Full Length Research Paper
ABSTRACT
Breast cancer is prevalent among women worldwide. African walnut (Tetracarpidium conophorum) seeds extract have been shown to have medicinal properties. This study is to determine the effects of feeding T. conophorum seeds lipid extract on 3-methylcholanthrene (MC) induced breast carcinogenesis and the expression of ethoxyresorufin-O-deethylase (CYP1A1), benzoxyresorufin-O-dealkylase (CYP1B1) and pentoxyresorufin-O-dealkylase (CYP2B1). The lipid was extracted using Soxhlet apparatus with n-hexane. Forty-five female Wistar rats of 21 days old were used, which were randomly divided into three major groups of 15 animals each. Groups A and B were fed for 12 weeks with diet containing T. conophorum seeds lipid extract (10%), and group B animals were administered MC (200 mg/kg body weight) intraperitoneally after 4 weeks of feeding. Group C animals were fed for 12 weeks with diet containing no T. conophorum seeds lipid extract and administered MC (200 mg/kg body weight) intraperitoneally after 4 weeks of feeding. Results indicated that CYP1A1, CYP1B1 and CYP2B1 were significantly (p < 0.05) reduced in Group B animals liver cells compared to group C with higher expression. Prolonged latency period, reduce tumor weight and size characterized group B animals compared to group C. Histopathology results showed normal morphology of the liver hepatocytes of animals in group B, while necrosis and steatosis were seen in group C. This study therefore showed that T. conophorum seeds lipid extract contains bioactive components that may oppose breast carcinogenesis induced by MC.
Key words: 3-Methylcholanthrene, carcinogenesis, Tetracarpidium conophorum, fatty acids.
INTRODUCTION
Walnuts are well known plants in Western Africa especially in Nigeria, and their edible seeds are widely cultivated for their delicacy (Uhunmwangho and Omoregie, 2017a). The tropical African walnut, known as Tetracarpidium conophorum belongs to the family Euphorbiaceae (Edem et al., 2009). Adebona et al. (1988) stated that some walnut species are found in the family Olacaceae. The plant African walnut is popularly known as, black walnut or Nigerian walnut (Nwaichi et al., 2017). In Nigeria, among the Yoruba tribe, it is known as awusa or asala, ukpa, or okeokpokirinyain Igbo and gawudibairi in Hausa, okhueor okwe among the Bini tribe of Edo State (Chijoke et al., 2015). The seeds are consumed as snacks (Chijoke et al., 2017). The plant is a woody perennial climber of about 6 to 18 m long (Nwachoko and Jack, 2015). The African walnut is widely grown in the western and eastern parts of Nigeria (Oke, 1995; Nwaichi et al., 2017). All parts of African walnut plant have been used ethnomedically (Janick and Paul, 2008). Nwauzoma and Dappa (2013) reported ethnobotanical uses of African walnut seed extract in the treatment of fibroids, high blood pressure, malaria (Ayoola et al., 2011; Ogunyinka et al., 2015; Uhunmwangho and Omoregie, 2017b). The nut oil contains 48 to 50% dry weight of oil, is golden yellow in colour, with a taste resembling linseed oil (Negi et al., 2011). The oil is highly rich in polyunsaturated fatty acids (Kanu et al., 2015). Dietary fats constitute essential nutrients and are important source of essential fatty acids (FAs) such as α-linolenic, dihomo-dietary-?-linolenic, docosahexaenoic and eicosapentaenoic acids (Uhunmwangho and Omoregie, 2017a). These FAs contribute to the inhibition of diseases involving abnormal and uncontrolled proliferation of cells (Hardman, 2014; Uhunmwangho et al., 2022a). The presence of these essential fatty acids in African walnut seed oil have been demonstrated (Dada and Aguda, 2015; Ayeni and Nuhu, 2018). Cytochromes P450 (CYPs) are enzymes that oxidize substances using iron and are able to metabolize a large variety of xenobiotic substances. CYP enzymes are linked to a wide array of reactions including and O-dealkylation, S-oxidation, epoxidation, and hydroxylation (Lee, 2013). The activity of the typical P450 cytochrome is influenced by a variety of factors, including herbal medications (Sparreboom et al., 2004). The objective of this work is to assess the dietary fats of T. conophorum seed oil in the modification of cancer metabolizing enzymes as cancers prevention phytomedicines.
MATERIALS AND METHODS
Ethical permission
Ethical permission was taken from UniMed Research Ethics Committee for the use of experimental rats for these studies.
Reagents/chemicals
All reagents used were of analytical grade.
Plant (sample collection)
Fresh T. conophorum fruits were obtained from farms in Ondo Town, Ondo State, Nigeria and authenticated by a taxonomist of the Botany Department, University of Medical Sciences, Ondo, Nigeria. At each harvest, 40 fruits were collected randomly. The collected fruits were cleaned and the seeds carefully separated from the fruits and dried at 65°C for 4 h in an oven, crushed with a laboratory mortar and pestle and were kept in a well labeled air tight screw-capped bottle at -4°C for extraction.
Extraction of oil from African walnut
The Soxhlet extraction method was according to AOAC (1996) as reported by Sankeshwari et al. (2018). The seeds of T. conophorum were shelled, cut into small pieces, and air dried at room temperature for 2 weeks. The dried seeds of African walnut were ground into powdered form using a blender and further air dried. Fifty grams of African walnut seed powder was packed in a muslin cloth bag and placed in soxhlet apparatus using n-hexane as solvent. At the end of extraction, the thimble was dried in an oven for about 30 min at 100°C to evaporate off the solvent and cool in a desiccator, which was weighed and kept in the refrigerator.
Feeding the animals with diet containing walnut seed oil
Female Wistar rats (21 day old) were obtained from the University of Medical Science, Ondo, and were housed in metal cages in a well-ventilated room and allowed access to water ad libitum. The animals were randomly divided into three major groups of 15 animals each. Group-A animals were fed for 12 weeks with diet containing 10% of T. conophorum seeds lipid extract only. Group B animals were fed for 12 weeks with diet containing 10% of T. conophorum seeds lipid extract and administered MC (200 mg/kg body weight) intraperitoneally after 4 weeks of feeding. Group C animals were fed for 12 weeks with diet containing no T. conophorum seeds lipid extracts and administered MC (200 mg/kg body weight) intraperitoneally after 4 weeks of feeding. The animals were palpated weekly to determine the time of appearance of tumors. Animals from each group were sacrificed after 12 week, mammary glands were exposed and tumors were excised. Tumor incidence, volume and weight were determined and tissues collected for enzymes and biochemical analysis. Portions of liver tissues were preserved in RNA for gene expression studies in formalin (10%) for histopathological studies.
Preparation of liver microsomes
The preparatory of liver microsomes were as reported by Rani and Kansa (2012).
Enzyme assays
Microsomal ethoxyresorufin-O-de-ethylase, benzoxyresorufin-O-dealkylase and pentoxyresorufin-O-dealkylase activities were assayed according to the procedure described by Burke et al. (1985) and modified by Teel and Huynh (1998).
PCR amplification and agarose gel electrophoresis
PCR amplification for the determination of genes whose primers are listed in Table 1 was done using the following protocol: PCR amplification was performed in a total of 25 µL volume reaction mixture containing 2 µL cDNA (10 ng), 2 µL primer (100 pmol) 12.5 µL, Ready Mix Taq PCR master mix and 8.5 µL nuclease-free water. Initial denaturation at 95°C for 5 min was followed by 20 cycles of amplification (denaturation at 95°C for 30 s, annealing for 30 s and extension at 72°C for 60 s) and ending with final extension at 72°C for 10 min. In all experiments, negative controls were included where reaction mixture has no cDNA. The amplicons were resolved on 1.5% agarose gel in Tris-Borate-EDTA buffer (pH 8.4).
Histopathology
The histopathology study was according to Avwioro (2010).
Statistical analysis
The values were expressed as mean ± SE. Kruskal-Wallis one-way analysis of variance (ANOVA) was used for the feed intake, tumor weight, tumor volume, and cancer metabolizing enzymes gene expression. A difference with P<0.05 was considered statistically significant. All the statistics were carried out in SAS (The SAS System for windows, v8; SAS Institute Inc., Cary, NC, USA).
RESULTS
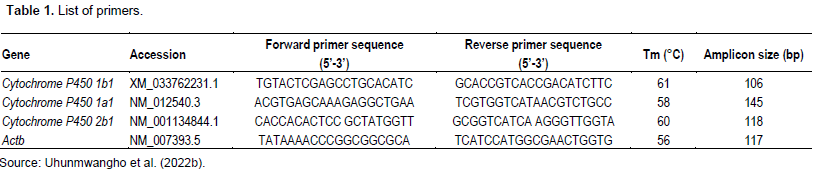
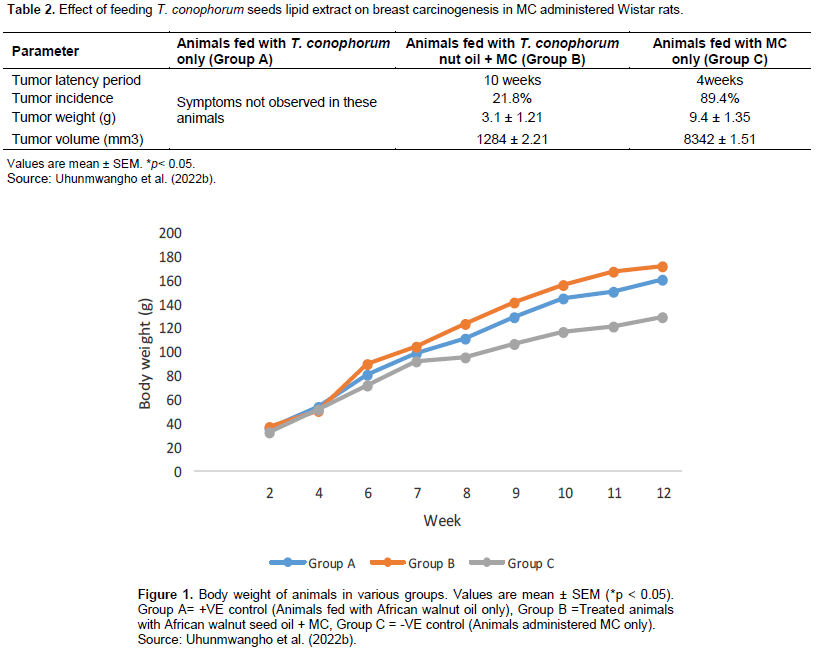
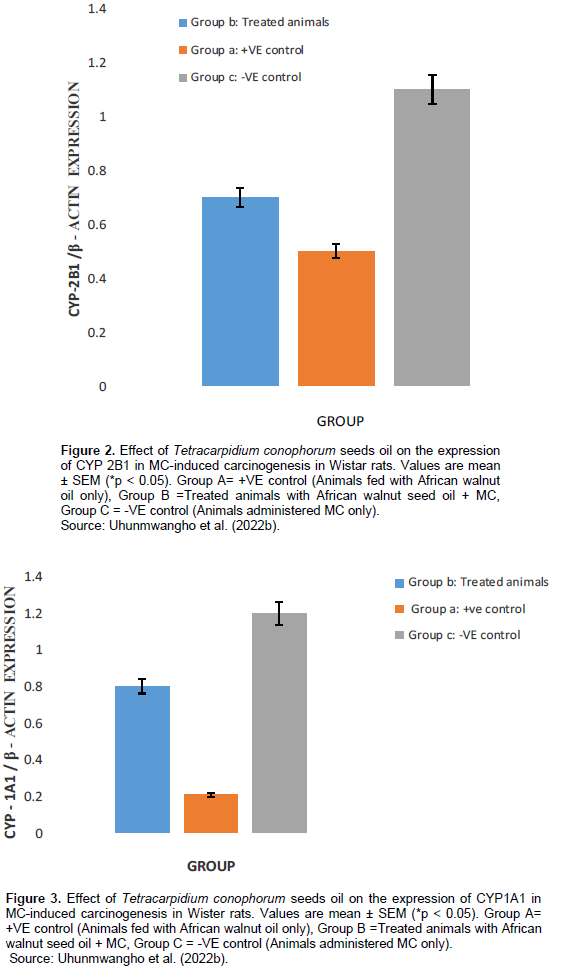
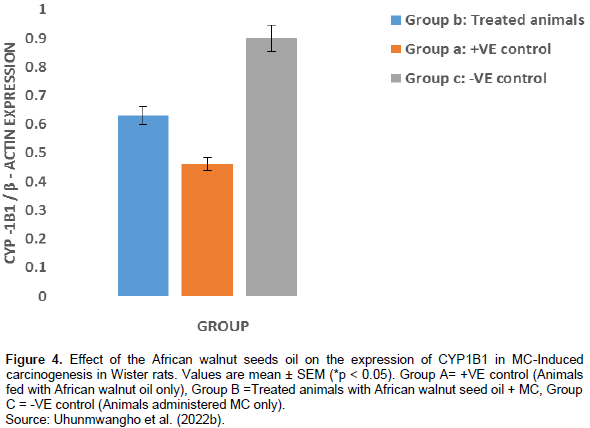
A total of 6 rats died within the 6 to 10th week due to carcinogen toxicity, while no mortality was observed in the group pretreated with T. conophorum seeds lipid extract and the group that was fed with T. conophorum seeds lipid extract only (Table 2). Figure 1 reveals that the growth of animals was similar in rats fed with diet containing T. conophorum seeds lipid extract for 12 weeks and the difference in body weight gained was not statistically significant except in animals administered MC. There was a significance increase in the expression of the activities of CYP1B1 and CYP2B1 in the group that received the carcinogen only (Figures 2 and 4). CYP1A1 activity in liver increased in animals in the group administered with carcinogen only and the magnitude of increase doubles activity in the animals in group treated with diet containing T. conophorum seeds lipid extract.
DISCUSSION
Phytotherapy is a potential therapeutic strategy involving the usage of traditional medicinal plants to target various molecular markers altered during cancer, thereby leading to the prevention and treatment of cancer, without inducing side effects (Alugoju et al., 2021). African walnut contain many bioactive components including specific fatty acids, which could be active against cancer (Batirel et al., 2018).T. conophorum have been found to have various medicinal properties such as antioxidant, anti-diabetic, anti-inflammatory, anti-proliferative effects, among others (Batirel et al., 2018; Ayeni and Nuhu, 2018). Figure 1 reveals that the growth of animals was similar in rats fed with diet containing Tetracarpidium conophorum seeds lipid extract for 12 weeks and the difference in body weight gained was not statistically significant except in animals administered MC. The dietary fat present in T. conophorum nut (Uhunmwangho and Omoregie, 2017a), may be responsible for the slight increase in weight compared to the animals in group not fed with diet containing Tetracarpidium conophorum seeds lipid extract. A total of 6 rats died within the 6 to 10th week due to carcinogen toxicity, while no mortality was observed in the group pretreated with T. conophorum seeds lipid extract and the group that was fed with T. conophorum seeds lipid extract only. Data on incidence, latency period, weight, size and volume of tumors in mammary gland are summarized in Table 1.
The incidence and latency period of tumors on T. conophorum seeds lipid extract pretreated animals was 21.8% and 10 weeks respectively, and was significantly (P<0.05) lower than animals administered carcinogen only with 89.4% and 4 weeks, respectively. The average size and volume of tumor was generally larger in MC administered group than in Tetracarpidium conophorum seeds lipid extract diet treated group. Hence, the feeding of animals which started during the pubescent period of mammary gland development might have resulted in the decreased tumor incidence and progression to malignancy. Histopathology results (Plates 1 to 3) showed normal morphology of the hepatocytes of animals fed with diet containing T. conophorum nut oil (groups A and B), while necrosis, steatosis and degeneration of cytoplasm with vacuoles were seen in group that was not fed with diet containing T. conophorum seeds lipid extract (group C).
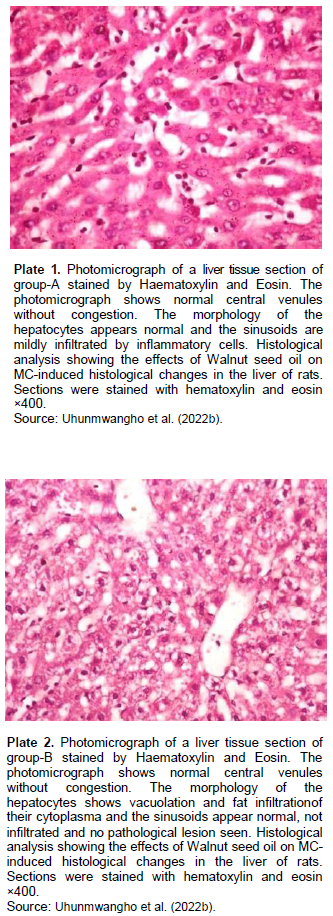
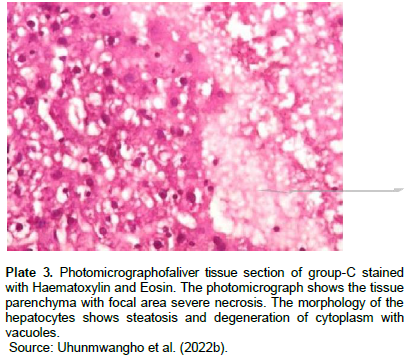
The metabolism of carcinogens involves phase-I and phase-II reactions; in the first reaction, carcinogens are metabolized to reactive molecules by phase-I enzymes, while the active metabolite gets detoxified by several phase-II enzymes. Thus, phase-I and phase-II enzymes reactive reactions would determine the extent of carcinogenesis. The phase-I cytochrome P450 enzymes are membrane bound and their activities are influenced by the fatty acids environment (Ogunyinka et al., 2015; Batirel et al., 2018; Ayeni and Nuhu, 2018). Hence, altering membrane lipid composition by feeding animals on singular source of fat might affect carcinogen metabolism (Talaska et al., 2006). CYP1A1 activity in liver increased in animals in the group administered with carcinogen only and the magnitude of increase doubles activity in the animals in group treated with diet containing T. conophorum seeds lipid extract (Figure 3). But the group fed with diet containing the extracted lipid only, the activity of CYP1A1 activity was the lowest. CYP1B1 and CYP2B1 activities in liver remained slightly affected the group treated with diet containing T. conophorum seeds lipid extract throughout the period of the experiment compared to group C (group fed with MC only), and there was a significant increase in the expression of the activities of CYP1B1 and CYP2B1 in the group that received the carcinogen only (Figures 2 and 4). T. conophorum seeds lipid extract which contains a large amount of saturated and unsaturated fatty acids can modify the degree of unsaturation in lipids and thereby change the physicochemical environment of the microsomal membranes, which may be responsible for the decrease CYP1A1, CYP1B2 and CYP1B1 activities observed in T. conophorum seeds lipid extract fed rats. The decrease of these phase I enzymes might have contributed to decreased incidence of mammary tumors in T. conophorum seeds lipid extract fed rats compared to animals administered with MC only. Conjugated linoleic acid (CLA) and docosahexaenoic acid are well-documented anticarcinogenic agent (Ip et al., 1996).
In an in vitro study, CLA (0.5 μM) was reported to inhibit CYP1A1, CYP1A2, and CYP1B1 activities in hamster liver microsome (Teel and Huynh, 1998). T. conophorum seeds lipid extract used in this study ontained rich amount of omega-3 fatty acids which include: alpha linolenic acid (ALA), eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA); omega-6 fatty acids which include: linoleic acid, arachidonic acid (AA), gammalinolenic acid (GLA), dihomo-gamma linolenic acid (DGLA); and omega-9 fatty acids which include: oleic acid, eicosenoic acid, erucic and nervonic acid (Uhunmwangho and Omoregie, 2017a). This indicates the great nutritional and health benefits of T. conophorum seed oil and its role in down regulation of the phase-I activities of carcinogen activation, CYP1A1, CYP1B1 and CYP2B1 in liver. These metabolic changes might have contributed to the decrease in 3-methylcholanthrene induced incidence of mammary tumors observed in Tetracarpidium conophorum seeds lipid extract fed rats compared to MC untreated animals.
CONCLUSION
The result suggests that the cytochrome P450 reactive enzymes could be modulated by biochemical compounds present in the T. conophorum nut oil. These biochemical compounds contain anti-cancer properties that may be important in the prevention and treatment of breast cancer.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
ACKNOWLEDGEMENT
The authors sincerely thank the cancer unit of the Biochemistry Department, University of Medical Sciences, Ondo, Nigeria, for supplying the reagents and chemicals used during this research.
REFERENCES
|
Adebona MB, Ogunsuo AO, Ologunde MO (1988). Development of conophornut based cereal snack food I-biscuits. Journal of Food and Agriculture 2:123-136. |
|
|
Alugoju P, Nyshadham SN, Krisnawamy VK, Pavan K (2021). Theranostic precision medicine approach for female specific cancer. Phytotherapy for Breast Cancer, pp. 29-163. |
|
|
AOAC International (1996). AOAC Official Method 994.10: Cholesterol in Foods-Direct Saponification-Gas Chromatographic Method. Association of Official Analytical Chemists, Arlington, VA. |
|
|
Avwioro OG (2010). Histochemistry and tissue pathology, principle and techniques. Claverianum press, Nigeria. |
|
|
Ayeni EA, Nuhu A (2018). Tetracarpidium conophorum (African walnut) Hutch and Dalziel: Ethnomedicinal uses and its therapeutic activities. Journal of Medicinal Plants for Economic Development 2(1):1-10. |
|
|
Ayoola PB, Adeyeye A, Onawumi OO, Faboya OOP (2011). Phytochemicaland nutrient evaluation of Tetracarpidium conophorum (Nigerian Walnut) Root. International Journal of Research and Reviews in Applied Sciences 7(2):197-202. |
|
|
Batirel S, Yilmaz AM, Sahin A, Perakakis N, KartalOzer N, Mantzoros CS (2018). Antitumor and antimetastatic effects of walnut oil in esophageal adenocarcinoma cells. Clinical Nutrition 37(6):2166-2171. |
|
|
Burke MD, Thompson S, Elcombe CR, Halpert JT, Mayer T (1985). Ethoxy-,pentoxy and benzyloxyphenoxazones and homologues: a series of substances to distinguish between different induced cytochrome P450. Biochemical Pharmacology 34(18):3337-3345. |
|
|
Chijoke OC, Anosike CA, Ani CC (2015). Studies on the phytochemical and nutritional properties of Tetracarpidium conophorum (black walnut) seeds. Journal of Global Bioscience 4(2):1366-1372. |
|
|
Chijoke OC, Assumpta AC, Lawrence E, Sunday U (2017). Effect of black walnut (Tetracarpidium conophorum) leaf extract on the reproductive organ of male albino rats. International Journal of Homeopathy and Natural Medicine 3(2):9-14. |
|
|
Dada AA, Aguda OE (2015). Dietary effects of Tetracarpidium conophorum on the reproductive indices in male African catfish (Clariasgariepinus) brood stock. Journal of Aquatic Sciences 30(1):107-118. |
|
|
Edem CA, Dosunmu IM, Bassey FI (2009). Determination of proximate composition, ascorbic acid and heavy metal content of African walnut (Tetracarpidium conophorum). Pakistan Journal of Nutrition 8(3):225-226. |
|
|
Hardman WE (2014). Walnuts have potential for cancer prevention and treatment in mice. Journal of Nutrition 144(4):555S-560S. |
|
|
Ip C, Briggs SP, Haegele AD, Thompson HJ, Storkson23. J, Scimeca JA (1996). The efficacy of conjugated linoleic acid in mammary cancer prevention is independent of the level or type of fat in the diet. Carcinogenesis 17(5):1045-1050. |
|
|
Janick J, Paul RE (2008). The encyclopedia of fruits and nuts. Cab. International. England. Oxfordshire. |
|
|
Kanu AM, Kalu JE, Okorie AC, Olabinri AB, Eniyansoro OO, Okoronkwo CO (2015). Evaluation of chelating ability of aqueous extract of Tetracarpidium Conophorum (African Walnut) in vitro. International Journal of Applied Research in Natural Products 3(3):13-18. |
|
|
Lee SJ (2013). Clinical application of CYP2C19 pharmacogenetics toward more personalized medicine Frontiers in Genetics 3:318. |
|
|
Negi AS, Luqma S, Srivastava V, Krishna N, Gupta MPD (2011). Antiproliferative and antioxidant activities of African Walnut (JuglanreginaL.) fruit extracts. Pharmaceutical Biology 49(6):669-705. |
|
|
Nwachoko N, Jack IR (2015). Phytochemical screening and anti-diarrhea activities of Tetracarpidium conophorum induced in albino rats. Sky Journal of Biochemistry Research 4(4):21-24. |
|
|
Nwaichi EO, Osuoha JO, Monanu MO (2017). Nutraceutical potential of Tetracarpidium conophorum and Buccholziacoriacea in diet-induced hyperlipidemia. Journal of Chemical Health Risks 7(3):157-170. |
|
|
Nwauzoma AB, Dappa MS (2013). Ethnobotanicals studies of Port Harcourt metropolis, Nigeria. International Scholarly Research Notices. |
|
|
Ogunyinka BI, Oyinloye BE, Adenowo AF, Kappo AP (2015). Potentials of some plant-derived foods in the management of diabetes and associated complications. African Journal of Traditional, Complementary and Alternative Medicines 12(6):12-20. |
|
|
Oke OL (1995). Leaf protein research in Nigeria, Ibadan University of Ibadan Press, Ibadan, Nigeria. |
|
|
Rani R, Kansa VK (2012). Study on cow ghee versus soybean oil on 7, 12-dimethylbenz (a)-anthracene induced mammary carcinogenesis & expression of cyclooxygenase-2 & peroxisome proliferators activated receptor- γ in rats. The Indian Journal of Medical Research 135(5):497-503. |
|
|
Sankeshwari RM, Ankola AV, Bhat K, Hullatti K (2018). Soxhlet versus cold maceration: Which method gives better antimicrobial activity to licorice extract against Streptococcus mutans? Journal of the Scientific Society 45(2):67-71. |
|
|
Sparreboom A, Cox MC, Acharya MR, Figg WD (2004). Herbal remedies in the United States: Potential adverse reactions with anticancer drugs. Journal of Clinical Oncology 22(12):2489-2503. |
|
|
Talaska G, Warshawsky D, Heffelfinger S, Gear R, Schnieder J, Schumann B (2006). Dietary Fat composition and intake affects DMBA metabolism and DNA adduct formation in breast organoids. 3rd Annual BCERC Early Environmental Exposures Conference, Berkeley, CA, November 2-3. |
|
|
Teel RW, Huynh H (1998). Modulation by phytochemicals of 12. Cytochrome P450- linked enzyme activity. Cancer Letters 133(2):135-41. |
|
|
Uhunmwangho ES, Omoregie ES (2017a). Changes in lipid Profile and Fatty Acid Composition during the Development of African Walnut (Tetracarpidium conophorum) Seeds. World Applied Sciences Journal 35(7):1174-1179. |
|
|
Uhunmwangho ES, Omoregie ES (2017b). Evaluation of nutritive, anti-nutritive and mineral content of Tetracarpidium conophorum (African Walnut) seed oil at different stages of fruit maturation. Haya: The Saudi Journal of Life Sciences 2(6):210-216. |
|
|
Uhunmwangho ES, Olafusi C, Akinyemi I (2022a). Anticancer potentials of Tetracarpidium conophorum (African walnut) Seed Oil on Prostate Carcinogenesis. Scientific Research and Essays 17(1):1-7. |
|
|
Uhunmwangho ES, Oyiborhoro O, Osungbemiro BW (2022b). African walnut Seed Oil Enriched Diet Reduces Prostate Cancer Incidence in Wistar Rats. European Journal of Pharmaceutical and Medical Research 9(5):431-437. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0