ABSTRACT
DNA-based methodology employing quantitative polymerase chain reaction (qPCR) has been successfully used to examine the incidence of genetically modified (GM) maize in Mali. This study aims to ascertain whether screening elements could also be used to detect GM maize. Fourteen maize varieties and one unknown dark color seeded variety from Mali were tested. DNA was extracted from three seeds of each variety. Three screening elements were used for qPCR amplification, the 35s promoter of the Cauliflower mosaic virus (CaMV), the nopaline synthase (NOS terminator) from Agrobacterium tumefaciens and the 35s promoter from the Figwort mosaic virus (FMV). The 14 varieties were negative for P35s CaMV (forward) and T-NOS (reverse) markers. In contrast, the unknown dark color seeded variety was positive with 94 bp PCR product. While, no DNA fragments were amplified using the FMV as the screening element. These data were supported by Ct values in which the 14 varieties had values above 50; whereas, the unknown variety showed values of 24.5 for P-35s-CaMV and 30 for the T-NOS. The study demonstrates the ability in detecting GM maize using screening elements and the usefulness of our laboratory in training and reinforcing regional concern about GMO circulation.
Key words: Genetically modified organism (GMO) detection, quantitative polymerase chain reaction (qPCR), capacity building, maize, Mali.
The human population in Sub-Saharan Africa is increasing at the rate of 2% per year (https://data.worldbank.org/region/sub-saharan-africa) and estimated at 1,061 billion in 2017 (https://data.worldbank.org/region/sub-saharan-africa). This exponential population growth in addition to erratic rainfall, climate variability (drought and flood) and agricultural pests has contributed to food shortages. This food insecurity may lead to population migration and further poverty in the Sahel region.
Adoption of genetically modified crops with improved grain yield and drought resistance is a mean to alleviate food shortage. Genetically modified organism (GMO) is defined as an organism in which the genetic material has been altered in a way that does not occur by mating and/or natural recombination (Plan and Van den Eede, 2010). Presently, genetically modified (GM) crops are a main agricultural product worldwide with GM crops having a global value of UDS$15.8 billion in 2016 (Briefs, 2017). In order to preserve the biodiversity, several countries have adopted the Cartagena protocol on biosafety (a legally binding global framework), that ensures the safe transport, handling and use of living modified organisms (LMO) created through gene engineering. The Cartagena protocol assists member country authorities in building the capacity to transfer technology and knowledge to prevent illegal shipment and accidental releases of GM products across member country boundaries. The Malian government has ratified the Cartagena protocol and has taken a set of regulations for importation, production, distribution and use of genetically living organisms (Law N0 08-042, December 2008). In addition, the Regional Biosafety Program of the Economic Community of West African States (ECOWAS) and the West African Economic Monetary Union (WAEMU) was implemented and has provided to each country member a platform to identify GMO crops and food product within its territorial region.
GM crops can be detected using several techniques (Cottenet et al., 2019; Dobnik et al., 2018; Fraiture et al., 2018). DNA-based approaches are more popular in detecting and quantifying GM crops than protein-based methods (Lipton et al., 2000), and real-time quantitative polymerase chain reaction (qPCR) is the standard in GMO analytics. The objective of the present study was to evaluate 15 maize varieties using PCR based strategies to detect GM varieties in germplasm from Mali.
Maize varieties
Fourteen maize varieties introduced in Mali between 1995 and 2014 and maintained at the Institut d’Ecomonie Rural (IER), the major national agriculture research institute in Mali, were selected based on availability of maize seeds from commercial fields in Mali. These are known to be non-GMO varieties (Table 1). Also, one unknown variety with dark colored seeds found in Bamako was investigated. Soybean specimen known as GMO was used as positive control and included in the test.
DNA extraction
DNA was extracted from seed samples using the DNA extraction kit from Biotecon Diagnostics (Potsdam, Germany). Briefly, three seeds were grounded with a mortar and pestle and 200 mg of homogenized sample was transferred to a centrifuge tube followed by the addition of 2 ml of extraction buffer. Samples were vortexed (Velp Scientifica, Europe) and incubated at room temperature for 30 min. After centrifugation at 12,000 x g for 10 min (Mikro 220R centrifuge Hettich, Tuttlingen, Germany), the supernatant was transferred to a 2 ml microcentrifuge tube containing 400 µl of fixative buffer and mixed by pipetting. Next, 80 µl of proteinase K (20 mg/ml, Bio-Rad, CA, USA) was added to the mixture and samples were incubated at 72°C for 10 min in a water bath (Fisher Scientific, Polystat 36, 5L/8662H). In order to precipitate the DNA, 200 µl of isopropanol was added and mixed by pipetting prior to transferring to a column with filter. The column was centrifuged at 5,000 x g for 1 min, transferred into a new eppendorf tube and
centrifuged at 5,000 x g for 1 min. The column was washed 3 times using 450 µl of washing solution at 5,000 x g for 1 minute. To remove residual washing buffer solution, the column was centrifuged for 10 s at 13,000 x g. Lastly, 200 µl of a warm elution buffer (70°C) was added to the column (placed in the sterile tube), incubated at 25°C for 5 min, and centrifugation at 5,000 x g for 5 min. Purified DNA was stored at -20°C prior to the PCR amplification.
Markers used for amplification
Three screening elements (Table 2) from the Foodproof®GMO screening 1 Lyokit (Biotecon Diagnostics, Potsdam, Germany) were used for qPCR amplification which were the 35s promoter of the Cauliflower mosaic virus (CaMV), the nopaline synthase (NOS terminator) from Agrobacterium tumefaciens and the 35s promoter from the Figwort mosaic virus (FMV). In addition, event markers such as bar, 35S-Pat, CTP2 were used for PCR amplification. The plant universal marker provided with the Kit Biotecon was used to amplify plant DNA.
Quantitative PCR (qPCR) to estimate the cycle threshold (Ct) value
The Foodproof®GMO screening 1 Lyokit was used to perform the qPCR. The DNA samples were diluted to 25 ng/µl and a 25 µl sample was added to an individual well containing the lyophilized PCR reagents. Negative (25 µl of sterile H2O) and positive controls (25 µl of Foodproof®GMO screening 1 control template) were included. Two steps qPCR were performed using StepOne Real Time PCR system (Applied Biosystems, Foster City CA, USA) with initial incubation for 1 cycle at 37°C for 4 min and denaturation process for 95°C for 10 min, followed by amplification step consisting of 50 cycles, a denaturation at 95°C for 15 s followed by annealing at 60°C for 60 s.
Gel electrophoresis
After amplification, 12 µl of each qPCR product was electrophoresed on a 2% agarose gel using 0.5X TBE running buffered (Euromedex, France). DNA fragments were stained with 0.3 mg/ml of ethidium bromide (Sigma, St-Louis, Mo, USA). Fragments were electrophoresed at 120 volts for 2 h and then photographed by UV transillumination with a KODAK EDAS 290 camera (Kodak, Rochester, NY, USA). The molecular weight of the products was estimated with DNA molecular weight marker 100 bps DNA ladder (Quick load, New England Biolabs, Ipswich, MA).
Ct estimations
The cycle threshold (Ct), the fractional cycle number at which the well’s accumulating fluorescence crosses a set threshold that is several standard deviations above base fluorescence, was determined. Any amplification curve below the threshold line between the first and the fifth cycles was considered as negative for a specific screening element marker.
The 14 maize varieties were negative for P35s CaMV and T-NOs markers (Table 4). In contrast, the unknown dark color seeded maize was positive with 94 bp PCR product for P35s CaMV (forward) and T-NOs (reverse) markers. In addition, the use of event markers did not produce PCR fragments (Table 3).
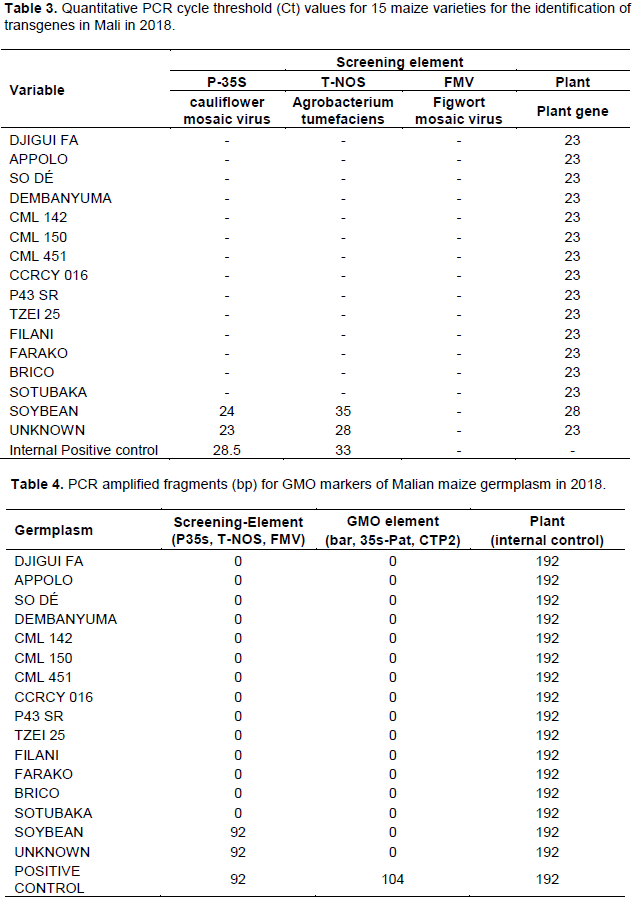
The presence of PCR fragment was consistent with the Ct values obtained during qPCR. The Ct values for the 14 varieties were above 50 (Table 3). In contrast, Ct values for the unknown variety were 23 for P-35s-CaMV and 28 for the T-NOS. This confirms the dark seeded maize variety was genetically modified maize with the genome containing the 35s promoter of the Cauliflower mosaic virus (CaMV) and the nopaline synthase (NOS terminator) from Agrobacterium tumefaciens. However, the 35s promoter sequence from the Figwort mosaic virus (FMV) was not amplified for the 15 varieties. In South Africa, 10% of varieties contained genes for insect resistance and 15% were associated with herbicide tolerant events (Iversen et al., 2014). These data along with the identification of a transgenic dark seeded maize variety from Mali would suggest additional screening of maize germplasm from Mali should be conducted.
The study demonstrates the ability in detecting the GM maize using screening elements and the usefulness of our laboratory in training and reinforcing regional concern about GMO circulation. The presence of molecular platform (qPCR and Sanger sequencing techniques) and immunological technique such as Elisa within in our laboratory constitutes a valuable asset. The next step will include reference material for GMO detection and quantification in food. Taken together, the country will be in a better position to screen all entering maize seeds and to fulfill the regulatory requirements such as the Cartagena Protocol.
The authors have not declared any conflict of interests.
The authors thank the Focal Point of the WAEMU Regional Biosafety program (Saïdou Kina and Zourata Ouedrago Lompo)through funding from GEF project (ID 2911), the European Commission Joint Research Center (Towards Global Harmonisation of GMO analysis by Creating and Supporting Regional Networks of Excellence” Project) through Dr Maddalena Quercy, the Convention for Biological Diversity (CBD) for support to IT and HD, the French IRD JEAI CoANA Dr. Valerie Verdier, Dr. Christiane Kouatchoua and also thank Dr Doumbia Lassina and Dr Diarra Youssouf for the review of the manuscript.
REFERENCES
|
Briefs ISAAA (2017). Global status of commercialized biotech/GM crops in 2017: biotech crop adoption surges as economic benefits accumulation in 22 years.
|
|
|
|
Cottenet G, Blancpain C, Sonnard V, Chuah PF (2019). Two FAST multiplex real-time PCR reactions to assess the presence of genetically modified organisms in food. Food Chemistry 274:760-765.
Crossref
|
|
|
|
Dobnik D, Demšar T, Huber I, Gerdes L, Broeders S, Roosens N, Žel J (2018). Inter-laboratory analysis of selected genetically modified plant reference materials with digital PCR. Analytical and Bioanalytical Chemistry 410(1):211-221.
Crossref
|
|
|
|
Fraiture MA, Saltykova A, Hoffman S, Winand R, Deforce D, Vanneste K, Roosens NH (2018). Nanopore sequencing technology: a new route for the fast detection of unauthorized GMO. Scientific Reports 8(1):7903.
Crossref
|
|
|
|
Iversen M, Grønsberg IM, van den Berg J, Fischer K, Aheto DW, Bøhn T (2014). Detection of transgenes in local maize varieties of small-scale farmers in Eastern Cape, South Africa. PloS One 9(12):e116147.
Crossref
|
|
|
|
Lipton CR, Dautlick JX, Grothaus GD, Hunst PL, Magin KM, Mihaliak CA, Stave JW (2000). Guidelines for the validation and use of immunoassays for determination of introduced proteins in biotechnology enhanced crops and derived food ingredients. Food and Agricultural Immunology 12(2):153-164.
Crossref
|
|
|
|
Plan D, Van den Eede G (2010). The EU legislation on GMOs. JRC Scientific and Technical Reports, EUR, 24279.
|