explore (NY) 2:446-449.
Crossref
|
|
|
|
|
Ludy MJ, Moore GE, Mattes RD (2012). The effects of capsaicin and capsiate on energy balance: critical review and meta analyses of studies in humans. Chemical Senses 37:103-121.
Crossref
|
|
|
|
|
Mahadevan R, Sridhar E (1982). Methods in Physiological Plant. Pathologyí Sivakin Publications.
|
|
|
|
|
Maji AK, Banerji P (2016). Phytochemistry and gastrointestinal benefits of the medicinal spice, Capsicum annuum L.(Chilli): a review. Journal of Complementary and Integrative Medicine 13:97-122.
Crossref
|
|
|
|
|
Makkar HPS, Blummel M, Borowy NK, Becker K (1993). Gravimetric determination of tannins and their correlations with chemical and protein precipitation methods. Journal of the Science of Food and Agriculture 61:161-165.
Crossref
|
|
|
|
|
McGraw LJ, Jager AK, Van Staden J (2002). Isolation of antibacterial fatty acids from Schotia brachypetala. Fitoterapia 73:431-433.
Crossref
|
|
|
|
|
MERCK (2005). Mineral deficiencies. The Merck Veterinary Manuel, Ninth Edition. Published by Merck and Co. Inc., Whitehouse Station, N.J., USA. pp. 2320-2330.
|
|
|
|
|
Muhammad A, Dangoggo SM, Tsafe AI, Itodo AU, Atiku FA (2011). Proximate, minerals and anti-nutritional factors of Gardenia aqualla (Gauden dutse) fruit pulp. Pakistan Journal of Nutrition 10(6):577-581.
Crossref
|
|
|
|
|
Neelam G, Madhu G, Darshana M, Bhupendra K (2016). Chemical composition, total phenolic and flavonoid contents, and in vitro antimicrobial and antioxidant activities of crude extracts from red chilli seeds (Capsicum frutescens L.). Journal of Taibah University for Science 10(4):462-470.
Crossref
|
|
|
|
|
O'Dell BL (1979). Effects of soy protein on trace mineral bioavailability. In "Soy protein and human Nutrition" (H.L Wilke, D.t. Hopkins and D.H Waggle) p. 187. Academic press, New York.
Crossref
|
|
|
|
|
Okaka JC, Akobundu ENT, Okaka ANC (2006). Food and human nutrition an integrated approach. OCJ. Academic publishers, Enugu. Nigeria. 135-368.
|
|
|
|
|
Okwu DE (2001). Evaluation of the chemical composition of indigenous spices and flavouring agents. Global Journal of Pure and Applied Sciences 7:455-459.
Crossref
|
|
|
|
|
Olatunji TL, Afolayan AJ (2020) Comparison of nutritional, antioxidant vitamins and capsaicin contents in Capsicum annuum and C. frutescens, International Journal of Vegetable Science 26(2):190-207.
Crossref
|
|
|
|
|
Onwuka GI (2005). Food analysis and instrumentation, proximate composition of food minerals 1st Edition. Naplithali print, a Division of H.G. support Nigerian Ltd, Nigeria pp. 64-81.
|
|
|
|
|
Otunola GA, Afolayan AJ, Ajayi EO, Odeyemi SW (2017). Characterization, Antibacterial and Antioxidant Properties of Silver Nanoparticles Synthesized from Aqueous Extracts of Allium sativum, Zingiber officinale, and Capsicum frutescens. Pharmacognosy Magazine 13(2):201-208.
Crossref
|
|
|
|
|
Papoiu AD, Yosipovitch G (2010). Topical capsaicin. The fire of a 'hot'medicine is reignited. Expert Opinion on Pharmacotherapy 11:1359-1371.
Crossref
|
|
|
|
|
Pawar SS, Bharude NV, Sonone SS, Deshmukh RS, Raut AK, Umarkar AR (2011). Chilles as food, spice and medicine: a perspective. International Journal of Pharma and Bio Sciences 1:311-318.
|
|
|
|
|
Pearson D (1976). The Chemical Analysis of Foods. Churchill Livingstone, Edinburgh.
|
|
|
|
|
Prior RL, Cao G (2000). Antioxidant phytochemicals in fruits and vegetables: Diet and health implications. HortScience 35(4):588-592.
Crossref
|
|
|
|
|
Saldanha LG (1995). Fibre in the diet of U.S. children: Result of national surveys. Pediatrics 96:994-996.
|
|
|
|
|
Sarpras M, Gaur R, Sharma V, Chhapekar SS, Das J, Kumar A, Yadava SK, Nltin M, Brahma V, Abraham SA, Ramchiary N (2016). Comparative analysis of fruit metabolites and pungency candidate genes expression between Bhut Jolokia and other Capsium species. PLoS ONE 11(12):e0167791.
Crossref
|
|
|
|
|
Shariati A, Pordeli HR, Khademian A, Aydani M (2010). Evaluation of the antibacterial effects of Capsicum Spp. extracts on the multi-resistant Staphylococcus aureus strains. Journal of Plant Science and Research 5:76-83.
|
|
|
|
|
Singletary K (2011). Red pepper: overview of potential health benefits. Nut Today 46:33-47.
Crossref
|
|
|
|
|
Soetan KO, Olaiya CO, Oyewole OE (2010). The importance of mineral elements for humans, domestic animals and plants- A review. African Journal of Food Science 4(5):200-222.
|
|
|
|
|
Soumya SL, Nair BR (2012). Antifungal efficacy of Capsicum frutescens L. extracts against some prevalent fungal strains associated with groundnut storage. Journal of Agricultural Technology 8:739-750.
|
|
|
|
|
Sukhdev SH, Suman PS, Gennaro L, Dev DR (2008). Extraction Technologies for Medicinal and Aromatic Plants. International Center for Science and High Technology, pp. 1-10.
|
|
|
|
|
Takahashi M, Arakaki M, Yonamine K, Hashimoto F, Takara K, Wada K (2018). Influence of fruit ripening on color, organic acid contents, capsaicinoids, aroma compounds, and antioxidant capacity of shimatogarashi (Capsicum frutescens). Journal of Oleo Science 67(1):113-123.
Crossref
|
|
|
|
|
Trease GE, Evans WC (1989). Pharmacognosy. 13th (ed). ELBS/Bailliere Tindall, London. pp. 345-773.
|
|
|
|
|
Unuofin JO, Otunola GA, Afolayan AJ (2017). Phytochemical screening and in vitro evaluation of antioxidant and antimicrobial activities of Kedrostis africana (L.) Cogn. Asian Pacific Journal of Tropical Biomedicine 7(10):901-908.
Crossref
|
|
|
|
|
Vinayaka KS, Nandini KC, Rakshitha MN, Ramya M, Shruthi J, Hegde SV, Kekuda TRP, Raghavendra HL (2010). Proximate composition, antibacterial and anthelmintic activity of Capsicum frutescens (L.) Var. Longa (Solanaceae) Leaves. Pharmacognosy Journal 2:486-491.
Crossref
|
|
|
|
|
Wei LS, Wee W, Siong JY, Syamsumir DF (2011). Characterizationof anticancer, antimicrobial, antioxidant properties and chemicalcompositions of Peperomia pellucida leaf extract. Acta Medica Iranica 49:670-674.
Crossref
|
|