Full Length Research Paper
ABSTRACT
Endophytic fungus Cercospora kikuchii lipase was immobilized on agroindustrial by-products and dried by oven, freeze and spray drying. Spray drying showed the best performance regarding the drying technologies evaluated. Microcrystalline cellulose and rice husk showed the best result since they retained almost 100% of lipase activity after drying. Immobilized derivatives obtained had decreased enzyme activity (» 30.0%) during a storage period of six months; and retained an average of 50.0% of the initial activity after five reuse cycles. Water content in immobilized derivatives varied between 4.2 and 6.1% and the water activities ranged from 0.14 to 0.30.
Key words: Enzyme immobilization, drying, Cercospora kikuchii, agricultural by-products.
Abbreviation: p-NPP, p-Nitrophenyl palmitate; BSA, bovine serum albumin; PDA, potato agar dextrose; MCC, microcrystalline cellulose.INTRODUCTION
Lipases (triacylglycerol acylhydrolase - EC 3.1.1.3) are enzymes formerly characterized by the ability to reacting with a wide range of substrate with a high enantio and regio selectivity (Singh and Mukhopadhyay, 2012). In fact, this enzyme has a considerable industrial potential and catalyze a number of useful reactions, such as esterification, transesterification, acidolysis, alcoholysis, aminolysis and resolution of racemic mixtures (Reetz, 2002). According to Adlercreutz (2013), the use of lipases in non-conventional media (for esterification and transesterification reactions) has expanded since the mid 1980s, allowing the efficient use of lipases in different industrial processes, in addition to the traditional hydrolysis reactions. Despite widespread research efforts in academics and industry, the application of enzymes can suffer from several drawbacks like instability towards temperature, pH and shear resulting in limited suitability or shelf life (Cowan and Fernandez-Lafuente, 2011). Moreover, soluble enzymes cannot be easily recovered from reaction medium and hence cannot be reused. These operational problems have been improved steadily over the years through the use of process alterations, genetic engineering or immobilization techniques (Polizzi et al., 2007). The last one is attractive for all types of enzymes, in particular lipases due to its use in organic media, bringing some industrial and economical advantages such as recovery and re-use, greater stability and continuous operation (Adlercreutz, 2013). So far, various carriers and methodologies have been used for enzyme immobilization in order to improve the properties of free enzyme (Castro et al., 2001; Freitas et al., 2010; Pereira et al., 2003; Costa-Silva et al., 2014a). Enzymes immobilization can be carried out on organic and inorganic supports; and the strategies generally used can be classified into three types: non-covalent adsorption, encapsulation, and covalent attachment, each with their proper advantages and disadvantages (Nisha et al., 2012). Adsorption of enzymes onto a support is one of the most basic methods of enzyme immobilization. It involves physical surface interactions between the enzyme and the support matrix and can be driven by combined hydrogen bonding, electrostatic attractive forces, and hydrophobic effects (Adlercreutz, 2013). This immobilization method is important as leaching and excessive denaturing is reduced because it can be conducted under mild conditions. Generally, the choice of a suitable immobilization strategy is determined by the physico-chemical properties of both supporting surface and the enzyme of interest (Khan and Alzohairy, 2010). In this context, the properties of the carrier materials have significant influence on enzyme immobilization. The supports should be readily available, nontoxic, resistant to mechanical stress and should offer a good biological compatibility for enzyme (Zhang et al., 2013). Researchers have used eco-friendly supports like coconut fibers, rice straw, wood cellulignin, chitin, cotton cloth and olive pomace having good results regarding enzyme stability and industrial application features (Brígida et al., 2008; Castro et al., 2001; Synowiecki et al., 1987; Shu et al., 2011). So, eco-friendly supports, mainly of biological origin, not only prevent emergence of ethical issues, but also cut down the production costs (Datta et al., 2013).
The performance of immobilized enzyme relies on several key factors including immobilization strategy, immobilization carrier materials, enzyme pre-treatment and enzyme loading (Zhang et al., 2013). Moreover, dehydration of the enzyme support systems is another important factor that affects the end properties of the immobilized enzyme. The water concentration, even when using organic solvents as reaction media, is a very important parameter that should be measured and adjusted before the immobilized enzyme application. For special case of lipases, water can promote negative effects on the rate of catalysed reactions due to: interference with substrate binding (inhibition), formation of a diffusion barrier for hydrophobic substrates and causes hydrolysis which competes with esterification or transesterification reaction (Adlercreutz, 2013). Several drying technologies can be utilized for the production of dried thermosensitive products like plant extracts, fruit juices, blood products, microorganisms, and enzymes, including freeze drying, spray drying, and spouted and fluidized bed drying (Costa-Silva et al., 2011; Souza and Oliveira, 2005; Samborska and Witrowa-Rajchert, 2005; Bott et al., 2010; Schutyser et al., 2012; Yang et al., 2012). Great attention has been paid to spray drying, a mild and cost-effective convective drying method, albeit in industrial practice for example freeze drying or freezing are often preferred dehydration method for biologics (Schutyser et al., 2012).
The aim of this work was to investigate the potential of several agricultural by-products as low-cost and eco-friendly supports for immobilization of lipase produced by endophytic fungus Cercospora kikuchii using the adsorption method followed by dehydration in different drying technologies: oven, freeze drying and spray drying. Thus, exploiting novel immobilization methods and carrier materials have an important significance on enzyme immobilization technology.
MATERIALS AND METHODS
Bradford reagent, p-nitrophenyl palmitate (p-NPP), bovine serum albumin (BSA), was purchased from Sigma-Aldrich (St. Louis, MO - USA); Potato Agar Dextrose (PDA) was purchased from Biolife (Milan, Italy). Microcrystalline cellulose (MCC) was purchased from Blanver Farmoquimica Ltda (Itapevi, Brazil). All other chemicals, media, and reagents were of analytical grade.
Microorganism and lipase production
The lipase used as model in this study was produced by the endophytic fungus C. kikuchii, isolated from Tithonia diversifolia. The lipase production was carried out in 250 mL Erlenmeyer flasks containing 100 mL of Vogel’s minimum medium supplemented with 2% soybean oil as the only carbon source (Vogel, 1956). The culture was incubated at 30°C in a rotary shaker at 120 rpm for 6 days and the mycelium obtained was removed by vacuum filtration through filter papers (No. 1 Whatmann filter paper, GE Health Care, São Paulo, Brazil) (Costa-Silva et al., 2011). The C. kikuchii lipase was biochemically characterized according to Costa-Silva et al. (2014).
Protein assay
Protein concentration was determined according to Bradford method, which involves the binding of Coomassie Brilliant Blue G-250 to protein. Bovine serum albumin was used as a standard (Bradford, 1976).
Enzymatic activity of the free and immobilized lipase
ρ-nitrophenyl palmitate (p-NPP)
Lipase activity assay was performed using ρ-nitrophenyl palmitate (p-NPP) as substrate according to Mayordomo et al. (2000). In brief, the reaction mixture consisted of 205 μL of buffer (200 mg of Triton X-100 and 50 mg of gum arabic in 50 mL of 50 mm phosphate buffer, pH 6.5), 45 μL of substrate (15 mg of p-NPP in 10 mL of 2-propanol), and 250 μL of enzyme solution (5 mgprot/mL). The mixture was incubated at 40°C for 30 min and then 0.5 mL of 2% trizma base was added. The optical density was measured at 410 nm. Enzymatic activity is given as μmol of pNP produced per minute per mg of enzyme (IU) under the conditions described above.
Olive oil emulsion
C. kikuchii lipase activity was also measured using an olive oil emulsion as substrate, according to the method described by Andrade et al. (2014). The substrate was prepared by mixing 50 g olive oil with 150 g Arabic gum solution (3 wt.%). The reaction mixture containing 5 mL emulsion, 5 mL 0.1 M phosphate buffer (pH 6.5), and immobilized (2.0 g to 2 mg of protein g-1 of support ) or soluble (0.250 mL - 5 mgprot/mL) lipase was incubated for 5 min at 40°C. The reaction was stopped by addition of 10 mL commercial ethanol. The fatty acids formed were titrated with 0.02 M sodium hydroxide solution in the presence of phenolphthalein as indicator. One international unit of activity was defined as the amount of enzyme that liberates 1 µmol free fatty acid per minute per mg of enzyme (IU) under the conditions described above.
Support
“In natura” Agricultural byproducts supplied by local farmers, were ground and sieved to obtain particle sizes between 50 and 150 mesh. These materials were then washed with distilled water and dried at 60°C before being used as the support matrix. Microcrystalline cellulose (MCC) was also used as a model support.
Support characterization
The specific surface area of supports and total volume and average pore diameter were determined on a Quantachrome equipment New Model 1200, equipped with software for data analysis from measures adsorption-desorption of N2. Before analysis, samples were subjected to heat treatment at 60°C under vacuum for 48 h to remove the water adsorbed during handling and possible condensate existing in the pores of the solids. The surface areas of the samples were calculated by the Brunauer, Emmett and Teller (BET) method and pore parameters were determined based on calculations BJH (Barrett-Joyner-Halenda). The technique encompasses external area and pore area evaluations to determine the total specific surface area in m2/g yielding important information in studying the effects of surface porosity and particle size in many applications. The specific surface area of a sample is determined by physical adsorption of a gas on the surface of the solid and by calculating the amount of adsorbate gas corresponding to a monomolecular layer on the surface (Fagerlund, 1973). The determination is usually carried out at the temperature of liquid nitrogen. The amount of adsorbed gas is dependent on its relative vapour pressure and is proportional to the total external and internal surface of the material (Fagerlund, 1973). The porosity, pore size distribution and density of the adsorbent material were obtained by mercury porosimetry (Autopore II brand Micromeritics) (Bedin et al., 2013; Ramos et al., 1998).
Lipase immobilization
Lipase was immobilized by adsorption in byproducts in the presence of polyethylene glycol (PEG 1500 MW) as stabilizing agent. Lipase-support (5 mg of protein.g-1 of support) system was maintained in contact for 1 h at room temperature under 250 rpm. Hence, the immobilized derivatives were dried by spray drying, oven and freeze drying methods. The activity retention (RAE %) was calculated following the equation 1:
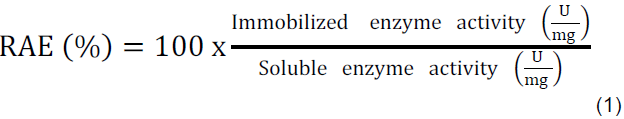
The immobilization efficiency, IE (η%), was determined by equation 2 (Menoncin et al., 2009), where P0 was the protein content in the lipase solution (mg) and P1 was the amount of protein adsorbed on the supports (mg). P1 was estimated by the difference between total protein content added to immobilization process and the protein content washed from the supports.
IE (%) = 100 × (P0/P1) (2)
Spray drying
Immobilized derivatives were dehydrated in a bench-top spray dryer (model SD-05, Lab-Plant, Huddersfield, U.K), with concurrent flow regime. The drying chamber has 215 mm in diameter and height of 500 mm. The main components of the system are a feed system of the drying gas, constituted by a blower and an air filter; a temperature control system of the drying gas and a product collect system (cyclone). The enzyme solution (soluble enzyme + supports: 5 mg of protein g-1 of support at pH 6.7) was fed to the spray dryer through a feed system, constituted by a peristaltic pump, a two fluid atomizer (inlet orifice diameter of 1.0 mm) and an air compressor. The spray drying conditions were determined in a previous study of optimization of spray drying of crude lipase extract (Costa-Silva et al., 2011). The feed flow rate of atomizing air was set in 17.0 L/min at pressure of 1.5 kgf/cm2 (Costa-Silva et al., 2014b). The flow rate of the drying air was maintained constant at 60 m3/h. The drying operation started with injection of the drying air into the SD-05 spray dryer. The air was heated to the desired temperature (100°C) and then the enzyme-support solution was fed at a preset flow rate together with the atomizing air. Measurements of the outlet gas temperature, Tgo, were taken at regular intervals in order to detect the moment when the dryer attained the steady state (± 15 min).
Freeze-drying
The experiments were performed with a vertical freeze-dryer (SNL 108 B – Thermo Fischer Scientific). Initially, the enzymatic solution (soluble enzyme + supports: 5 mg of protein g-1 of support at pH 6.7) was maintained in contact for 1 h at room temperature at 250 rpm. Then the immobilized derivative was frozen in a freezer (refrigerator) at -80°C and posteriorly was submitted to freeze drying. The chamber temperature was maintained at approximately -50°C and 0.05 mbar. The frozen samples were lyophilized for 24 h.
Oven drying
The drying operations were performed using an oven dryer model: Fanem, mod. 315 SE – Guarulhos, Brazil. The enzymatic solution (soluble enzyme + supports: 5 mg of protein g-1 of support and pH 6.7) was maintained in contact for 1 h at room temperature at 250 rpm. Then the immobilized derivative was recovered and posteriorly was submitted to oven drying at 40°C for 24 h.
Dryer performance and product properties
Samples of the dried product, using all drying equipment, were collected and used to evaluate the dryer performance and product properties through the following procedures:
Enzymatic activity
The lipase activity assay was performed using p-NPP as the substrate, with some modifications (Mayordomo et al., 2000). The difference was the use of 1 g immobilized derivative (5 mg of protein g-1 of support) in 50 mM of phosphate buffer, pH 6.5. The solution was used to evaluate the residual activity.
Enzymatic activity of immobilized derivatives after reuse cycles
Residual enzymatic activity was determined for the immobilized lipase derivatives after each batch of reaction (Andrade et al., 2014). The substrate was prepared by mixing 50 g olive oil with 150 g Arabic gum solution (3 wt.%). The reaction mixture containing 5 mL emulsion, 5 mL 0.1 M phosphate buffer (pH 6.5), and immobilized (2.0 g to 2 mg of protein g-1 of support) or soluble (0.250 mL to 5 mg prot/mL) lipase was incubated for 5 min at 40°C. The reaction was stopped by addition of 10 mL commercial ethanol. The fatty acids formed were titrated with 0.02 M sodium hydroxide solution in the presence of phenolphthalein as indicator. The residual activity of the biocatalyst was calculated in terms of percentage of activity (U) of the immobilized enzyme measured after each cycle compared with the activity of the immobilized enzyme before the first cycle.
Efficiency of the powder production
The spray-drying performance was evaluated by mass balance, through the determination of the product recovery (REC), defined as the ratio between the total mass of the product recovered to the mass of enzyme-support composition fed to the system (dry basis).
Product moisture content
The moisture content of the spray-dried product was determined by the oven drying method at 105°C up to a constant weight and was calculated from triplicate analyses and by Karl Fischer method (WHO, 1998; Mendham et al., 2002).
Water activity (Aw)
Water activity was determined in an AQUALAB 4TEV-Decagon according to Norenã et al. (1996).
Enzyme stabilization
The stability of the immobilized enzyme derivatives after spray drying was assessed by monitoring the retention of the enzyme activity during a storage period of 6 months at 5°C.
RESULTS
For industrial use as biocatalysts, soluble enzymes have to be immobilized in order to be reused for several processing cycles. In addition, some other critical enzyme properties need to be improved, including stability, activity, and selectivity. Enzyme immobilization by physical adsorption traditionally refers to binding of the enzymes via weak attractive forces to an inert carrier that has not been chemically modified. Because the carrier is directly involved in binding to the enzyme, both morphologic and chemical characteristics play important roles. Table 1 shows selected physical characteristics of supports used, important for adsorption. The agro industrial by-products showed high specific surface compared to commercial beads (Accurel MP1000: specific surface area was mea-sured at 78.92 cm2/g or controlled-pore glass beads surface area 22.7 m2 g-1) which makes them suitable to be used as carriers, particularly for enzymes adsorption studies (Séverac et al., 2011; Gunnlaugsdottir et al., 1998).
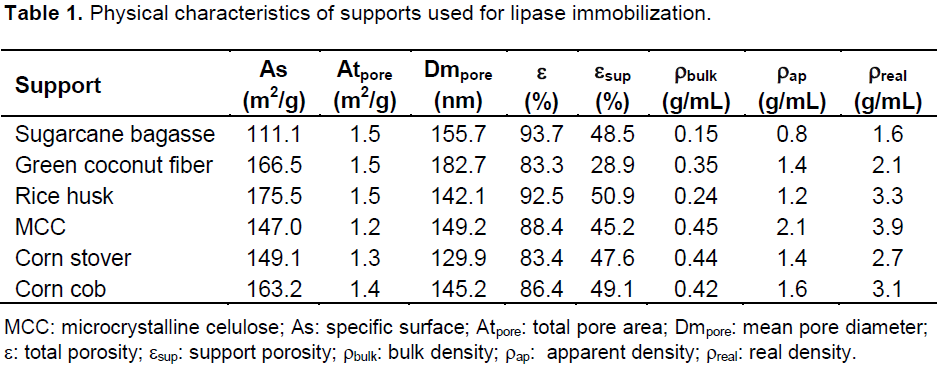
The immobilization efficiency was consistent with the values ​​of enzymatic activity retention and the specific surface of the supports evaluated. The coconut husk, corn cobs, rice husk and microcrystalline cellulose showed higher values ​​for the surface area and hence greater immobilization efficiency (Tables 1 and 2) evidencing the existence of a relationship between specific surface and the support adsorption capacity. From the results presented in Table 1 and 2, it can be observed that in general the greater the supports specific surface, the higher is the immobilization efficiency (Figure 1). In this set of experiments, the green coconut fiber, rice husk and corn cob presented the highest values of specific surface and immobilization efficiency.
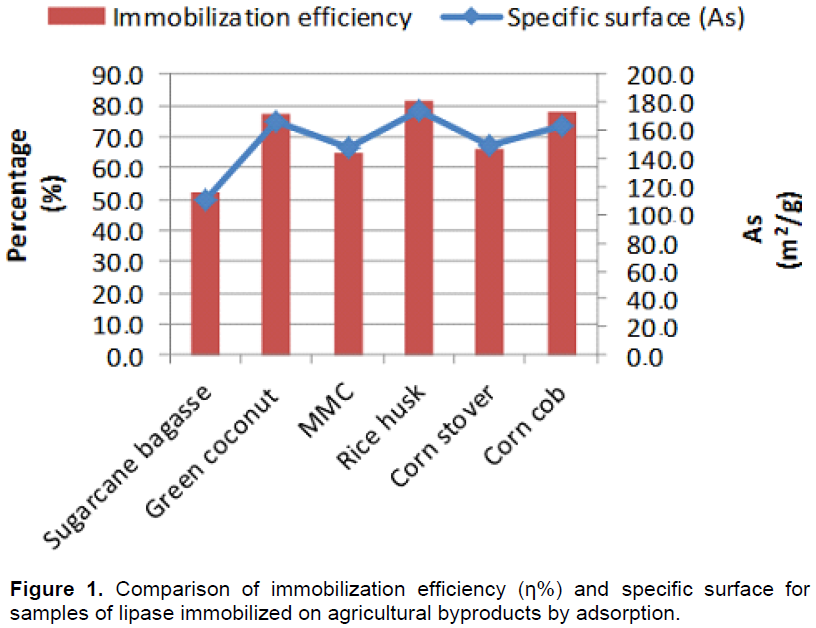
Although, the knowledge of the sample surface area is important, the pore size distribution is even more critical, since it greatly affects the activity-coupling yield of the biologic immobilization because of the diffusion-controlled phenomena. The samples showed structures with different levels of porosity, with pores diameters larger than 50 nm which classifies them as macroporous materials. When enzyme is immobilized within a porous support, there could also present resistance to internal diffusion, since it must diffuse through the pores in order to contact the biocatalyst, in addition to external mass-transfer effects. Decreasing the dimensions of the porous support containing the biocatalyst can contribute to reduce this additional effect, since the path length the substrate should pass through is significantly reduced, leading to a decrease in the substrate concentration gradient (Soares et al., 1999). For the present work, it was used as a substrate with lower molecular weight, p-NPP, and higher enzyme activity retention was obtained compared with olive oil as substrate. In literature the lignocellulosic material density is characterized by an inhomogeneous value. This is understandable since the variations of the characteristics are determined by many factors, such as moisture, particle size, influence the milling mechanism, structure composition, among others. It is important to know the particles density given that it is coupled with the porosity of the sample and, therefore, with the adsorption potential. Besides, it is also important in the productive chain mainly due to fluid dynamic behavior, and product behavior during transport and storage.
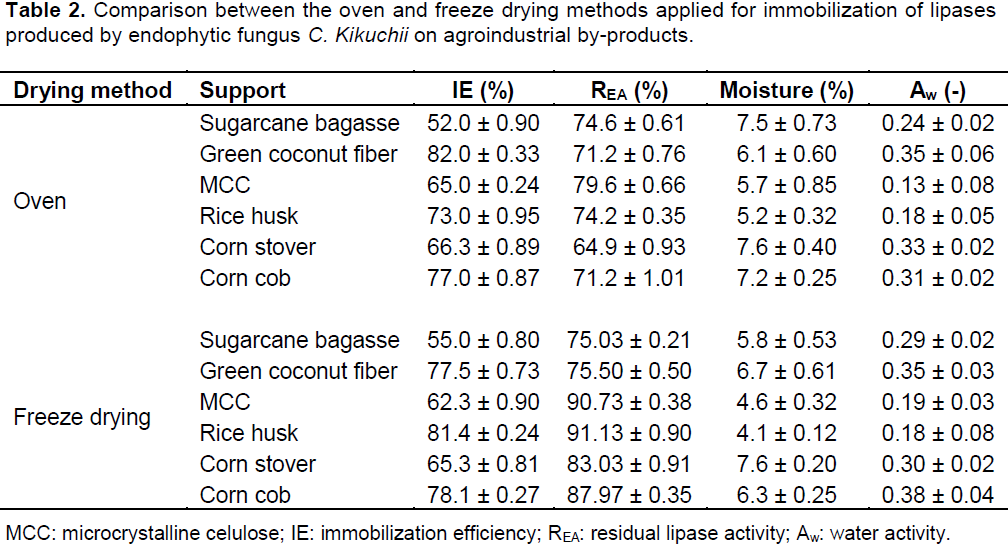
One of the main concerns of enzyme drying is the retention of enzyme activity, which must be retained during all product shelf life. In this work, we combined the advantages of the drying and immobilization processes and made them a unique step using a spray dryer (that showed the best result for enzyme activity retention), making industrial-scale application an economically feasible process. Table 2 shows the effects of the oven and freeze drying methods on properties of the final product. The residual enzymatic activity of the product generated by both drying procedures was in the range of 67.9 to 91.1%. The freeze drying method showed the best results for the residual enzymatic activity. The rice husk was the best support used, maintaining 91.1% of activity after drying. This was followed by microcrystalline cellulose (90.7%), corn cob (87.9%) and corn stover (83.0%). The utilization of moisture as a quality indicator is of particular interest, because water is a key determinant of both the integrity of the solid matrix and support–protein interactions. Water content in the obtained powders ranged between 4.1 and 7.6%. These low values are important since the dehydration could provide an acceptable protein shelf life, and protect the biological activity of these molecules (Namaldi et al., 2006). Water activity is another factor that affects the enzyme stability. Higher values of water activity could provide feasible conditions for microorganism growth and occurrence of degradation reactions. The water activities of the immobilized derivatives were in the range of 0.13 to 0.38; which are considered safe to avoid micro-organism development (Beauchat, 1981).
The spray drying was the third method evaluated. This drying process is a mild technique due to its very short drying times and the relatively low temperatures to which the product is exposed mainly when compared with others convective air-drying methods (Mazza et al., 2003). Table 3 shows the results of product recovery, residual lipase activity, water content, storage stability and enzyme activity after reuse cycles of the spray dried immobilized derivatives. The products recoveries were in the range of 41.3 to 77.0%, which are common values for bench-top spray dryers. Cyclone efficiency and powder deposition in the spray-drying chamber contributes to product loss. The residual enzymatic activity after spray drying was in the range of 85.5 to 98.6%. Therefore, spray dryer was the best drying equipment used for immobilized derivatives dehydration, in terms of retention of enzyme activity. Among all support evaluated, microcrystalline cellulose and rice husk showed the best result because it maintained almost 100% of activity after drying. For the natural lipase substrate, olive oil, the average of enzyme activity retention was 72.7%. Lipase immobilized on microcrystalline cellulose showed the best results, presenting 78.1% of the original activity after spray drying, followed by rice husk (73.9%), green coconut husk (71.3%), sugarcane bagasse (70.7%) and corn cobs (67.8%). The reason for this lower activity could be due to substrate nature: the oil chain is higher than pNPP chain, so the access to the biocatalyst is hampered compared with the synthetic substrate.
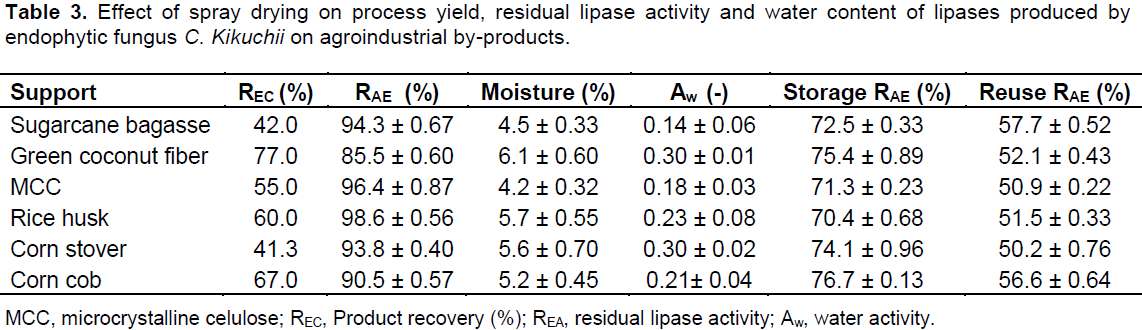
During spray drying the rapid changes in droplet temperature and moisture content has influence on enzyme conformation and consequently its activity. Other possible stress factors that the protein experiences during spray drying are: adsorption, shearing stress and liquid/air interfacial expansion (Lee, 2002). However, optimum drying conditions and tailored matrix formula-tions are required to avoid severe structural damage of enzyme chain leading to loss in enzyme activity. In this study, the positive interaction between the lipase and supports during the drying process could be responsible for the high enzyme activity retention. In our previews study, the effect of spray drying conditions on the retention of enzyme activity of lipase, in the presence of carbohydrates have been investigated. The residual enzyme activity after drying with 10% (w/v) of lactose, b-cyclodextrin, maltodextrin, mannitol, gum arabic, and trehalose ranged from 63 to 100% (Costa-Silva et al., 2011). The enzyme activity was lost in the absence of adjuvants. Therefore, the addition of some drying adjuvants (or supports/carries) offers a way to prevent direct contact of enzyme with the high-temperature air and is one of the key techniques for the encapsulation of pharmaceutical enzymes by spray drying.
Stability tests were performed for all spray dried samples, which were stored at 5°C for up to 6 months. The immobilized derivatives obtained had decreased enzyme activity with an average of only 30.0%, whereas the free enzyme form lost 85.8% of its initial activity in the same period. These results are an indicator of the feasibility of using the spray drying as a way to protect the enzyme properties and to control their stability. The ability to reuse the biocatalyst is of practical and economical importance. In this work, the operational stability of immobilized derivatives was determined using olive oil as the substrate.
The results are also summarized in Table 3. It can be observed that the biocatalysts prepared retained an average of 53.2% of the initial activity after five reuse cycles. Lipase immobilized on sugarcane bagasse showed the best results of operational stability, presenting 68.1% of the original activity after first activity cycle, followed by 63.9% (cycle 2), 61.1% (cycle 3), 58.9% (cycle 4) and 57.7% after five reuse activity cycle. In general, low values of moisture content (and water activity as well) are excellent for product stability. Water content in the obtained powders varied between 4.2 and 6.1% and the water activities of the dried immobilized derivatives were in the range 0.14 to 0.30. The presence of water can accelerate degradation reactions in the solid state, such as deamidation, oxidation, disulfide cross-linking, and Maillard reactions. In particular for proteins, water can affect a complex matrix of protein movements, ranging from oscillatory and rotational motion of individual amino acid groups, to segmental and internal fluctuations that increase their dynamic mobility and thereby decrease their conformational stability (Bone, 1994). Besides, another important observation about drying process is that the water content of immobilized enzymes could be associated with their application. Industrially, lipases are applied mainly in organic reactions and the major of these processes must be performed in the absence of water.
CONCLUSION
A practical simultaneous immobilization and drying method to load lipase onto non-conventional supports was developed. In this work, it was demonstrated that cheap eco-friendly supports were biocompatible with lipases, rendering immobilized derivatives with characteristics similar to or even better than those previously obtained with natural and synthetic polymers, such as chitosan and silica matrices. It was also demonstrated that spray drying can be successfully used for drying thermally sensitive materials, such as immobilized enzymes, considering the high relative enzymatic activity achieved after the dehydration step. Thus, the procedures established in this paper have promising capability to be applied for immobilization of other enzymes of industrial interest.
CONFLICT OF INTERESTS
The authors did not declare any conflict of interest.
REFERENCES
|
Adlercreutz P (2013). Immobilization and application of lipases in organic media. Chem. Soc. Rev. 42: 6406-6436. Crossref |
||||
| Beauchat LR (1981). Microbial stability as affected by water activity. Cereal Food World 26: 345–349. | ||||
| Bedin S, Oliveira MF, Vieira MGA, Vieira MGA, Silva MGC, Santos OAA (2013). Adsorption of Toluene in Batch System in Natural Clay and Organoclay. Chem. Eng. Trans. 32: 313-318. | ||||
|
Bone S (1994). Dielectric studies of native, unfolded and intermediate borms of â-lactamase. Phys. Med. Biol. 39: 1801-1809. Crossref |
||||
|
Bott RF, Labuza TP, Oliveira WP (2012). Stability testing of spray- and spouted bed dried extracts of Passiflora alata. Drying Technol. 28: 1255–1265. Crossref |
||||
|
Bradford MM (1976). A rapid and sensitive method for the quantification of microgram quantities for protein utilizing the principle of protein–dye binding. Anal. Biochem. 72: 156–171. Crossref |
||||
|
Brígida AIS, Pinheiro ADT, Ferreira ALO, Gonçalves LRB (2008). Immobilization of Candida antarctica Lipase B by adsorption to green coconut fiber. Appl. Biochem. Biotechnol. 146: 173-187. Crossref |
||||
|
Castro HF, Lima R, Roberto CI (2001). Rice straw as a support for immobilization of microbial lipase. Biotechnol. Prog. 17: 1061-1064. Crossref |
||||
| Costa-Silva TA, Marques PS, Souza CRF, Said S, Oliveira WP (2014b). Enzyme encapsulation in magnetic chitosan-FeO3 microparticles. J. Microencapsul. 12:1-6. | ||||
|
Costa-Silva TA, Nogueira MA, Souza CRF, Oliveira WP, Said S (2011). Lipase production by endophytic fungus Cercospora Kikuchii: Stability of enzymatic activity after spray drying in the presence of carbohydrates. Drying Technol. 29:1112–1119. Crossref |
||||
|
Costa-Silva TA, Souza CRF, Oliveira WP, Said S (2014a). Characterization and spray drying of lipase produced by the endophytic fungus Cercospora kikuchii. Braz. J. Chem. Eng. 31:849-858. Crossref |
||||
|
Cowan DA, Fernandez-Lafuente R (2011). Enhancing the functional properties of thermophilic enzymes by chemical modification and immobilization. Enzyme Microb. Technol. 49: 326– 346 Crossref |
||||
| Datta S, Christena LR, Rajaram YRS (2013). Enzyme immobilization: an overview on techniques and support materials. 3 Biotech. 3: 1–9. | ||||
| Fagerlund G (1973). Determination of specific surface by the BET method. Matieriaux et constructions 6: 33-53. | ||||
|
Freitas L, Paula AV, Santos JC, Zanin GM, Castro HF (2010). Enzymatic synthesis of monoglycerides by esterification reaction using Penicillium camembertii lipase immobilized on epoxy SiO2-PVA composite. J. Mol. Catal. B Enzym. 65: 87–90. Crossref |
||||
|
Gunnlaugsdottir H, Wannerberger K, Sivik B (1998). Alcoholysis and glyceride synthesis with immobilized lipase on controlled pore glass of varying hydrophobicity in supercritical carbon dioxide. Enzyme Microb. Technol. 22: 360–367. Crossref |
||||
|
Kahn AA, Alzohairy MA (2010). Recent advances and applications of immobilized enzyme technologies: A review. Res. J. Biol. Sci. 5: 565-575. Crossref |
||||
|
Lee G (2002). Spray drying of proteins. In Rational Design of Stable Protein Formulations; Carpenter, J.F., Manning, M.C., Eds.; Plenum Press: New York, pp. 135-158. Crossref |
||||
|
Mayordomo I, Randez-Gil F, Pietro JA (2000). Isolation, purification, and characterization of a cold-active lipase from Aspergillus nidulans. J. Agric. Food. Chem. 48:105-109. Crossref |
||||
|
Mazza MGG, Brandao LEB, Wildhagen GS (2003). Characterization of the residence time distribution in spray dryers. Drying Technol. 21:525-538. Crossref |
||||
| Mendham J, Denney RC, Barnes JD, Thomas HJK (2002). Analise Química Quantitativa; LTC Editora S/A: Rio de Janeiro, Brazil. | ||||
|
Menoncin S, Domingues NM, Freire DMG, Oliveira JV, Di Luccio M, Treichel H (2009). Imobilização de lipases produzidas por fermentação em estado sólido utilizando Penicillium verrucosum em suportes hidrofóbicos. Ciênc. Tecnol. Aliment. 29:440-443. Crossref |
||||
|
Namaldi A, Çalik P, Uludag Y (2006). Effects of spray drying temperature and adjuvants on the stability of serine alkaline protease powders. Drying Technol. 24: 1495–1500. Crossref |
||||
| Nisha S, Arun KS, Gobi N (2012). A Review on Methods, Application and Properties of Immobilized Enzyme. Chem. Sci. Rev. Lett. 1:148-155. | ||||
| Norenã CZ, Hubinger MD, Menegalli FC (1996). Técnicas básicas de determinação de atividade de água: Uma revisão. SBCTA 30: 91–96. | ||||
|
Pereira EB, Zanin GM, Castro HF (2003). Immobilization and catalytic properties of lipase on chitosan for hydrolysis and esterification reactions. Braz. J. Chem. Eng. 20: 343-355. Crossref |
||||
|
Polizzi KM, Bommarius AS, Broering JM, Chaparro-Riggers JF (2007). Stability of biocatalysts. Curr. Opin. Chem. Biol. 11: 220–225. Crossref |
||||
|
Ramos MA, Gil MH, Schact E, Matthys G, Mondelaers W, Figueiredo MM (1998). Physical and chemical characterization of some silicas and silica derivatives. Powder Technol. 99: 79–85. Crossref |
||||
|
Reetz MT (2002). Lipases as practical biocatalysts. Curr. Opin. Chem. Biol. 6:145–150. Crossref |
||||
|
Samborska K, Witrowa-Rajchert D (2005). Spray-drying of a-amylase - The effect of process variables on the enzyme inactivation. Drying Technol. 23:941-953. Crossref |
||||
|
Schutyser MAI, Perdana J, Boom RM (2012). Single droplet drying for optimal spray drying of enzymes and probiotics. Trends Food Sci. Technol. 27: 73-82. Crossref |
||||
|
Séverac E, Galya O, Turond F, Pantele CA, Condorete J-S, Monsana P, Marty A (2011). Selection of CalB immobilization method to be used in continuous oil transesterification: Analysis of the economical impact. Enzyme Microb. Technol. 48:61-70. Crossref |
||||
|
Shu C, Caia J, Huanga L, Zhua X, Xua Z (2011). Biocatalytic production of ethyl butyrate from butyric acid with immobilized Candida rugosa lipase on cotton cloth. J. Mol. Catal. B Enzym. 72:139-144. Crossref |
||||
|
Singh AK, Mukhopadhyay M (2012). Overview of Fungal Lipase: A Review. Appl. Biochem. Biotechnol. 166:486-520. Crossref |
||||
|
Soares CM, Castro HF, Moraes FF, Zanin GM (1999). Characterization and utilization of Candida rugosa lipase immobilized on controlled pore silica. Appl. Biochem. Biotechnol. 77: 745-756. Crossref |
||||
|
Souza CRF, Oliveira WP (2005). Spouted bed drying of Bauhinia forficata Link extract: The effects of feed atomizer position and operating conditions on equipment performance and product properties. Braz. J. Chem. Eng. 22:239-247. Crossref |
||||
|
Synowiecki J, Siondalska SA, El-Bedawey AF (1987). Adsorption of enzymes on krill chitin modified with carbon disulfide. Biotechnol. Bioeng. 29:352-354. Crossref |
||||
| Vogel HJ (1956). A convenient growth medium for Neurospora crassa. Microb. Genet. Bull. 13:42-43. | ||||
| WHO: World Health Organization (1998). Quality Control Methods for Medical Plants Materials; World Health Organization: Geneva, p.235. | ||||
|
Yang S, Mao X-Y, Li F-F, Zhang D, Leng X-J, Ren F-Z, Teng G-X (2012). The improving effect of spray-drying encapsulation process on the bitter taste and stability of whey protein hydrolysate. Eur. Food Res. Technol. 235:91-97. Crossref |
||||
|
Zhang D-H, Yuwen L-X, Peng L-J (2013). Parameters Affecting the Performance of Immobilized Enzyme. J. Chem. 2013: 1-7. Crossref |
||||
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0