Full Length Research Paper
ABSTRACT
Salinity is major abiotic stress limiting plant growth worldwide. Plant adaptation to salinity stress involves diverse physiological and metabolic pathways. In this study, we assessed the effects of foliar application of zinc oxide nanoparticles (ZnONPs) and Moringa leaf extract (MLE) on salt tolerance in faba beans (cultivar, Sakha 4). Morphological, chemical, and biochemical parameters of plants grown under saline condition (50 and 100 mM NaCl) were assessed 60 days after sowing. Salt stress caused a remarkable reduction in growth traits, photosynthetic pigments, proline, minerals, total phenol, and enzyme activity of the faba bean variety. The results showed that foliar spraying of MLE and ZnONPs on faba bean grown under salt-stressed conditions promoted growth parameters (that is, shoot length, numbers of leaves, relative water content, shoot, roots fresh and dry weights), photosynthetic pigments (that is, chl a, b, total chlorophyll and carotenoids), proline, mineral elements (Na+, K+, Ca+2, and Zn+2), total phenol and enzyme activity (POX, PPO, APX, and CAT) compared to control plants. Based on these findings, the potential of foliar spraying application of MLE and ZnONPs may help alleviate the negative effect of salinity on growth, photosynthesis efficiency, and biochemical properties of faba bean.
Key words: Faba bean, Moringa oleifera, antioxidant enzyme activity, ZnO nanoparticles, salt stress, proline.
INTRODUCTION
Faba bean (Vicia faba L.) is the third most important legume crop grown in more than 60 countries as a cool-season legume (Bohra et al., 2014). It is a high-protein grain legume that provides 20 to 36% of protein to human and animal diets (Bulut and Akinci, 2010). Additionally, faba bean is rich in Fe, Mg, Zn, K, and Ca, as well as amino acids, carbohydrates, vitamins, and essential nutraceuticals (Koivunen et al., 2016). However, faba bean production is adversely affected by many abiotic stresses among them salinity and water deficiency. High concentrations of Na+ and Cl- ions in the soil adversely affect faba bean growth and photosynthesis due to chlorophyll degradation and the damaging effects of both ions on photosystem II (PSII) (Tavakkoli et al., 2010). Additionally, salinity affects seed germination, seedling growth, vegetative growth, reproductive phase, maturity, and grain production (Latef et al., 2021). Overall, salinity limits faba bean productivity in semi-arid regions, causing yield losses of up to 50% (Farooq et al., 2017). Moringa oleifera leaf extract (MLE) has been utilized as a natural plant growth enhancer in other crops, boosting plant growth and biomass development while developing tolerance to salt stress (Yasmeen et al., 2013). MLE is high in ascorbates, phenolic compounds, potassium, and calcium, all of which are used as exogenous plant growth stimulants (Waqas et al., 2017).
MLE is a natural plant growth regulator since it includes zeatin, a natural derivative of cytokinin, proteins, vitamins E, phenolics, ascorbic acid, vital amino acids, and different mineral components, making it a possible natural growth booster (El-Hack et al., 2018). MLE foliar spray improved crop production by promoting vigorous plant growth, preserving optimum tissue water status, improving membrane integrity, and increasing antioxidant content (Rehman et al., 2014). Foliar application of NPS is considered a more convenient and straight forward technique since plants directly absorb unlike the application of chemical fertilizers, reducing soil contamination (Kah et al., 2018). The nanoparticle fertilisers are a technique for boosting the availability of nutrients to plant leaves, hence enhancing the efficiency of plant nutrient absorption and yield (Vishekaii et al., 2019). In agriculture, ZnONPs are employed as fertilizers, growth regulators, pesticides, and herbicides (Khot et al., 2012). The use of ZnONPs has enhanced height, leaf number, fresh and dry weight of leaves, chlorophyll, essential oil, and phosphorus concentration (Vafa et al., 2015). Application of ZnONPs significantly increased Cyamopsis tetragonoloba plant biomass, chlorophyll, protein synthesis, rhizospheric microbial population, acid and alkaline phosphatase and phytase activity in the bean rhizosphere (Raliya and Tarafdar, 2013). ZnONPs supplemented with MS medium promoted somatic embryogenesis, shooting, plantlet regeneration, and increased proline synthesis and the activities of superoxide dismutase, catalase, and peroxidase, which improved resistance to biotic stress (Helaly et al., 2014). The green strategy for nanoparticle synthesis is based on the plant supply and the organic molecules present in plants (enzymes, amino acids, proteins, saccharides, vitamins, and organic acids) that may operate as reducing and/or capping agents during metal nanoparticle formation (Zafar et al., 2020). ZnONPs are one of the most widely employed nanoparticles in agriculture because they are linked to secondary metabolite pigment production, protein, and sugar content nutrient translocation, and they scavenge free oxygen radicals generated in stressed plant tissues (Zafar et al., 2016). The natural extract from Moringa oleifera (MO) leaves has been shown to be an efficient reducing/oxidizing, capping, and stabilizing agent in the synthesis of ZnO nanoparticles (Matinise et al., 2017).
The present study, therefore investigated the effect of foliar application of nanoparticle and Moringa leaf extract on the growth parameters, biochemical contents, photosynthetic pigment, and antioxidant enzymes in faba bean plants.
MATERIALS AND METHODS
Plant and field experiments
Field experiments were carried out in a greenhouse at the Jomo Kenyatta University of Agriculture and Technology (JKUAT) in Kenya. The experiments were placed from December 2020 to May 2021. Faba bean cultivar seeds (Sakha 4) were obtained from the Agricultural Research Center in Sakha, Kafr El Sheikh, Egypt, and were selected for size and colour consistency. The selected seeds were washed with distilled water sterilized in 1% (v/v) NaClO for 2 min, then rinsed with distilled water and left to dry at room temperature for 2 h. The seeds were then put in a Petri dish on sterilized, moistened filter paper and stacked in darkness for 48 h. Then, the seeds were planted in plastic pots (25 × 40 cm) that contained 91.44% sand, 6.56% silt, and 4.0% clay, and had a pH of 7.5 and EC of 1.3 dSm-1. The seed was grown in a plant growth chamber, the temperature was set to 28°C during the day and 20°C at night, and the relative humidity was maintained at 70 to 80%. The experiment was set up in the form of a Randomized Complete Blocks Design (RCBD) with 3 replications. Each pot was irrigated with tap water (control plants) and two salinity levels (50 and 100 mM NaCl).
Experimental treatments
The salinity levels were obtained by adding appropriate amounts of dry NaCl to water. After that, the pots were irrigated for a day after two days with tap water for 7 days. The treatments were administered 21 days after the seeds were sown. After that, the salt treatments were administered to each pot at 7-day intervals for 90 days. Foliar application of zinc oxide nanoparticles (ZnONPs) 50 mg/L and MLE 50 ml/L was done twice a week from the 30th day after sowing. Tween 20 (0.05%) was added to spray solutions as a wetting agent.
Preparation of extraction from M. oleifera plant leaves
The leaves of M. oleifera were gathered from the JKUAT garden. The leaves were taken from the stems and washed with distilled water and left to dry. Phytochemicals from the dried leaves were then extracted with distilled water, according to Pervez et al. (2017). Briefly, the leaves were dried and finely ground to a fine powder. The MLE extraction was done by 100 g of powdered soaking in 1000 mL of distilled water. The mixture was macerated for 48 h at 35°C and later filtered through (Whatman No.1) filter paper and the extraction was stored at -20°C for further uses.
Synthesis of zinc oxide nanoparticles from leaf extraction
Precipitation method
ZnONPs were formed from the reaction of zinc nitrate with a variety of other chemicals, as described by Pal et al. (2018). Briefly, 4.75 g zinc nitrate Zn [NO3]2.6H2O was dissolved in 90 mL of dh2o and stirred for 40 min for complete dissolution. For the synthesis of NPs, a dropwise addition of M. oleifera leaf extract solution (10 mL) was made into the zinc nitrate solution with vigorous stirring at 80°C for 3 h. The solution gradually becomes murky yellow in colour. The solution was then centrifuged at 10,000 rpm for 10 min and washed with dh2o to remove any contaminants or ions that have been absorbed. Finally, the product was dried in a laboratory oven at 70°C for 48 h.
Sample characterizations
Optical properties of Nanopowder from the amount of Green synthesis of zinc oxide nanoparticles (ZnONPs) were characterized based on UV-Vis Spectrophotometer absorption spectra with the wavelength range of 200 to 600 nm. X-ray diffraction (XRD) patterns of the as-synthesized ZnONPs were determined through the Rigaku D/Max-lllC X-ray diffractometer (Rigaku Int. Corp.Tokyo, Japan). It produced diffractions at a scanning rate of 20 min-1 in the 2 to 500 at room temperature with a CuKa radiation set at 40 kV and 20 mA. Fourier transforms infrared (FTIR) spectra were recorded on Jasco FT-IR5300 model spectrophotometer in KBr pellets. High-resolution transmission electron microscopy (SEM) was used to determine the particle size and characteristics of the samples.
Data collection
Ten randomized plants were selected from each plot at 60 days after sowing (DAS) to determine the plant's development factors, such as height (cm), the number of leaves/plants (shoots, roots, fresh and dry weight), antioxidant enzyme activity, photosynthetic activity, and biochemical content.
Measurement of plant biomass and growth parameters
Growth measurements for the plants exposed to salt treatments were taken after 30 days of treatment and after 40% of the plants at the highest concentration (100 mM), they are dried. The three replicates taken for each treatment were used to calculate the mean of each measurement. The mean of each measurement was calculated based on the three replicates that were obtained for each treatment. The length of the shoot (SFW), the number of plant leaves, and the size of the root were all measured (RFW). The samples were packaged and kept in an oven at 60°C for 72 h. Following that, the samples were completely dehydrated, and the root dry weight (RDW) and shoot dry weight (SDW) were determined.
Leaf relative water content
The relative water content (RWC) of the sample was measured and calculated according to Gulen and Eris (2003)using the following formula:
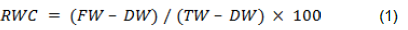
where FW = fresh weight, TW = turgid weight, and DW = oven dry weight.
Analysis of pigment contents
The contents of chlorophyll (Chl a, Chl b and carotenoids) in fresh leaves were measured using spectrophotometry (Khalilzadeh et al., 2016). The fresh leaves were taken from the midst of five major leaves (60 days after sowing). Approximately 1 g of leaf tissue was extracted by grinding in a mortar using 20 ml (80% v/v) acetone and 0.5 g calcium carbonate to equalize the cellular sap acidity. The extract was filtered through a No. 2 filter paper. After complete filtrates were obtained in new test tubes and absorbance was taken at 645, 663, and 470 nm wavelengths using a spectrophotometer. To calculate chlorophyll contents following equations were used.

where V is the total volume in milliliter (mL) of 80% (v/v) acetone and W is the fresh weight (FW) of the sample (g).
Proline content
The proline content in leaves following the anthesis stage, which occurred 60 days after planting, was determined with some modifications by Bates et al. (1973). Briefly, approximately 0.5 g sample of fresh leaves were crushed and ground in a mortar with 10 ml of sulfosalicylic acid (3.0%). The homogenate was filtered through Whatman No. 2-filter paper; after that, 2 ml of the extract was mixed with ninhydrin reagent (2 mL) and 2 mL glacial acetic acid then placed in a boiling water bath for 1 h at 100°C until the appearance of red colour. The tubes were then cooled in the ice. Toluene 4 mL was added to the test tube, and the mixture was mixed until the upper coloured layered appeared. Then this layer was separated from the mixture in other test tubes and absorbance was taken at 520 nm. Proline concentration was determined from a standard curve and calculated fresh weight mmol proline (mg. g-1 FW).
Determination of mineral ion contents
Fresh samples were dried for 48 h at 35°C. A total of 0.3 g of leaves were pulverized into a powder and burnt at 560°C. It was then digested for 1 to 2 h with 10 ml of an acid combination comprising HNO3: HClO4 (2:1 v/v) until the red NO2 emissions stopped. After full digestion, distilled water was used to dilute the colourless digests (2-3 ml) to a volume of 20 ml, which was then filtered using Whatman No. 1 filter paper. The ions Na+, K+, Zn+2, and Ca+2 were determined by inductively coupled plasma atomic absorption spectrometry using aliquots from this solution (Optima2000DV, Perkin Elmer, USA). The content was determined following the earlier procedure (Stateras and Moustakas, 2018).
Measurement of phenolic compounds
The sample extraction method is described with modifications (Neugart et al., 2015). Fresh leaves (20 mg) were immersed in 10 mL of 50% aqueous methanol for 60 min, with sonication below 40°C. The samples were then centrifuged at 14,000× g for 15 min, and the supernatants were collected kept at 4°C for further analysis. Total phenolic content was assayed using the Folin-Ciocalteu colourimetric method. In brief, 0.125 ml of methanolic extract solution was mixed with 0.5 ml of deionized water and 0.125 ml of the Folin-Ciocalteu reagent. Then the mixture was incubated for 1 min before adding 1.25 ml of 7% sodium carbonate (Na2CO3) solution. Then incubation for 90 min in the dark at room temperature, the absorbance was measured by a spectrophotometer at 760 nm as described by Lim et al. (2014)with some modifications.
Estimation of the antioxidant enzymes activities
Fresh leaf samples were used to determine the activities of antioxidant enzymes. Extraction of samples and preparation of supernatants was carried out according to the method (Ahmad et al., 2016). Briefly,1 g leaf samples of faba bean plants were homogenized in an extraction buffer containing potassium phosphate buffer (0.1 M, pH 7.5) and ethylenediaminetetraacetic acid (EDTA, 0.5 mM). Then centrifugation at 12,000 rpm for 10 min at 4°C, the supernatants were collected for the measurements for enzyme assay.
Catalase (CAT) activities
Briefly, 3 ml reaction mixture contained 1.5 ml of 50 mM sodium phosphate buffer (pH 7.0), 1 ml of 18 mM H2O2 and 50 μl enzyme extract. The catalase was estimated by measuring the decrease in the absorbance at 240 nm according to the method of Aebi (1984).
Ascorbate peroxidase (APX) activities
In brief, fresh extract assay by 3 ml of enzyme reaction mixture contained 1.5 ml of 50 mM potassium phosphate buffer (pH 7.0), 0.5 ml of 0.5 mM ascorbic acid, 0.1 ml of 0.1 mM EDTA, 100 μl enzyme extract, 0.7 ml of H2O and 0.1 ml of mM H2O2. The reaction was initiated by adding 0.1 ml of 3 mM H2O2. A decrease followed the H2O2 dependent oxidation of ascorbic acid in the absorbance measured at 290 nm for 3 min at the interval of 30 s (Nakano and Asada, 1981).
Polyphenol oxidase activity (PPO)
Leaf tissue (1 g) ground in 10 ml of 100 mM sodium phosphate buffer, pH 6.5. The homogenate was centrifuged for 10 min at 4°C at 12,000 rpm, and the supernatant was employed in the enzyme assays. The reaction mixture contained 1 ml of pyrogallol (10 mM pyrogallol), 1.5 ml of 10 mM phosphate buffer, pH 6.5, and the reaction was initiated by adding 0.5 ml of enzyme extract. The changes in the colour due to the oxidized pyrogallol were read at 420 nm for 1 min at an interval of 15 s. The enzyme activity was expressed as (mg/g FW) (Kar and Mishra, 1976).
Peroxidase activity (POX)
About 50 μL of plant extract was added to 1.35 mL 0.1 M potassium phosphate buffer (pH 6.0), 100 μL 45 mM guaiacol, and 500 μL 44 mM hydrogen peroxide. Then, using a UV-Vis spectrophotometer, we recorded kinetic changes in absorbance at 470 nm over a 10-s interval for 30 s at 25°C (MacAdam et al., 1992).
Statistical analysis
Plant growth data, biomass, photosynthetic pigment contents (chlorophyll a, b, and carotenoids), mineral contents, total phenol content, and antioxidant enzymes were assessed periodically. The experimental data were computed using two-way analysis of variance (ANOVA) and the treatment effects were ascertained through mean comparison. Means were compared using Tukey’s honestly significant difference (HSD) test at P<0.05%.
RESULTS
Physical and chemical properties of soil mixture
Soil properties after harvesting
Irrigation with saline water (50 and 100 mM NaCl) significantly reduced K+, HCO3, and SO4 levels while increasing Na+, Cl-, pH, and EC concentrations in postharvest soil samples to the salt-stressed plants, which utilized distilled water for irrigation. After harvesting, no carbonate was detected in the soil solution. Two levels of salt increased the Na+ concentration in the soil (Table 1).
Ultraviolet (UV-Vis) analysis of ZnONPs from M. oleifera leaf extract
The presence of secondary metabolite in plant (M. oleifera extract) causes zinc ions in the solution to be reduced to zinc oxide. The plant extract not only functions as a reducing agent but also acts as a stabilizing agent. This was validated by doing a UV–visible spectrum investigation in the region of 300 to 600 nm. The spectra revealed a peak at 370 nm, which is specific to ZnO nanoparticles (Figure 1).
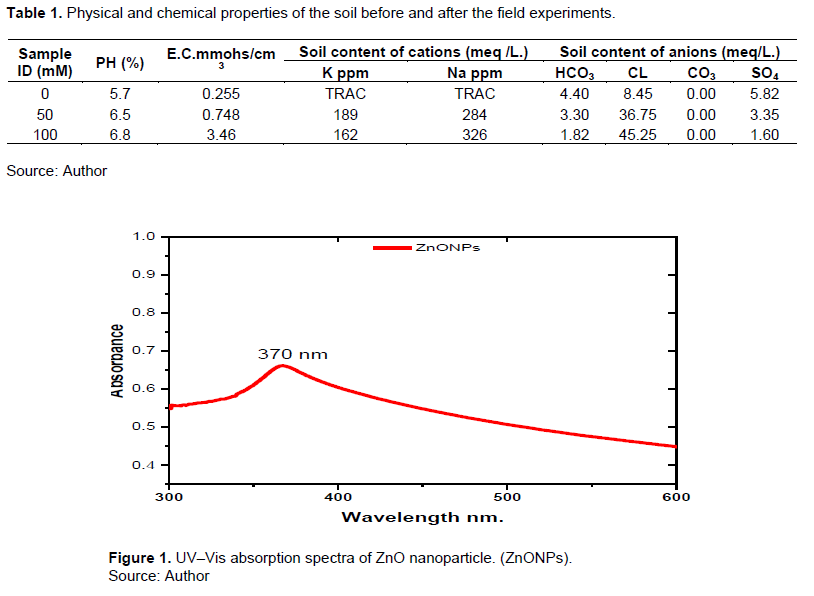
FTIR analysis
The Fourier transform infrared spectroscopy (FTIR) was utilized to identify the various functional groups included in the produced nanoparticles. The peaks were utilized to identify the functional groups in ZnO nanoparticles, which were then characterized (Figure 2), including M. oleifera leaf extract, which revealed an absorption band at 3421,3394 cm-1, which is attributable to the asymmetric and symmetric stretching vibrations of the alcohol O-H group, respectively. However, the absorption peak shows the C=C bonding of alkene at 1595 cm-1. Stretching vibrations at 1401 cm-1 revealed the methyl group’s C-H stretching. The peak in ZnO represented the amide band of the random coil of protein at about 1401 cm-1. The peak indicates the stretching vibrations of ZnO nanoparticles at 734 cm-1. A metal-oxygen bond is allocated a range between 400 and 600 cm-1. In addition to the absorption bands of the biomolecules utilized as reduction and stabilization (capping agents), the presence of ZnONPs is confirmed by the absorption peak at 477 cm-1 owing to Zn-O stretching vibration.
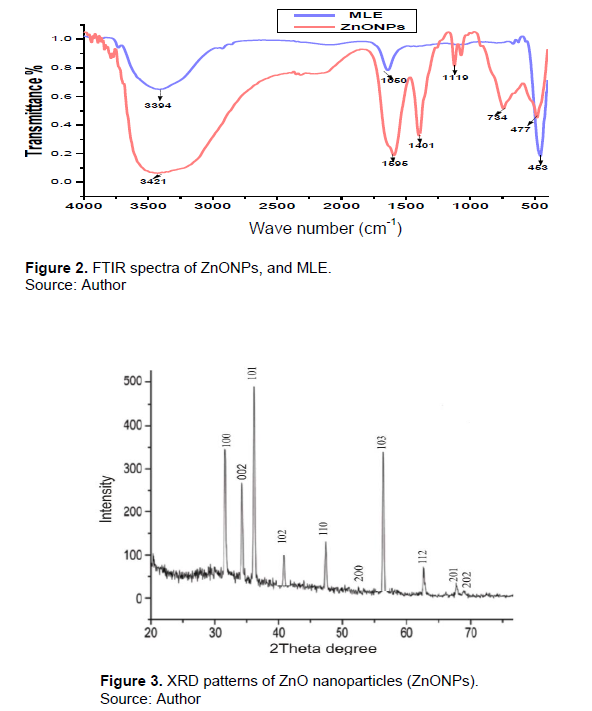
Analysis of crystal structure (XRD) of ZnONPs from M. oleifera leaf extract
The structure and phase purity of the nanoparticles sample produced strong, intense peaks in the spectrum
of 2θ values ranging from 25 to 70 that were identified from XRD patterns (Figure 3). The sharp diffraction peaks were observed at 2θ values 26.3, 29.1, 36.33, 39.29, 45.33, 51.52, 53.50, 52.50, 56.23, 64.84, and 67.79°. Diffraction peaks of XRD matched very well with the hexagonal wurtzite structure by comparison with the data from JCPDS card No. 89-1397. All the reflection peaks obtained were corresponding to (100), (002), (101), (102), (110), (103), (200), (112), (201), and (202) diffraction lattice planes, respectively which confirm the hexagonal wurtzite structure for the synthesized nanoparticles.
Scanning-electron microscope (SEM) analysis of ZnO NPs from M. oleifera leaf extract
As shown in Figure 4, scanning electron microscopy (SEM) images were acquired at various magnifications to investigate the form and size of the nanoparticles that were manufactured. The evolution of nanoparticles in their agglomerated condition may be seen in the surface morphology. According to detailed structural parameters, the synthesized products have a spherical and crystalline structure, with diameters in the range of 215 nm. The findings of the SEM analysis indicated that the size and form of the nanoparticles were influenced by the various precursors used in their preparation.
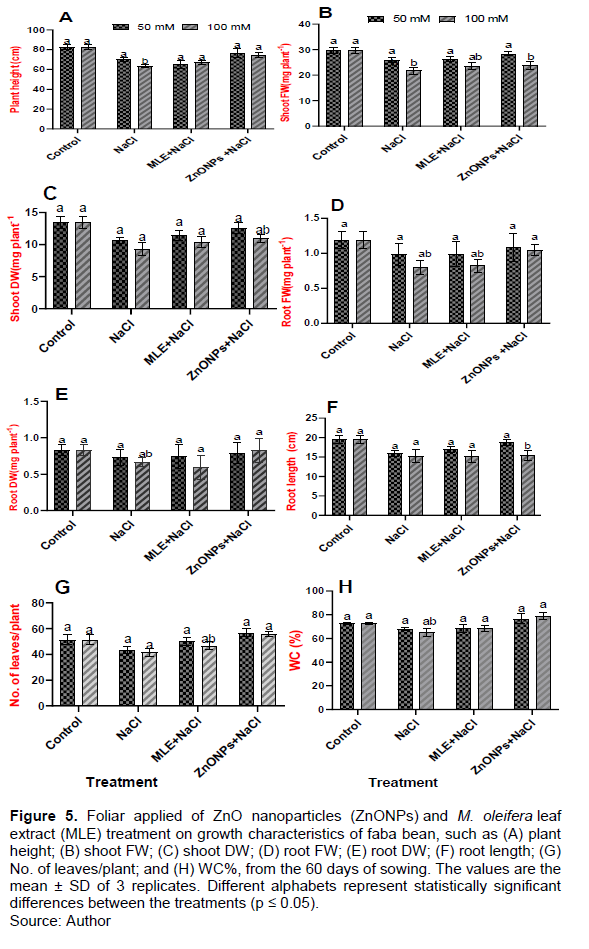
Effects of MLE and ZnONPs application on growth-related parameters
The growth responses of faba bean under saline conditions were evaluated by measuring growth-related parameters to assess their salt-tolerance capacity. Leaf number, plant height, and total DW of cultivar decreased with the increasing salinity levels (Figure 5). Specifically, when NaCl concentration was increased from 50 to 100 mM, growth-related parameters, such as plant height, leaf number, WC, FW, and DW, were reduced compared to the control plants (Figure 5). Compared with the control, plant height reduced in the 50 and 100 mM NaCl by 84.67 and 76.96%, respectively (Figure 5A). Foliar spray of faba bean plants with MLE and ZnONPs improved plant height (92.71,105.60, 108.90 and 116.83%) at 50 and 100 mM NaCl levels compared to plants sprayed with NaCl alone (Figure 5A). However, the shoot FW (SFW) decreased considerably at 50 and 100 mm NaCl by 87.15 and 73.29% respectively, as compared to control plants (Figure 5B). In contrast, salt levels with treatments (MLE and ZnONPs) had increased SFW compared to salt-stressed plants (101.89, 108.69, 109.70, and 109.10%), respectively (Figure 5B).

The shoot DW(SDW) had a pronounced decrease with alt-stress against control plants by 79.12 and 69.01%, respectively. The treatment with MLE and ZnONPs markedly boosted SDW compared to salt-stressed plants (107.51, 111.84, 117.93, and 117.86%), respectively (Figure 5C). Salt stress reduced the root FW; the reduction was noticed at 50 and 100 mM NaCl than control plants (82.35 and 67.22%, respectively). MLE and ZnONPs application caused a significant increase in root FW in plants grown under salt treatments compared to salt-stressed plants (101.02, 105, 110.20, and 130%), respectively (Figure 5D). Root DW decreased significantly in 50 and 100 mM NaCl treatment compared to non-salt stressed plants by 87.95 and 79.51%, respectively. In contrast, the root DW was increased significantly in all the treatments compared to salt-stressed plants (101.36, 89.39, 108.21, and 124.24%), respectively (Figure 5E). Faba bean stress alone reduced root length by 52 and 60% at 50 and 100 mM NaCl levels, respectively, over the control plants. Foliar spray of faba bean plants with MLE and ZnONPs improved root length (106.65, 99.27, 117.82, and 101.38%), respectively (Figure 5F). Furthermore, a significant decrease in leaf number was observed in 50 and 100 mM NaCl compared to control plants (84.06 and 80.85%, respectively). Foliar spray of MLE and ZnONPs increases the leaf number versus plants exposed to NaCl alone (116.27, 112.40, 131.14, and 134.54%), respectively (Figure 5G). Additionally, the water content (WC%) of faba bean decreased in salt-stressed plants compared to control plants (93.61 and 89.64%, respectively). The application of MLE and ZnONPs increased the water content significantly compared to plants treated with NaCl alone (100.70, 105.36, 112.75, and 121.64%), respectively (Figure 5H).
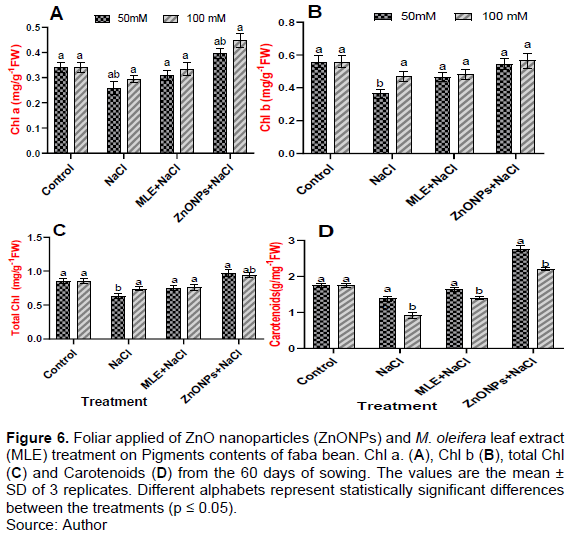
Effects of MLE and ZnONPs application on pigment contents and photosynthetic characteristics in faba bean
To evaluate the protective roles of the photosynthetic pigments under NaCl stress, the contents of photosynthetic pigments (Chl a, Chl b, Chl a + b, and carotenoids) in salt-exposed faba bean leaves were determined (Figure 6). Compared with the control sample, there was a significant decrease in Chl a content under different salinity levels (50 and 100 mM NaCl) by 73.52 and 85.29%, respectively. However, plants grown under salt conditions and received MLE and ZnONPs supply restored the concentration of Chl a compared with their salt-stressed plants by 119.37, 113.31, 153.48, and 152.55%, respectively (Figure 6A). On the other hand, it was noticed from the results that the Chl b of faba bean under different salinity levels (50 and 100 mM NaCl) decreased significantly in bean leaves with salinity stress as compared to control plants by 65.45 and 85.45%, respectively. The exogenous application of MLE and ZnONPs increased the concentration of Chl b compared with their salt-stressed plants by (127.77, 102.12, 147.94, and 119.14%), respectively (Figure 6B). However, the results showed that increasing salinity decreased the total chlorophyll of faba bean significantly under different salinity levels (50 and 100 mM NaCl) compared to control plants by 74.11 and 85.88%, respectively. In addition, the foliar application of MLE and ZnONPs increased the total chlorophyll concentration compared with their salt-stressed plants by 117.46, 102.73, 152.38, and 128.76%, respectively (Figure 6C). Increasing salinity decreased the carotenoids of faba bean significantly under different salinity levels (50 and 100 mM NaCl) compared to control by 79.31 and 52.29%, respectively. In contrast, spraying of MLE and ZnONPs increased the concentration of carotenoids compared with their salt-stressed plants by 118.11, 153.84, 200.36, and 241.75%, respectively (Figure 6D).
Effects of MLE and ZnONPs on proline content of faba bean under salt stress
The proline content of faba bean showed that NaCl treatment significantly increased, whereas 100 mM treatment significantly decreased free proline contents, and MLE and ZnONPs treatment significantly enhanced the proline accumulation (Figure 7). The proline content increased significantly at the salinity level of 100 mM NaCl, then decreased at 50 mM NaCl to control plants by 168 and 136%, respectively. However, the MLE and ZnONPs, when applied to plants, showed a further significant increase in proline accumulation content than the salt-stressed plants (85.29, 73.80, 97.05, and 85.71%), respectively (Figure 7).
Effects of MLE and ZnONPs application on mineral ion contents in faba under salt stress
Among the mineral ions, Na+ contents of shoot gradually increased with increasing salinity in salinity-treated plants. The Na+ contents of the shoot were also higher than control plants in the combined treatment of MLE + NaCl and ZnONPs + NaCl compared to salt-stressed plants (Figure 8). Compared with control, the plants treated with 50 and 100 mM NaCl had higher Na+ concentrations in their shoots by 321.42 and 307.14%, respectively (Figure 8A). However, the concentration of Na+ in the shoot of plants treated with MLE+50 mM NaCl and ZnONPs+50 mM NaCl was reduced by 57.77 and 80.21%, respectively, when compared with 50 mM NaCl plants (Figure 8A). Moreover, the amount of Na+ in the shoot of plants treated with MLE+100 mM NaCl and ZnONPs+100 mM NaCl was reduced by 76.74 and 78.13%, respectively, compared to the content in 100 mM NaCl plants (Figure 8A). Faba bean plants treated with the salinity levels (50 and 100 mM NaCl) compared to the control sample had a significant increase in leaf K+ content (274.40 and 275.20%), respectively (Figure 8B). Moreover, for the plants treated with the MLE and ZnONPs, the K+ was increased in the shoot by 245.60, 256.80, 237.60, and 257.60%, respectively, against the control plants (Figure 8B). Salinity significantly decreased Ca+2 contents in leaves of faba bean plants versus non-stress plants by 82.29 and 80.20%, respectively (Figure 8C). However, foliar application of MLE and ZnONPs significantly improved the accumulation of Ca+2 contents in leaves of faba bean plants compared to only salt-stressed plants by 142.85, 114.28, 132.91, and 127.27%, respectively (Figure 8C).
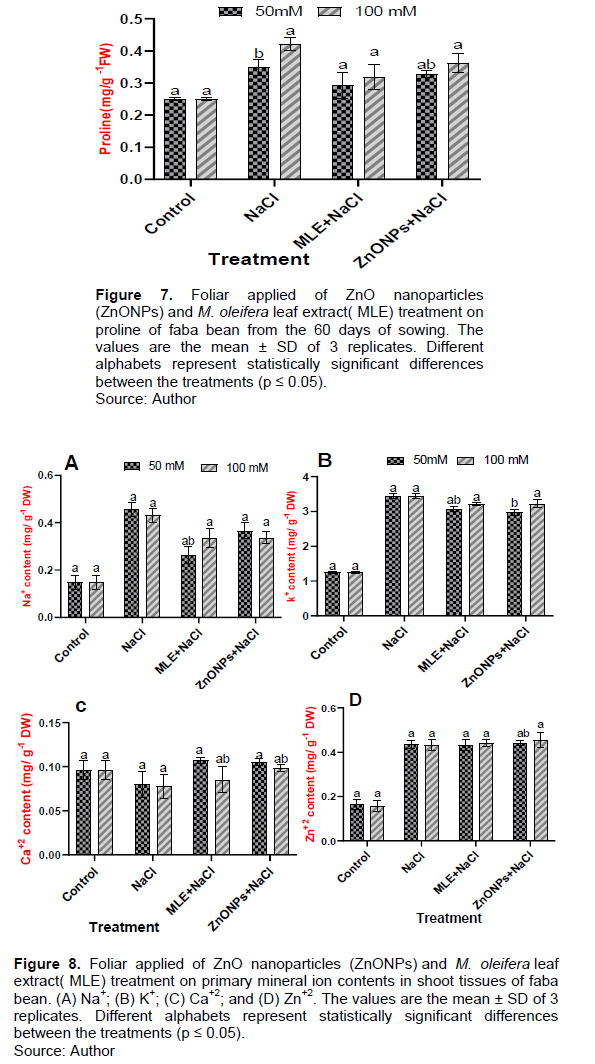
On the other hand, salinity significantly increased the Zn+2 content in salt-stressed plants than in the control plants for the shoot of faba bean by 268.75 and 286.66%, respectively (Figure 8D). Moreover, foliar application of MLE and ZnONPs plants showed a further significant increase in the amount of Zn2+ in the shoot of faba bean plants versus non-stress plants by 263.41, 293.33, 270.12 and 290.44%, respectively (Figure 8D).
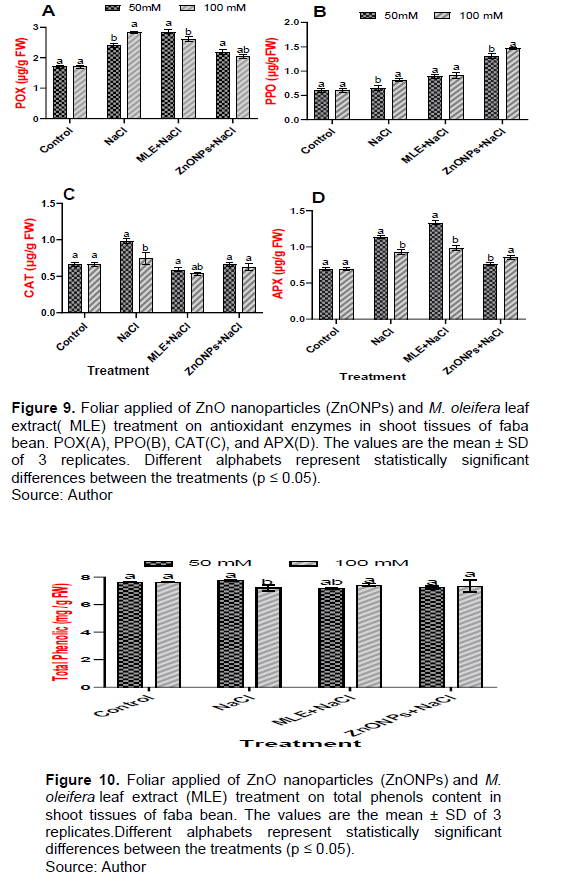
Effects of MLE and ZnO NPs application on antioxidant enzymes of faba bean under salt stress
The roles of MLE extract and ZnONPs against oxidative stress were assessed by determining the levels of the enzyme activities POX, PPO, CAT, and APX in the leaves of faba bean plants under salinity conditions (Figure 9). In salt-stressed faba bean plants grown under 50 and 100 mM, NaCl levels showed an augmented level of POX, PPO, CAT, and APX activities which increased significantly over the control plants by 141.17, 166.47, 110.16, 135.59, 148.48, 112.12, 163.76, and 134.78%, respectively (Figure 9A to D). Further foliar application of MLE and ZnO NPs significantly increased the activities of antioxidant enzymes such as POX, PPO, CAT, and APX in plants compared to the salt-stressed plants (Figure 9A to D).
Effects of MLE and ZnONPs application on total phenolic compounds of faba bean under salt stress
Faba bean plants grown in 50 and 100 mM NaCl levels showed a drastic increase in the total phenols content compared to the untreated control sample by 101.57 and 94.62%, respectively (Figure 10). Foliar application of MLE and ZnONPs to NaCl-treated plants slightly increased the total phenols content by 92.77, 102.63, 93.54 and 101.66%, respectively, compared with NaCl-treated plants (Figure 10).
DISCUSSION
M. oleifera leaf extract was used to prepare eco-friendly ZnONPs because the biomolecules in the extract are efficient in stabilizing ZnONPs. The formation of yellow colour in the extracted colour with ZnNO3 solution confirmed the synthesis of ZnONPs (Karnan and Selvakumar, 2016). The shape of ZnONPs was confirmed by XRD analysis (Zak et al., 2011). Scanning electron micrographs (SEM) show several agglomerated particles with uneven shapes in addition to individual particles with hexagonal or elongated structures (Rajendran and Sengodan, 2017). The various functional groups of synthesized nanoparticles were determined using FTIR spectroscopy. Further, proteins, alcohols, aromatic hydrocarbons, aldehydes, ketones, carboxylic acids, and stretching and bending vibrations were related to ZnONPs (Ahmad et al., 2020; Ogunyemi et al., 2019). However, the optical characteristics of the samples were investigated using UV-visible spectroscopy.
Salinity increases the osmotic potential of plants, limiting growth and productivity by imposing toxicity and adverse effects on plant growth. Salt stress affects many aspects of plant metabolism, and, as a result, growth and yields are reduced. Saline soils and saline irrigations are significant production concerns for many crops since saline conditions limit plant growth (Yildirim et al., 2008). The current study shows that salinity adversely affected faba bean growth parameters compared to the control plant. These results agree with Zhu et al. (2004), who detected that treated cucumber plants with 25 50, 100, and 190 mM NaCl reduced several growth parameters, such as fresh and dry weight in the shoot and root. However, compared to non-treated salt stress plants, MLE and ZnONPs applications enhanced the fresh and dry weight of shoot and root, leaf number, plant height, and water content (WC) of faba bean plants. Similar findings were reported byDhoke et al. (2013), who revealed that ZnONPs treatment increased root biomass and above-ground tissues of Vigna radiata seedlings. Additionally, under stress that limits soil water-deficits, plants can absorb water and nutrients due to the provision of ZnONPs, which improves the activity and expression of genes linked to phytohormones (for example, ABA and cytokinin), which promote root growth (Semida et al., 2021; Zhao et al., 2017)found that foliar spraying of ZnONPs on eggplant tissue improves water status in water-stressed plants; it can help preserve cell membrane integrity and increase RWC as metabolically accessible water, indicating plant metabolic activities. In addition, M. olifera leaf extract (MLE) significantly enhanced the growth parameters of rocket (Eruca sativa) due to its high protein content, which is required for protoplasm synthesis, as well as growth-promoting hormones including auxins and cytokinins (Abdalla, 2013). The high levels of growth-promoting compounds in MLE may be accountable for improving growth traits (Figure 5). This might be attributed to using MLE and ZnONPs, which maintained photosynthesis by improving physiological and biochemical metabolism and enhanced plant growth parameters against salt stress (Figure 5). These positive foliar applications MLE and ZnONPs might be attributed to improved membrane integrity, RWC, and increasing growth parameters (Hafez et al., 2020). This enhancement in growth might be attributed to salinity-induced positive regulation of oxidative and ionic stress (Choudhary et al., 2017).
The chlorophyll content of faba bean leaves reduced as the saline level increased. These findings are in line with those of St?pie? and K?bus (2006), who indicated that chlorophyll content decreased significantly in the leaves of spinach and cucumber plants when NaCl concentration increased. Parida and Das (2005)reported that salt stress considerably decreased the chlorophyll and total carotenoid concentrations in the leaves of many crops. The decrease of these pigments in cells under salt stress is due to sluggish synthesis or quick degradation of the pigments in cells (Ashraf, 2003). This study showed the highest concentrations of photosynthetic pigments (Chl a, b, total Chl, and carotenoids) in faba bean leaves when using an application of MLE and ZnONPs combined with plants under salt stress. Yasmeen et al. (2014)indicated that enhanced chlorophyll concentrations of tomato attributable to MLE application correspond to delayed leaf senescence due to zeatin, ascorbate, and potassium present in MLE. The high amount of chemicals involved in the chlorophyll structure in moringa leaves, including carotenoids and minerals such as Mg, P, and Ca, may be responsible for the increasing chlorophyll and carotenoid concentrations (El Sohaimy et al., 2015). Further, foliar spraying of MLE and ZnONPs may improve photosynthetic efficiency, increasing metabolites and photosynthesis for faba bean growth. One of the explanations for the decline in photosynthetic activity induced by salt stress might be a decrease in pigment content (Silveira and Carvalho, 2016).
Proline is a critical osmolyte for osmoregulation and the stability of various other macromolecules (Curá et al., 2017). The present study observed that proline levels were significantly higher in salt-stressed plants than in control plants. Plants in the current study had higher proline concentrations, which might be related to treating faba bean plants with MLE and ZnONPs, which relieved salt stress. Our findings are similar to the results obtained in barley (Dbira et al., 2018). Proline has been widely exploited as an effective marker in plant salt tolerance mechanisms due to its critical involvement in plant osmotic adjustment (Uddin et al., 2012). It may be possible that MLE and ZnONPs treatments enhanced proline accumulation by activating proline metabolism enzymes.
Our study indicated that salt stresses caused decreased Ca+2 contents (Fig.8C) and a significant increase in other ions concentration (Na+, K+, and Zn+2) compared to non-stressed plants. In this study, the foliar application of MLE and ZnO NPs led to a significant increase in the mineral content (Na+, K+, Ca+2, and Zn+2) compared to untreated plants under salt stress. This might be because NPs can permeate tissues and move to other organs through the phloem (Pérez-de-Luque, 2017). The Na+ concentration in plant tissues is an essential indicator of salt tolerance since low content indicates minimal ion uptake (Tahir et al., 2006).
Furthermore, the treatments increased K+ concentrations in plant tissues, leading to a K+/Na+ ratio regulation in stressed plants compared with the non-stress plants. Rady et al. (2019)demonstrated that under abiotic stress conditions, foliar spraying with MLE induced a decrease in Na+ and increased the content of K+ and Ca+2 in common bean plants compared to untreated plants. In addition, calcium (Ca+2) is essential for processes such as maintaining the structural and functional integrity of plant membranes, stabilizing cell wall structures, regulating ion transport and selectivity and controlling ion-exchange behaviour and cell wall enzyme activities (Yildirim et al., 2008). Intracellular Ca+2 can influence plant responses to drought and salinity, which has also been attributed to the transmission of drought and salt-stress responses in plants, which play an essential part in osmoregulation under these environments (Bartels and Sunkar, 2005).
Antioxidant enzymes (e.g., PPO, POX, CAT, and APX.) are induced in faba bean plants as a response to abiotic stress as well as a vital part of the antioxidant defense system to prevent biomarkers of oxidative stress, such as hydroxyl radicals responsible for lipid peroxidation in cell membranes and destructive effects on plant productivity (Abogadallah, 2010). This study showed that the foliar spraying of ZnONPs and MLE enhanced the enzymes activities of faba bean under salt stress. These results agreed with Trivedi et al. (2018), who suggest that with increasing levels of salt stress, polyphenol oxidase activity (PPO) increased in green gram plants. Similarly, Purohit et al. (2020)reported that higher concentrations of salt stress increased the activity of antioxidant enzymes such as catalase (CAT) in groundnut plants. Pal et al. (2004)noted that the antioxidant enzymes such as ascorbate peroxidase (APX) increased in salinity tolerant and sensitive rice cultivars. These findings may be attributable to the high potency active components present in MLE and ZnONPs, which increased enzymatic antioxidants in faba beans, as shown in Figure 9.
Phenolic compounds are the most common secondary metabolite in plants, which have redox characteristics that help plants absorb and scavenge free radicals, quench singlet and triplet oxygen, and decompose peroxides (Osawa, 1994). Consequently, phenolics are potent antioxidants and play an essential role in plant defense against environmental stressors (Elzaawely et al., 2007). The present study showed that the application of MLE and ZnONPs significantly increased total phenolics in faba bean leaves compared to salt stress plants (Figure 9). This results in agreement with Basra et al. (2011)in maize. The result obtained showed that foliar application of ZnONPs and MLE increased total phenolics compared with untreated stress plants.
CONCLUSION
In this study, ZnONPS were synthesized using Moringa leaf extract. The UV spectra revealed a peak at 370 nm, which is within the wavelength range of green ZnONPs. XRD peaks confirmed the effectiveness of the crystalline feature and synthesis procedure. The average size of ZnONPs was confirmed by SEM examination and was revealed to be 215 nm. Through FTIR analysis, functional groups of phytoconstituents that act as capping and stabilizing agents were found, endorsed the formation of ZnONPs. Further, the foliar applications of MLE and ZnONPs can reduce the adverse effects of salt stress in faba bean plants by increasing chlorophyll content, photosynthetic activity, growth parameters, biochemical characteristics such as chlorophyll a, chlorophyll b, total chlorophyll, carotenoids, proline, mineral content, antioxidant enzyme activity, and total phenolic. Thus, helping plants to exhibit salt tolerance. Based on these findings, the MLE and ZnONPs treatments could help reduce the adverse effects of salinity on faba bean growth.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
ACKNOWLEDGEMENT
The authors thank the African Union through the Pan African University Institute of Basic Science, Technology and Innovation (PAUSTI) for funding the research.
REFERENCES
|
Abdalla MM (2013). The potential of Moringa oleifera extract as a biostimulant in enhancing the growth, biochemical and hormonal contents in rocket (Eruca vesicaria subsp. sativa) plants. International Journal of Plant Physiology and Biochemistry 5(3):42-49. |
|
|
Abogadallah GM (2010). Insights into the significance of antioxidative defense under salt stress. Plant Signaling and Behavior 5(4):369-374. |
|
|
Aebi H (1984). Catalase in vitro." Methods in enzymology.Academic Press, Inc. 105:121-126. |
|
|
Ahmad H, Venugopal K, Rajagopal K , Britto S, Nandini B, Pushpalatha HG, Konappa N, Udayashankar AC, Geetha N, Jogaiah S (2020). Green synthesis and characterization of zinc oxide nanoparticles using Eucalyptus globules and their fungicidal ability against pathogenic fungi of apple orchards. Biomolecules 10(3):425. |
|
|
Ahmad P, Abdel Latef AA, Hashem A, Abd Allah EF, Gucel S, Tran LSP (2016). Nitric oxide mitigates salt stress by regulating levels of osmolytes and antioxidant enzymes in chickpea. Frontiers in Plant Science 7:347. |
|
|
Ashraf M (2003). Relationships between leaf gas exchange characteristics and growth of differently adapted populations of Blue panicgrass (Panicum antidotale Retz.) under salinity or water logging. Plant Science 165(1):69-75. |
|
|
Bartels D, Sunkar R (2005). Drought and salt tolerance in plants. Critical Reviews in Plant Science 24(1):23-58. |
|
|
Basra SMA, Iftikhar MN, Afzal I (2011). Potential of moringa (Moringa oleifera) leaf extract as priming agent for hybrid maize seeds. International Journal of Agriculture and Biology 13(6). |
|
|
Bates LS, Waldren RP, Teare ID (1973). Rapid determination of free proline for water-stress studies. Plant and Soil 39(1):205-207. |
|
|
Bohra A, Pandey MK, Jha UC, Singh B, Singh IP, Datta D, Chaturvedi SK, Nadarajan N, Varshney RK (2014). Genomics-assisted breeding in four major pulse crops of developing countries: present status and prospects. Theoretical and Applied Genetics 127(6):1263-1291. |
|
|
Bulut F, Akinci S (2010). The effect of salinity on growth and nutrient composition in broad bean (Vicia faba L.) seedlings. F Fresenius Environmental Bulletin 19(12):2901-2910. |
|
|
Choudhary RC, Kumaraswamy RV, Kumari S, Sharma SS, Pal A, Raliya R, Biswas P, Saharan V (2017). Cu-chitosan nanoparticle boost defense responses and plant growth in maize (Zea mays L.). Scientific Reports 7(1):1-11. |
|
|
Curá JA, Franz DR, Filosofía JE, Balestrasse KB, Burgueño LE (2017). Inoculation with Azospirillum sp. and Herbaspirillum sp. bacteria increases the tolerance of maize to drought stress. Microorganisms 5(3):41. |
|
|
Dbira S, Al Hassan M, Gramazio P, Ferchichi A, Vicente O, Prohens J, Boscaiu M (2018). Variable levels of tolerance to water stress (drought) and associated biochemical markers in Tunisian barley landraces. Molecules 23(3):613. |
|
|
Dhoke SK, Mahajan P, Kamble R, Khanna A (2013). Effect of nanoparticles suspension on the growth of mung (Vigna radiata) seedlings by foliar spray method. Nanotechnology Development 3(1):e1. |
|
|
El-Hack ME, Alagawany M, Elrys AS, Desoky E-SM, Tolba HMN, Elnahal ASM, Elnesr SS, Swelum AA (2018). Effect of Forage Moringa oleifera L. on Animal Health and Nutrition and Its Beneficial Applications in Soil, Plants and Water Purification. Agriculture 8(9):145. |
|
|
El Sohaimy SA, Hamad GM, Mohamed SE, Amar MH, Al-Hindi RR (2015). Biochemical and functional properties of Moringa oleifera leaves and their potential as a functional food. Global Advanced Research Journal of Agricultural Science 4(4):188-199. |
|
|
Elzaawely AA, Xuan TD, Koyama H, Tawata S (2007). Antioxidant activity and contents of essential oil and phenolic compounds in flowers and seeds of Alpinia zerumbet (Pers.) BL Burtt. and RM Sm. Food Chemistry 104(4):1648-1653. |
|
|
Farooq M, Gogoi N, Hussain M, Barthakur S, Paul S, Bharadwaj N, Migdadi HM, Alghamdi SS, Siddique KHM (2017). Effects, tolerance mechanisms and management of salt stress in grain legumes. Plant Physiology and Biochemistry 118:199-217. |
|
|
Gulen H, Eris A (2003). Some physiological changes in strawberry (Fragaria× ananassa 'Camarosa') plants under heat stress. Journal of Horticultural Science and Biotechnology 78(6):894-898. |
|
|
Hafez Y, Attia K, Alamery S, Ghazy A, Al-Doss A, Ibrahim E, Rashwan E, El-Maghraby L, Awad A, Abdelaal K (2020). Beneficial effects of biochar and chitosan on antioxidative capacity, osmolytes accumulation, and anatomical characters of water-stressed barley plants. Agronomy 10(5):630. |
|
|
Helaly MN, El-Metwally MA, El-Hoseiny H, Omar SA, El-Sheery NI (2014). Effect of nanoparticles on biological contamination of'in vitro'cultures and organogenic regeneration of banana. Australian Journal of Crop Science 8(4):612-624. |
|
|
Kah M, Kookana RS, Gogos A, Bucheli TD (2018). A critical evaluation of nanopesticides and nanofertilizers against their conventional analogues. Nature Nanotechnology 13(8):677-684. |
|
|
Kar M, Mishra D (1976). Catalase, peroxidase, and polyphenoloxidase activities during rice leaf senescence. Plant Physiology 57(2):315-319. |
|
|
Karnan T, Selvakumar SAS (2016). Biosynthesis of ZnO nanoparticles using rambutan (Nephelium lappaceum L.) peel extract and their photocatalytic activity on methyl orange dye. Journal of Molecular Structure 1125:358-365. |
|
|
Khalilzadeh R, Sharifi RS, Jalilian J (2016). Antioxidant status and physiological responses of wheat (Triticum aestivum L.) to cycocel application and bio fertilizers under water limitation condition. Journal of Plant Interactions 11(1):130-137. |
|
|
Khot LR, Sankaran S, Maja JM, Ehsani R, Schuster EW (2012). Applications of nanomaterials in agricultural production and crop protection: a review. Crop Protection 35:64-70. |
|
|
Koivunen E, Partanen K, Perttilä S, Palander S, Tuunainen P, Valaja J (2016). Digestibility and energy value of pea (Pisum sativum L.), faba bean (Vicia faba L.) and blue lupin (narrow-leaf)(Lupinus angustifolius) seeds in broilers. Animal Feed Science and Technology 218:120-127. |
|
|
Latef AAA, Hasanuzzaman M, Tahjib-Ul-Arif M (2021). Mitigation of salinity stress by exogenous application of cytokinin in faba bean (Vicia faba L.). Notulae Botanicae Horti Agrobotanici Cluj-Napoca 49(1):12192. |
|
|
Lim YJ, Oh CS, Park YD, Eom SH, Kim DO, Kim UJ, Cho YS (2014). Physiological components of kiwi fruits with in vitro antioxidant and acetylcholinesterase inhibitory activities. Food Science and Biotechnology 23(3):943-949. |
|
|
MacAdam JW, Nelson CJ, Sharp RE (1992). Peroxidase activity in the leaf elongation zone of tall fescue: I. Spatial distribution of ionically bound peroxidase activity in genotypes differing in length of the elongation zone. Plant Physiology 99(3):879-885. |
|
|
Matinise N, Fuku XG, Kaviyarasu K, Mayedwa N, Maaza MJ (2017). ZnO nanoparticles via Moringa oleifera green synthesis: Physical properties & mechanism of formation. Applied Surface Science 406:339-347. |
|
|
Nakano Y, Asada K (1981). Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant and Cell Physiology 22(5):867-880. |
|
|
Neugart S, Rohn S, Schreiner M (2015). Identification of complex, naturally occurring flavonoid glycosides in Vicia faba and Pisum sativum leaves by HPLC-DAD-ESI-MSn and the genotypic effect on their flavonoid profile. Food Research International 76:114-121. |
|
|
Ogunyemi SO, Abdallah Y, Zhang M, Fouad H, Hong X, Ibrahim E, Masum MMI, Hossain A, Mo J, Li B (2019). Green synthesis of zinc oxide nanoparticles using different plant extracts and their antibacterial activity against Xanthomonas oryzae pv. oryzae. Artificial Cells, Nanomedicine, and Biotechnology 47(1):341-352. |
|
|
Osawa T (1994). Novel natural antioxidants for utilization in food and biological system. In: Uritani I, Garcia VV, Mendoza EM (eds) Post-harvest bSiochemistry of plant food-materials in the tropics. Journal of the Physical Society of Japan 241-251. |
|
|
Pal M, Singh DK, Rao LS, Singh KP (2004). Photosynthetic characteristics and activity of antioxidant enzymes in salinity tolerant and sensitive rice cultivars. Indian Journal of Plant Physiology 9(4):407-412. |
|
|
Pal S, Mondal S, Maity J, Mukherjee R (2018). Synthesis and characterization of ZnO nanoparticles using Moringa oleifera leaf extract: investigation of photocatalytic and antibacterial activity. International Journal of Nanoscience and Nanotechnology 14(2):111-119. |
|
|
Parida AK, Das AB (2005). Salt tolerance and salinity effects on plants: a review. Ecotoxicology and Environmental Safety 60(3):324-349. |
|
|
Pérez-de-Luque A (2017). Interaction of nanomaterials with plants: what do we need for real applications in agriculture. Frontiers in Environmental Science 5:12. |
|
|
Pervez K, Ullah F, Mehmood S, Khattak A (2017). Effect of Moringa oleifera Lam. leaf aqueous extract on growth attributes and cell wall bound phenolics accumulation in maize (Zea mays L.) under drought stress. Kuwait Journal of Science 44(4):110-118. |
|
|
Purohit HB, Patel RS, Talavia BP, Kandoliya UK (2020). Effect of gibberellic acid, potassium nitrate and silicic acid on antioxidative enzymes in groundnut (Arachis hypogaea L.) seedling irrigated with saline water. Journal of Pharmacognosy and Phytochemistry 9(4):1867-1873. |
|
|
Rady MM, Talaat NB, Abdelhamid MT, Shawky BT, Desoky ESM (2019). Maize (Zea mays L.) grains extract mitigates the deleterious effects of salt stress on common bean (Phaseolus vulgaris L.) growth and physiology. Journal of Horticultural Science and Biotechnology 94(6):777-789. |
|
|
Rajendran S P, Sengodan K (2017). Synthesis and characterization of zinc oxide and iron oxide nanoparticles using Sesbania grandiflora leaf extract as reducing agent. Journal of Nanoscience (17):1-7. |
|
|
Raliya R, Tarafdar JC (2013). ZnO Nanoparticle Biosynthesis and Its Effect on Phosphorous-Mobilizing Enzyme Secretion and Gum Contents in Clusterbean (Cyamopsis tetragonoloba L.). Agricultural Research 2(1):48-57. |
|
|
Rehman H, Nawaz Q, Basra SMA, Afzal I, Yasmeen A (2014). Seed priming influence on early crop growth, phenological development and yield performance of linola (Linum usitatissimum L.). Journal of Integrative Agriculture 13(5):990-996. |
|
|
Semida WM, Abdelkhalik A, Mohamed G, El-Mageed A, Taia A, El-Mageed A, Shimaa A, Rady MM, Ali EF (2021). Foliar application of zinc oxide nanoparticles promotes drought stress tolerance in eggplant (Solanum melongena L.). Plants 10(2):421. |
|
|
Silveira JAG, Carvalho FEL (2016). Proteomics, photosynthesis and salt resistance in crops: An integrative view. Journal of Proteomics 143:24-35. |
|
|
Stateras DC, Moustakas NK (2018). Seasonal changes of macro-and micro-nutrients concentration in olive leaves. Journal of Plant Nutrition 41(2):186-196. |
|
|
St?pie? P, K?bus G (2006). Water relations and photosynthesis in Cucumis sativus L. leaves under salt stress. Biologia Plantarum 50(4):610-616. |
|
|
Tahir MA, Rahmatullah T, Aziz M, Ashraf S, Kanwal S, Maqsood MA (2006). Beneficial effects of silicon in wheat (Triticum aestivum L.) under salinity stress. Pakistan Journal of Botany 38(5):1715-1722. |
|
|
Tavakkoli E, Rengasamy P, McDonald GK (2010). High concentrations of Na+ and Cl-ions in soil solution have simultaneous detrimental effects on growth of faba bean under salinity stress. Journal of Experimental Botany 61(15):4449-4459. |
|
|
Trivedi SK, Solanki MV, Kandoliya UK, BA G (2018). Effect of exogenous application of salicylic acid on antioxidative enzymes in green gram (Vigna radiate L.) irrigated with saline water. International Journal of Chemical Studies 6(4):2668-2674. |
|
|
Uddin M, Juraimi AS, Ismail M, Hossain M, Othman R, Abdul Rahim A (2012). Physiological and growth responses of six turfgrass species relative to salinity tolerance. Scientific World Journal 10 p. |
|
|
Vafa ZN, Sirousmehr AR, Ghanbari A, Khammari I, Falahi N (2015). Effects of nano zinc and humic acid on quantitative and qualitative characteristics of savory (Satureja hortensis L.). International Journal of Biosciences 6(3):124-136. |
|
|
Vishekaii ZR, Soleimani A, Fallahi E, Ghasemnezhad M, Hasani A (2019). The impact of foliar application of boron nano-chelated fertilizer and boric acid on fruit yield, oil content, and quality attributes in olive (Olea europaea L.). Scientia Horticulturae 257:108689. |
|
|
Waqas MA, Khan I, Akhter MJ, Noor MA, Ashraf U (2017). Exogenous application of plant growth regulators (PGRs) induces chilling tolerance in short-duration hybrid maize. Environmental Science and Pollution Research 24(12):11459-11471. |
|
|
Yasmeen A, Basra SMA, Wahid A, Farooq M, Nouman W, Hussain N (2013). Improving drought resistance in wheat (Triticum aestivum) by exogenous application of growth enhancers. International Journal of Agriculture and Biology 15(6):1560-8530. |
|
|
Yasmeen A, Nouman W, Basra SMA, Wahid A, Hussain N, Afzal I (2014). Morphological and physiological response of tomato (Solanum lycopersicum L.) to natural and synthetic cytokinin sources: a comparative study. Acta Physiologiae Plantarum 36(12):3147-3155. |
|
|
Yildirim E, Turan M, Guvenc I (2008). Effect of foliar salicylic acid applications on growth, chlorophyll, and mineral content of cucumber grown under salt stress. Journal of Plant Nutrition 31(3):593-612. |
|
|
Zafar H, Ali A, Ali JS, Haq IU, Zia M (2016). Effect of ZnO nanoparticles on Brassica nigra seedlings and stem explants: growth dynamics and antioxidative response. Frontiers in Plant Science 7:535. |
|
|
Zafar S, Ashraf A, Ijaz MU, Muzammil S, Siddique MH, Afzal S, Andleeb R, Al-Ghanim KA, Al-Misned F, Ahmed Z (2020). Eco-friendly synthesis of antibacterial zinc nanoparticles using Sesamum indicum L. extract. Journal of King Saud University-Science 32(1):1116-1122. |
|
|
Zak AK, Razali R, AbdMajid WH, Darroudi M (2011). Synthesis and characterization of a narrow size distribution of zinc oxide nanoparticles. International Journal of Nanomedicine 6:1399-1403. |
|
|
Zhao LS, Li K, Wang QM, Song XY, Su HN, Xie BB, Zhang XY, Huang F, Chen XL, Zhou BC (2017). Nitrogen starvation impacts the photosynthetic performance of Porphyridium cruentum as revealed by chlorophyll a fluorescence. Scientific Reports 7(1):1-11. |
|
|
Zhu Z, Wei G, Li J, Qian Q, Yu J (2004). Silicon alleviates salt stress and increases antioxidant enzymes activity in leaves of salt-stressed cucumber (Cucumis sativus L.). Plant Science 167(3):527-533. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0