ABSTRACT
1,3-Propanediol (1,3-PDO) is a bifunctional molecule, and used in applications similar to those of ethylene glycol, propylene glycol, 1,3-butanediol and 1,4-butanediol. The use of glycerol as a feedstock is an alternative to reduce production costs for both 1,3-PDO and biodiesel, since biodiesel glycerol can be used for the production of 1,3-PDO by bacteria. Also, using metabolic engineering, it is possible to manipulate the metabolic routes and obtain high value products, reduce or eliminate the formation of undesirable byproducts. The aim of the study was to produce 1,3-propanediol in E. coli cloned with dha genes from Klebsiella pneumoniae GLC29. Six genes responsible for 1,3-PDO production in Klebsiella pneumoniae GLC29 were cloned. These genes were assembled in pSB1C3 as an expression vector: Genes dhaB1, dhaB2, dhaB3 and dhaT (pSB1C3dhaB123T), and another vector with genes dhaF and dhaG (pSB1C3dhaB123TFG) were derived using Gibson's Assembly technique. Escherichia coli TCS099 and SZ63 stains were used as hosts for 1,3-PDO production, and kept at -80°C for long-term storage. Glycerol was used as the sole or main carbon source in all experiments. Fermentations were performed in flasks in aerobic and anaerobic conditions using minimal media. Also, two stage fermentation (aerobic-anaerobic) was performed for 1,3-propanediol production. Only pSB1C3dhaB123TFG was able to produce high amounts of 1,3-PDO in shake flasks experiments, producing 2.5 g/L in micro-aerobic conditions, using E. coli TCS099 as host. Besides, E. coli SZ63 hosting pSB1C3dhaB123TFG was able to produce high amounts of 1,3-PDO, corresponding to 11.3 g/L of 1,3-PDO using a two-stage fermentation process using low concentration of vitamin B12 (1 mg/L). Plasmid pSB1C3dhaB123TFG shows potential for producing high amounts of 1,3-PDO, specially because of dhaF and dhaG, reaffirming the importance of this genes on 1,3-PDO production, especially with the addition of low amounts of vitamin B12, which is an expensive compound.
Key words: 1,3-Propanediol, Escherichia coli, glycerol, Klebsiella pneumoniae, metabolic engineering.
1,3-Propanediol (1,3-PDO) is a starting point for a new generation of polymers with improved properties for the textile industry. It can be obtained by chemical or biochemical route, however in the chemical route the co-production of its isomer 1,2-propanediol cannot be avoided, being produced in a 1:1 ratio, resulting in a costly separation process. DuPont and Genencor International uses a genetically modified Escherichia coli strain to produce 1,3-PDO from glucose (Maervoet et al., 2011). Glucose is a high cost feedstock, and biodiesel-derived glycerol is becoming an abundant alternative feedstock, due to the increasing biodiesel production (Pyne, 2014). The price of corn-derived glucose is approximately US$ 0.28/kg (Gallardo et al., 2014) while the current price of biodiesel-derived glycerol varies from US$ 0.04 to 0.11/kg (Quispe et al., 2013). Some processes of biodiesel production generate crude glycerol a byproduct considered as waste, generally with no commercial value or acceptance, and its disposal costs is attributed to the biodiesel producers (Yazdani and Gonzalez, 2007).
Glycerol may be used as carbon source in many bioprocesses, and one promising exploitation is to produce 1,3-PDO by Klebsiella pneumoniae and Clostridium butyricum (Papanikolaou and Aggelis, 2009; Rymowicz et al., 2006). Production from crude glycerol from biodiesel can contribute to the reduction of environmental pollution and commercial valorization of this carbon source and to lower the production of 1,3-propanediol. But intrinsic bottlenecks limiting these processes are the potential pathogenicity of K. pneumoniae, and the requirement of total anaerobic conditions for Clostridium spp., which although it is not vitamin dependent, the enzyme from Clostridium spp. is oxygen sensitive, requiring therefore, totally anaerobic cultures (Kaur et al., 2012).
The production of 1,3-PDO is connected to the process of glycerol oxidation. Glycerol enters the cell by glpF (glycerol facilitated transport), or by diffusion (Maervoet et al., 2011). After entering the cell, it may follow two routes. In the first one, it suffers oxidative dehydrogenation by a NAD+ dependent glycerol dehydrogenase, becoming dihydroxyacetone (DHA). DHA is then phosphorylated to dihydroxyacetone phosphate by an ATP-dependent DHA kinase. Through a parallel process, glycerol is dehydrated to form 3-hydroxypropionaldehyde (3-HPA) by glycerol dehydratase (EC 4.2.1.30), which in K. pneumoniae is B12-dependent, composed by three peptides encoded by dhab1, dhaB2, and dhaB3. Then, 3-HPA is reduced to 1,3-PDO by 1,3-propanediol oxidoreductase (EC 1.1.1.202) linked to NADH (Oh et al., 2012; Yazdani and Gonzalez, 2007).
In K. pneumoniae the overall reductive reaction rate is limited, firstly because this reaction is mediated by cyanocobalamin (vitamin B12). Furthermore, substrate inhibition may occur, with an irreversible binding of cobalamin with the enzyme to form alkylcobalamines. However, reactivation factors, encoded by genes gdrA and gdrB (or dhaF and dhaG), swap the inactivated cobalamin for a new molecule of vitamin B12, requiring the presence of magnesium ions (Mg2+) and with consumption of 1 ATP. The resultant Apo enzyme rebinds coenzyme B12, and glycerol conversion to 3-HPA resumes. To avoid low activity of the enzyme, the amount of glycerol should be controlled and vitamin B12 to the medium should be added (Yamanishi et al., 2012; Nakamura and Whited, 2003; Shibata et al., 2002; Kajiura et al., 2001, Daniel et al., 1998). As a consequence of the normal catalytic cycle with glycerol, the coenzyme B12 is occasionally rendered inactive (B12-inact). The B12-inact remains tightly bound to the dehydratase and catalysis ceases. An auxiliary enzyme, glycerol dehydratase reactivase, facilitates the dissociation of the B12-inact and glycerol dehydratase (EC 4.2.1.30). The resultant apoenzyme rebinds and glycerol conversion to 3-HPA resumes (Nakamura and Whited, 2003).
E. coli naturally grows on glycerol under aerobic conditions, but several researchers have been trying to genetically modify it to produce 1,3-PDO, thus making glycerol a valuable carbon source (Ma et al., 2009). By metabolic engineering, it is possible to manipulate metabolic routes, obtain high value products, and reduce or eliminate the production of undesirable byproducts (Cheng et al., 2005). E. coli, which does not have a dha system, is unable to grow anaerobically on glycerol without an exogenous electron acceptor and does not produce 1,3-PDO (Tong et al., 1991). Although several researches have been done on 1,3-PDO production using glycerol, the 1,3-PDO productivities and, in particular, the product concentrations obtainable with engineered organisms harboring the 1,3-PDO pathway have been low (less than 0.1 g/L with recombinant S. cerevisiae and 6.5 g/L with recombinant E. coli AG1) compared to those of natural 1,3-PD producers (Biebl et al., 1999). While other papers have reported high amounts of 1,3-PDO produced by Engineered E. coli harboring genes from Clostridium sp., the limitation of being very sensitive enzymes to oxygen is a drawback in large scale use. Also, very few papers were successful using genes from K. pneumoniae, which is a great natural producer of 1,3-PDO and requires little vitamin B12, as we demonstrated in this work.
The objective of this study was to construct two plasmids, expressing genes related to synthesis from K. pneumoniae GLC29 in order to produce 1,3-PDO from glycerol: One plasmid harboring the genes dhaB1, dhaB2, dhaB3, encoding glycerol dehydratase, and dhaT encoding 1,3-PDO oxidoreductase, under control of the R0010 promoter, while the other plas mid had in addition the coding DNA for the glycerol dehydratase reactivation factors dhaF and dhaG. This approach was done using standard parts from iGEM and genes extracted from a wild type bacterium (K. pneumoniae GLC29), which have been previously reported as a good 1,3-PDO producer. Furthermore, Gibson's Assembly (Gibson et al., 2009) was used for all cloning, eliminating the need to previously sequence or modify the wild genes for traditional cloning, cloning multiple genes at once, and eliminating scars from restriction enzymes. Both constructs for 1,3-PDO production were compared in micro-aerobic and anaerobic conditions, and then evaluated for 1,3-PDO production in bioreactors using anaerobic conditions and two-stage fermentations.
Strains and maintenance
K. pneumoniae CLG29 was isolated from bryophytes grown on the base of leaf stalks of Terminalia catappa at UNESP – Universidade Estadual Paulista, Rio Claro, Brazil and characterized as a new 1,3-PDO producer (da Silva et al., 2014). E. coli TCS099 - ΔmgsA, ΔldhA, ΔfdrA, Δzwf, Δndh, ΔmaeB, Δpta, ΔpoxB, ΔmhpF, ΔadhP and ΔadhE (Trinh and Srienc, 2009), was used at University of Tennessee Knoxville, and E. coli SZ63, W3110 mutant, ΔfocA-pflB::FRT, ΔfrdBC ΔadhE::FRT, ackA::FRT (Zhou et al., 2003) was kindly sent from the University of Florida to the Department of Biochemistry and Microbiology, Biosciences Institute of Rio Claro, Univ. Estadual Paulista – UNESP. Both used as hosts for vectors assembled and experiments. Cultures were kept at -80°C for long-term storage, and reactivated in Luria-Bertani medium (LB) prior to experiments.
Cloning
Plasmid backbone pSB1C3_RFP containing BBa_R0010 promoter, sensitive to LacI and CAP protein, and BBa_B0015 double terminator (Figure 1) was used as template for the plasmid backbone. BBa_B0034 was designed within the primers, which sequence was obtained from iGEM. Primers corresponding to genes were designed using known sequences from K. pneumoniae strains 342, MGH78578, and KTCC 2242, in which complete genome is available at ncbi.nlm.nih.gov (three genomes homology regions were identical to the primers designed). All primers were designed using Gibson’s Assembly (Gibson et al., 2009), with a 40 base pair overlap with the next sequence (Table 1), dhaB1_F was designed with a 40 base pair overlap with the promoter and dhaT_term_R was designed with a 40 base pair terminator overlap. Genes were isolated from K. pneumoniae GLC29 using PCR and the primers listed in Table 1.
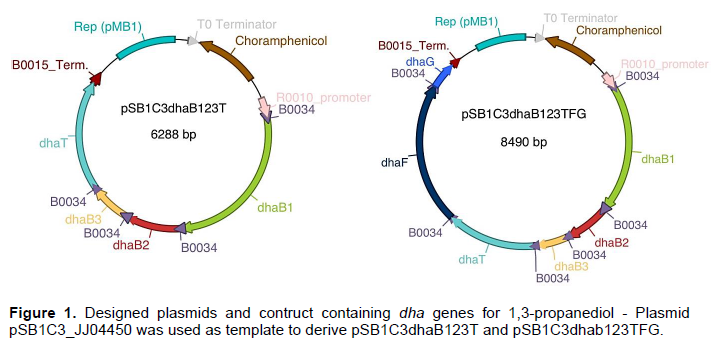

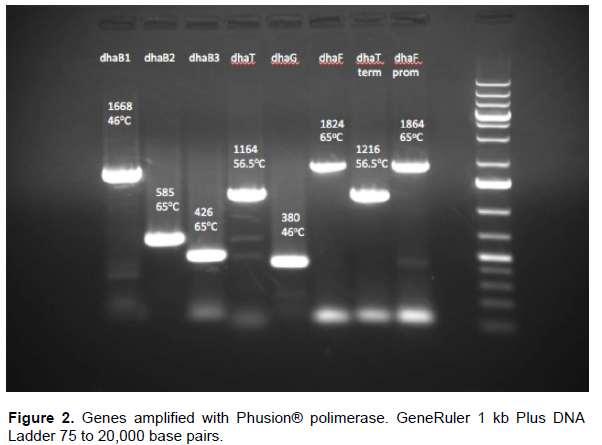
All genes (Figure 2) used for cloning were amplified using the primers designed using Phusion® polymerase HF, gel and purified using Zymo Research® Zymoclean™ Gel DNA Recovery Kit and ligated using Gibson Assembly (2009), cleaned and concentrated using Zymo Research® DNA Clean and Concentrator™. Plasmids were extracted from E. coli cultures after 6 h growth in LB media with 50 µg/ml chloramphenicol, using Zymo Research® - Plasmid Miniprep™ - Classic. Construction of plasmids were performed using Gibson’s Assembly (GA) isothermal protocol (Gibson et al., 2009). Plasmid pSB1C3-dhaB123T carries the genes dhaB1, dhaB2, dhaB3 (glycerol dehydratase), dhaT (1,3-propanediol oxidoreductase), while pSB1C3-dhaB123TFG harbors the above-mentioned genes and also dhaF and dhaG (glycerol dehydratase reactivase). Plasmids were sequenced by capillary electrophoresis ABI 3730 genetic analyzer.
Production of 1,3-propanediol
Confirmed plasmids were extracted from Top10 using plasmid mini-prep and transformed into competent E. coli TCS099 by heat shock. Transformed E. coli TCS099 hosting pSB1C3dhaB123T and pSB1C3dhaB123TFG were characterized for 1,3-PDO production in minimal medium (glycerol 20.0 g/L; KH2PO4 3.5 g/L; K2HPO4 5.0 g/L; (NH4)2HPO4 3.5 g/L; MgSO4.7H2O 0.25 g/L; CaCl2.2H2O 0.015 g/L; vitamin B12 0.25 mg/l; chloramphenicol 30.0 µg/ml). Experiments were performed in test tubes in aerobic, anaerobic and micro-aerobic conditions. Periodically, samples of 1 ml were collected and centrifuged at 10,000 g for 10 min. The cell-free supernatant was filtered (0.22 µm) and analyzed by high performance liquid chromatography (HPLC) using ion exchange column Phenomenex Rezex ROA (300 mm × 7.8 mm) at 60°C and 0.005 M H2SO4 solution as mobile phase at 0.5 ml/min flow rate, equipped with UV and RI detectors. External standards were ethanol, 1,3-PDO, propionic acid, acetic acid, 2,3-butanediol and glycerol.
Two-stage fed-batch fermentations were performed with four different aeration conditions on initial 24 h (first stage). Medium composition was glycerol 20 to 45 g/L, tryptone 10 g/L, KH2PO4 0.136 g/L, (NH4)2HPO4 3.5 g/L, MgSO4.7H2O 0.48 g/L, CaCl2 0.15 g/L, vitamin B12 1 mg/L, sodium selenite 1 µM, and chloramphenicol 30 µg/ml. After 24 h, remaining glycerol was quantified, and one pulse of glycerol was fed to reach 50 g/L in the bioreactor. The second stage was set into anaerobic conditions, and pure nitrogen gas was pumped at 0.05 L/min. Samples were collected and analyzed as described previously.
Production of 1,3-propanediol
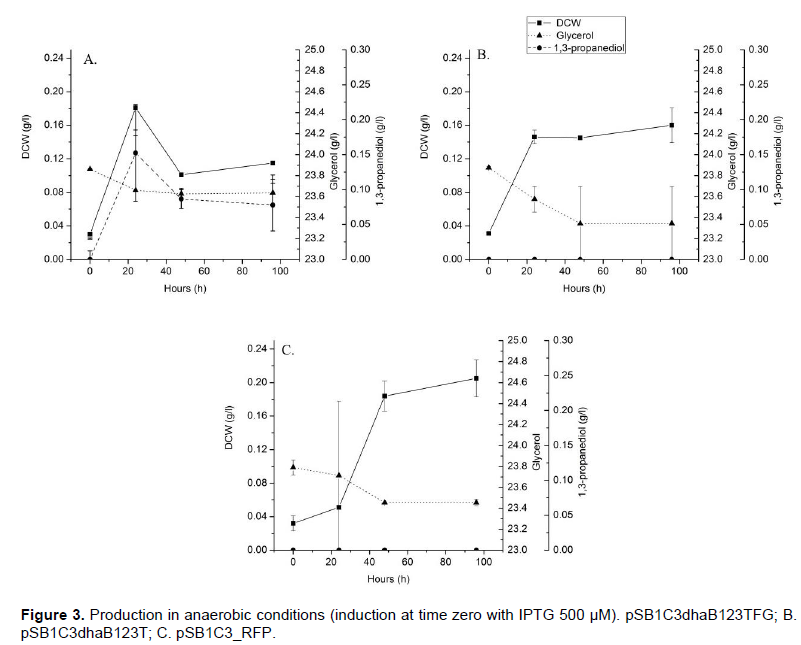
Production of 1,3-PDO and influence of glycerol dehydratase reactivase (dhaF and dhaG) were evaluated in micro-aerobic and anaerobic conditions. Induction was performed with 500 µM of IPTG at the first minute of the process and TCS099 with pSB1C3_RFP was used as negative control. Anaerobic culture showed little growth and low 1,3-PDO production. Host with pSB1C3dhaB1234TFG presented the fastest growth and the best production, followed by pSB1C3dhaB123T, both presented faster growth than the negative control (Figure 3).
Using micro-aerobic condition (Figure 4), growth and 1,3-PDO production were higher than in anaerobic cultures, in which the strain containing pSB1C3dhaB123TFG reached up to 2.5 g/L of 1,3-PDO in 72 h (0.41 g/g yield). Glycerol consumption was more consistent and it was possible to verify that pSB1C3dhaB123TFG consumed more glycerol than the other experiments.
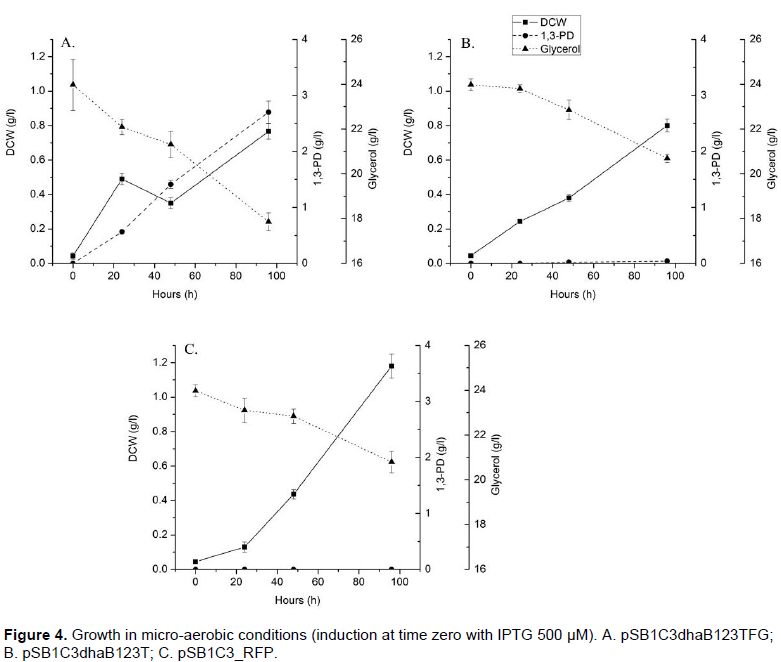
It is important to note that in this experiment, 250 µg/l of vitamin B12 was added to a minimal medium; besides, fermentation strategies and media were not optimized yet. In a similar result, Skraly et al. (1998) demonstrated production of 1,3-PDO up to 6.3 g/L out of 9.33 g/L of glycerol in a 4-liter fed-batch E. coli AG1/pTC53 fermentation using 14 µg/l of vitamin B12. Using statistical design, Zhang et al. (2006) constructed a novel E. coli recombinant using the complete dhaB gene set comprised of three different subunits dhaBCE, (2.8 kb) from Citrobacter freundii assembled into pHsh-yqhD. By the experimental design using 61.8 g/L of glycerol, 6.2 g/L of yeast extract, and 49 mg/l of vitamin B12, Zhang et al. (2006) produced 43.8 g/L of 1,3-PDO in a 5-liter bioreactor in aerobic conditions (0.8 vvm). However, due to the current high price of vitamin B12, the use of 49 mg/l is not economically feasible.
Cameron et al. (1998) cloned from K. pneumoniae the genes dhaB and dhaT to E. coli, including several ORFs (dhaB3, dhaB3a, dhaB4, and dhaB4a) from dhaB complex. A series of synthetic plasmids with dhaB and dhaT genes disposed in the same transcription direction and under the same promoter were built, and 1,3-PDO concentration reached over 70 g/L in 5 L fermenter, using fed batch fermentations, reaching a yield of 0.39 g1,3-PDO/gglycerol (0.48 mol/mol), but using aerobic conditions. The main byproduct was 2,3-butanediol, reaching nearly 20 g/L. Importantly, in our case; no 2,3-butanediol was detected on fermentations using the different plasmids and strains. Different from this work, the aim was to produce 1,3-PDO anaerobically using glycerol.
Reactor fermentation with E. coli SZ63
In an effort to maximize 1,3-PDO yield and concomitantly minimize production time and byproduct, Tang et al. (2009) established a two-stage two-substrate fermentation for producing 1,3-PDO by an engineered E. coli K-12 ER2925 strain, and dhaB1 and dhaB2 from C. butyricum SYU 20108 were cloned and expressed in the host strain. On the first stage from 0 to 10 h, dissolved oxygen was maintained above 40% air saturation, glucose was added continuously to maintain up to2 5g/L until a final biomass of 26 g/L DCW was reached. Then, the second stage involved replacement of glucose medium and byproducts from the first stage with new fresh glycerol fermentation medium every 2 h, shifting the temperature to 42°C, 1,3-PDO the authors claim reaching a final concentration of 104.4 g/L. Therefore a similar approach was figured out enriching the culture with pO2 and comparing with a anaerobic control.
E. coli SZ63 harboring pSB1C3dhaB123TFG was used for these experiments in bioreactors at UNESP - Universidade Estadual Paulista, Brazil, since E. coli TCS099 was not available for importation from the host university. Fermentations in bioreactors are shown in Figure 5. The first fermentation (Figure 5a) started anaerobically while nitrogen gas was used to purge oxygen out of the fermenter prior to the start. Despite the 18 g/L of glycerol consumed in the initial 24 h, little 1,3-PDO was produced (1.69 g/L) and 0.41 g/L of DCW was reached. E. coli SZ63 harboring our plasmid improved DCW by 3 fold, glycerol consumption improved 90 fold, and 1,3-PDO production improved by 11 fold on the first 24 h of process when compared to essays in tubes using E. coli TCS099 and minimal media. Glycerol was fed (18 g/L) at 24 h, but even though recreating Gonzalez (2012) optimal conditions for glycerol fermentation had tried, only 3.23 g/L of 1,3-PDO was produced, and 6.12 g/L of glycerol consumed after 84 h. However, this represents a 9-fold improvement in 1,3-PDO production. Still, conversion rates were limited by the amount of cells, due to slow growth and consequent low cell density. No 2,3-butanediol was observed, but 1.4 g/L of ethanol was detected in the end of the fermentation, that could be residual from the antibiotic mixture dissolved in ethanol.
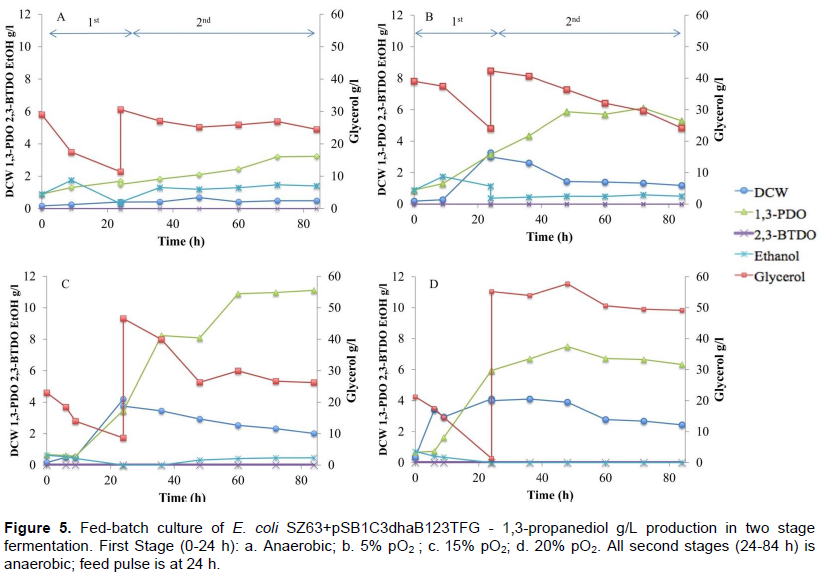
Setting up to 5% pO2 for the initial 24 h fermentation (Figure 5b) resulted in 15 g/L of glycerol consumed, 3.27 g/L of DCW and 3.15 g/L of 1,3-PDO produced in the initial 24 h. This means that more cells were able to produce more 1,3-PDO. In the second stage, fermentation was shifted to anaerobic conditions with nitrogen purge, 18 g/L of glycerol was fed into the bioreactor, and after 60 h, 6.1 g/L of 1,3-PDO was reached. This result suggests that E. coli SZ63 is not able to grow in anaerobic condition using glycerol as sole carbon source, even when 1,3-PDO pathway was inserted as a way to recycle NADH, so 1,3-PDO could be the last electron acceptor. It is suggested that micro-aerobic conditions are therefore necessary for growth using glycerol as sole carbon source.
The best conditions were reached using 15% pO2 (Figure 5c), which resulted in 3.5 g/L of 1,3-PDO, 4.2 g/L of DCW, and 14.4 g/L of glycerol consumed on the initial 24 h. Compared to initial essays in tubes previously described, DCW was improved 8.5-fold, 1,3-PDO was improved 6.5-fold, and glycerol consumption was improved 16.8 fold. Production continued on the second stage in anaerobioc conditions, reaching 11.1 g/L after 60 h, which improved 4.1-fold. No 2,3-butanediol was observed, and residual 0.5 g/l of ethanol was detected.
When 20% pO2 was employed (Figure 5d), glycerol was depleted after 24 h. During the first 24 h, 6.5 g/L of 1,3-PDO was produced. On the second stage, in anaerobic conditions, however, there was no consumption of glycerol nor substantial 1,3-PDO production. This could mean that cells were stressed from glycerol depletion on the initial 24 h. Similar to the other experiments, no 2,3-butanediol or ethanol was observed during the 84 h of experiment.
Transferring a biosynthetic pathway to a non-native producer faces several difficulties, such as the non-native pathways overexpression can disrupt the intrinsic metabolism in the host, using most of the essential precursors for growth or maintenance. Furthermore, pathways re-engineering frequently leads to imbalanced gene expression, which creates bottlenecks in the biosynthetic pathway that could that reduce production of the wanted compound (Atsumi et al., 2008). New investigations on protein expression or mRNA could elucidate better comprehension on limiting factors on the production of 1,3-PDO in this work.
Previous studies on D-lactate production have shown that an initial period of aeration in complex media can be used to boost the growth of D-lactate-producing E. coli strains containing mutations in phosphoenolpyruvate carboxylase and phosphotransacetylase genes resulting in shorter time for fermentation. Initial aeration of an SZ58 culture eliminated the lag phase resulting in 10-fold increase in cell yield within the initial 24 h, which accelerated glucose conversion to lactate and reduced the time required to complete the process (Zhou et al., 2003).
Further fermentations should be performed to optimize these conditions. Among the evaluated strains, glycerol was not efficiently fermented to support cell growth and 1,3-PDO production. Glycerol is a highly-reduced substrate and maintenance of redox balance is challenging specially in anaerobic conditions. In the absence of oxygen or other electron acceptor, E. coli is not able to use glycerol as sole carbon source efficiently. The incorporation of glycerol as a carbon source to cell mass results in production of reducing equivalents, in which H2 plays an important role, participating as electron donor for several reactions. If H2 is decreased, this does not happen and fermentation proceeds. Increasing headspace dilutes H2, and also flushing it out with an inert gas, such as argon, nitrogen, or CO2, improves fermentation (Gonzalez, 2012).
Glycerol can be oxidized to dihydroxyacetone (DHA) by the GldA enzyme, a type II glycerol dehydrogenase, which is encoded by gldA in E. coli, however it is usually not expressed in wild type strains. Activation of this gene requires inactivation of glpK, glpR and glpD followed by mutagenesis and selection procedures, which resulted in a strain that recovered the ability to metabolize glycerol, but not the ability to ferment glycerol (Gonzalez, 2012). In E. coli, there are two glycerol-3-phosphate dehydrogenases, but only one can use NAD+ as an electron acceptor (Lin, 1976). Only one of these enzymes is able to donate electrons to the fumarate reductase complex, producing succinate from fumarate. It was demonstrated that the quantity of succinate produced by their E. coli strain corresponded to only 4% of the glycerol metabolized, therefore, it is not able to grow in anaerobic environment (Skraly et al., 1998). Production and growth of 1,3-PDO was reported by Tong et al. (1991) using a cosmid harboring dha genes from K. pneumoniae ATCC 25955, in which E. coli AG1/pTC1 produced up to 0.46 mol/mol of 1,3-PDO after 120 h, but little 1-3-PDO was produced. DHA and glycerol were added to a defined medium, and also the cosmid had a dha kinase and glycerol dehydrogenase from K. pneumoniae, which might explain the cell growth reported in anaerobic environment. New experiments cloning dha kinase from K. pneumoniae should be performed to evaluate cell growth in anaerobic conditons.
Optimum glycerol fermentation by E. coli occurs at slightly acid pH of 6.3, 10-20% CO2 or higher, high concentrations of glycerol, up to 100 g/l, 200 rpm, 37°C, 0.01 L/min argon or nitrogen, low potassium (less than 10 mM) and phosphate concentrations (from 50 to less than 1.3 mM) are preferred, because high concentration of these ions inhibits glycerol dehydrogenase and DHA kinase, and furthermore increases methylglyoxal toxicity. Tryptone supplementation is additionally required when DHA is not added (Gonzalez, 2012). These conditions were replicated in these experiments on bioreactors, but fermentation of glycerol did not result in good cell growth and high productivity.
High concentration of glycerol is required to GldA, due to its low Km, and acid condition favors its reductive activity, while neutral or alkaline conditions increases its oxidative activity. Also, alkaline conditions increase methylglyoxal toxicity. To prevent cytoplasmic acidification, E. coli produces CO2 and H2 from formic acid, but H2 can negatively influence glycerol fermentation (Gonzalez, 2012). Besides, high concentrations of glycerol, over 49 g/L, is known to decrease 1,3-PDO production, since it favors the inactivation of glycerol dehydratase (GDHt) (da Silva et al., 2014).
The overexpression of GDHt leads to serious growth deficiency of K. pneumoniae. Instability of the plasmids bearing the genes encoding GDHt and/or 1,3-PDO oxidoreductase were responsible for the observed phenomena due to an imbalanced conversion of glycerol to 3-HPA and its toxicity. Similar research using resting cell systems, in which growth was stopped while metabolic activity was maintained, eliminates disturbances associated with cell growth. Overexpression of 1,3-PDO oxidoreductase led to faster glycerol conversion and 1,3-PDO production. After 12-h conversion, it improves 1,3-PDO yield by 20.4%, and boosts product/substrate yield from 50.8 to 59.8% (mol/mol) (Song et al., 2010). Further investigations should be done to address if 3-HPA is occurring in E. coli SZ63 during 1,3-propanediol production and see the need to overexpress 1,3-PDO oxidoreductase.
Zhang et al. (2006) constructed a similar plasmid in E. coli with genes from Citrobacter freundii, and using experimental design from fixed concentrations: 61.8 g/L glycerol, 6.2 g/L of yeast extract and 49 mg/L of vitamin B12, achieved 1,3-PDO production of 41.3 g/L. Ma et al. (2009) constructed a plasmid containing dhaB and dhaT genes in E. coli and expressed both genes in the same direction, successfully producing 11.3 g/l of 1,3-PDO from 40 g/l glycerol.
E. coli hosting pSB1C3dhaB123TFG was able to produce higher amounts of 1,3-propanediol than the plasmid pSB1C3dhaB123T. Genes dhaF and dhaG are very important for the production of 1,3-propanediol, specially with lower concentrations of vitamin B12 (from 0.25 to 1 mg/L). Moreover, aeration on the initial 24 h up to 15% pO2 is able to increase 1,3-PDO production and productivity with E. coli SZ63, reached 11.3 g/L of 1,3-PDO produced in 60 h. Bottlenecks on glycerol fermenta-tion by E. coli still need to be addressed, adding 1,3-PDO as a NADH recycle metabolic pathway did not improve E. coli growth on glycerol on the absence of oxygen, that could mean regulation issues such as 3-HPA toxicity or an anaerobic glycerol-3-phosphate dehydrogenase deficiency from E. coli. New fermentation strategies could be performed to improve productivity and production, such as using glucose and glycerol together, and further engineer E. coli to efficiently ferment glycerol. Also, protein expression and mRNA analysis could elucidate bottlenecks on glycerol metabolism by E. coli hosting pSB1C3dhaB123TFG and improve 1,3-PDO and cell growth. Improving glycerol uptake via cloning K. pneumoniae dhaD, dhaK, dhaL and dhaM to convert glycerol to DHA and then to glyceronephosphate and pyruvate could increase cell growth in anaerobioc conditions, furthermore generating more NADH for 1,3-PDO production.
The authors have not declared any conflict of interests.
REFERENCES
|
Atsumi S, Cann AF, Connor MR, Shen CR, Smith KM, Brynildsen MP, Chou KJY, Hanai T, Liao JC (2008). Metabolic engineering of Escherichia coli for 1-butanol production. Metab. Eng. 10(6):305-311.
Crossref
|
|
|
|
Biebl H, Menzel K, Zeng A-P, Deckwer W-D (1999). Microbial production of 1,3-propanediol. App. Microbio. Biotechnol. 52:289-297.
Crossref
|
|
|
|
|
Cameron DC, Altaras NE, Hoffman ML, Shaw A J (1998). Metabolic engineering of propanediol pathways. Biotechnol. Prog. 14(1):116-125.
Crossref
|
|
|
|
|
Cheng K-K, Liu H-J, Liu D-H (2005). Multiple growth inhibition of Klebsiella pneumoniae in 1,3-propanediol fermentation. Biotechnol. Lett. 27(1):19-22.
Crossref
|
|
|
|
|
Da Silva GP, de Lima CJB, Contiero J (2014). Production and productivity of 1,3-propanediol from glycerol by Klebsiella pneumoniae GLC29. Catal. Today 257(2):259-266.
|
|
|
|
|
Gallardo R, Alves M, Rodrigues LR (2014). Modulation of crude glycerol fermentation by Clostridium pasteurianum DSM 525 towards the production of butanol. Biomass Bioenergy 71:134-143.
Crossref
|
|
|
|
|
Gibson DG, Young L, Chuang R, Venter JC, Iii CAH, Smith HO, America N (2009). Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 6:343-345.
Crossref
|
|
|
|
|
Gonzalez R (2012). Anaerobic Fermentation of Glycerol. WO Pat. 2,007,115,228.
|
|
|
|
|
Kaur G, Srivastava AK, Chand S (2012). Advances in biotechnological production of 1,3-propanediol. Biochem. Eng. J. 64:106-118.
Crossref
|
|
|
|
|
Lin E (1976). Glycerol dissimilation and its regulation in bacteria. Annu. Rev. Microbiol. 30:535-578.
Crossref
|
|
|
|
|
Ma Z, Rao Z, Xu L, Liao X, Fang H (2009). Production of 1, 3-propanediol from glycerol by engineered using a novel coexpression vector. Afr. J. Biotechnol. 8(20):5500-5505.
|
|
|
|
|
Maervoet VET, De Mey M, Beauprez J, De Maeseneire S, Soetaert WK (2011). Enhancing the Microbial Conversion of Glycerol to 1,3-Propanediol Using Metabolic Engineering. Org. Process Res. Dev. 15(1):189-202.
Crossref
|
|
|
|
|
Oh BR, Seo JW, Heo SY, Hong WK, Luo LH, Son JH, Park DH, Kim CH (2012). Fermentation strategies for 1,3-propanediol production from glycerol using a genetically engineered Klebsiella pneumoniae strain to eliminate by-product formation. Bioprocess Biosyst. Eng. 35:159-165.
Crossref
|
|
|
|
|
Papanikolaou S, Aggelis G (2009). Biotechnological valorization of biodiesel derived glycerol waste through production of single cell oil and citric acid by Yarrowia lipolytica. Lipid Technol. 21(4):83-87.
Crossref
|
|
|
|
|
Pyne M (2014). Development of genetic tools for metabolic engineering of Clostridium pasteurianum. University of Waterloo.
|
|
|
|
|
Quispe CAG, Coronado CJR, Carvalho Jr. JA (2013). Glycerol: Production, consumption, prices, characterization and new trends in combustion. Renew. Sustain. Energy Rev. 27:475-493.
Crossref
|
|
|
|
|
Rymowicz W, Rywińska A, Żarowska B, Juszczyk P (2006). Citric acid production from raw glycerol by acetate mutants of Yarrowia lipolytica. Chem. Pap. 60(5):391-394.
|
|
|
|
|
Skraly FA, Lytle BL, Cameron DC (1998). Construction and characterization of a 1,3-propanediol operon. Appl. Environ. Microbiol. 64(1):98-105.
|
|
|
|
|
Song Y, Xu Y, Liu D (2010). 1,2 and 1,3 Propanediol, Microbial Production Methods. Encyclopedia of Industrial Biotechnology. 1:1-16.
|
|
|
|
|
Tang X, Tan Y, Zhu H, Zhao K, Shen W (2009). Microbial conversion of glycerol to 1,3-propanediol by an engineered strain of Escherichia coli. Appl. Environ. Microbiol. 75(6):1628-1634.
Crossref
|
|
|
|
|
Tong T, Liao H H, Cameron D (1991). 1,3-Propanediol Production by Escherichia coli Expressing Genes from the Klebsiella pneumoniae dha Regulon. Appl. Environ. Microbiol. 57(12):3541-3546
|
|
|
|
|
Trinh CT, Srienc F (2009). Metabolic engineering of Escherichia coli for efficient conversion of glycerol to ethanol. Appl. Environ. Microbiol. 75(21):6696-6705.
Crossref
|
|
|
|
|
Yazdani SS, Gonzalez R (2007). Anaerobic fermentation of glycerol: A path to economic viability for the biofuels industry. Curr. Opin. Biotechnol. 18(3):213-219.
Crossref
|
|
|
|
|
Zhang X, Li Y, Zhuge B, Tang X, Shen W, Rao Z, Fang H, Zhuge J (2006). Construction of a novel recombinant Escherichia coli strain capable of producing 1,3–propanediol and optimization of fermentation parameters by statistical design. World J. Microbiol. Biotechnol. 22(9):945-952.
Crossref
|
|
|
|
|
Zhou S, Causey TB, Hasona A, Shanmugam KT, Ingram LO (2003). Production of optically pure D-lactic acid in mineral salts medium by metabolically engineered Escherichia coli W3110. Appl. Environ. Microbiol. 69(1):399-407.
Crossref
|
|