The plant Siparuna guianensis is used in traditional medicine and has been the target of studies on the development of new drugs for the control of pests and vector insects. The present study was aimed to evaluate the seasonal influence on the content and composition of the essential oil of S. guianensis. The experiment was conducted for 12 months evaluating the yield of the essential oil of leaves throughout the seasons (autumn, winter, spring and summer). The chemical composition of the essential oil was obtained by gas chromatography-mass spectrometry (GC-MS). The highest oil yield was in autumn and winter, comprising 0.33 and 0.29% (w/w), respectively. The major compound identified was β-myrcene (48.59-24.2%), followed by epicurzerenone (27.24-13.7%) being the most abundant; germacrene D showed lower values of 9.93% in autumn and 13.5-14.34% in the other seasons, besides curzerene that had no production in autumn. The É£-elemene component had a higher production of 7.29% in autumn. Compound 2-undecanone did not show significant seasonal changes, with percentages being between 7.26 and 5.43%. The monoterpenes and sesquiterpenes showed similar levels in autumn; however, in the other seasons (winter, spring, and summer), the sesquiterpenes presented higher concentration, reaching 68.54% in summer. The components identified in the essential oil of S. guianensis exhibit interesting biological activities, making this essential oil a promising compound for the development of new biodegradable drugs, repellents, and insecticides. The knowledge about the yield and seasonal composition is fundamental to optimize and maximize the obtainment of compounds of interest for the production of new drugs.
Medicinal plants have been used since ancient times owing to their biological activities and aromatic properties. Among the products obtained, the essential oils are remarkable and have attracted the attention of researchers. Essential oils are complex mixtures of volatile and lipophilic substances produced by the secondary metabolism of plants, obtained primarily by distillation, where leaves are normally used, although different parts of the plant can also be used in this process. These compounds are primarily used in perfumes, cosmetics, food and pharmaceutical industries (Teixeira et al., 2013; Okereke et al., 2017).
Siparuna guianensis Aublet is a neotropical species, and in Brazil, it is primarily found in the Cerrado biome, where it is both native and abundant and primarily known by the popular name of “negramina.” The use of this plant was identified in Central and South America in the traditional medicine through remedy baths, and juice and tea were also prepared using its leaves to combat fevers, rheumatic diseases, cramps, and high blood pressures. It was also used as an antibiotic, aromatic, diuretic, and stimulant (Approbato and Godoy, 2006; Furtado, 2006; Souza and Felfile, 2006; Valentini et al., 2010a; Pereira and Silva, 2017).
Other ethnobotanical studies have revealed the traditional use of this plant for combating sinuses, headaches, migraines, and body aches, acting as an analgesic and anti-inflammatory agent (Valentini et al., 2010a; Andrade et al., 2015). It has also been reported to be used for fluctuation of consciousness between hypoactive and hyperactive states, neck pains, muscle strains, fears, vomiting, and seizures (Pagania et al., 2017). The essential oil of this plant has also been studied for the biological control of pests, wherein it has been recently used for the control of Aedes aegypti Linnaeus, 1762 (Diptera: Culicidae) and Culex quinquefasciatus Say, 1823 (Diptera: Culicidae) with interesting results (Aguiar et al., 2015).
The plant has also been evaluated for its activity against pathological bacteria such as Escherichia coli, Listeria monocytogenes, Pseudomonas aeruginosa, Salmonella choleraesuis and Staphylococcus aureus; against the filamentous fungi such as Aspergillus flavus, Aspergillus niger, Aspergillus carbonarius, and Penicillium commune; and against the epimastigotes of Trypanosoma cruzi (Andrade et al., 2015). It has also been evaluated for its activity against the promastigotes of Leishmania amazonensis (Andrade et al., 2016) and as a repellent (Aguiar et al., 2015).
Several promising results can be observed in the literature confirming the biological activity of S. guianensis for various applications. However, less information is available on the variation of the chemical composition of the oil of this plant in relation to the period of harvest of the plant. Therefore, the objective of this study was to evaluate the influence of seasonality on the content and yield of the essential oil of S. guianensis, with the intention of subsidizing the production of essential oil for commercial purposes.
Study area
Leaves were collected from S. guianensis in Gurupi-TO, Brazil (11°43ʹ45ʺ S, 49°04ʹ07ʺ W), for the extraction of the essential oil. Taxonomic identification was confirmed by experts at the herbarium of the Federal University of Tocantins (Campus-Porto Nacional), where the samples were deposited with reference number 10.496. The research was registered and approved by Conselho Nacional de Desenvolvimento Científico e Tecnológico, CNPq, no. 010580/2013-1.
Obtaining the essential oil
The leaves of S. guianensis were removed from the plant between 7:00 and 8:00 AM. They were then crushed and submitted to hydrodistillation extraction in a Clevenger apparatus (Aguiar et al., 2015). A mixture of 300 g of fresh crushed leaves and 1000 mL of distilled water was heated in a 2-L flask, and the vapors generated were directed to a condensation column coupled to a cooling system. At the end of the distillation process, an aromatic watery phase and another less dense organic phase containing the wet essential oil were obtained, and the organic phase was dried using sodium sulfate. The yield was calculated in relation to the weight of the dried essential oil with the weight of the leaves in natura (Silva et al., 2016). The collection period of S. guianensis included the four seasons of the year, with six extractions in each season.
Gas chromatography and mass spectrophometry (GC-MS)
The chemical composition of the essential oil was assessed in triplicate and identified by gas chromatography (GC-FID) using a Chemito 8510 GC instrument (Chemito Technologies Ltd, Mumbai, India Pvt.). The separation of the constituents of the sample was performed by a large caliber capillary column BP-5 (30 × 0.53mm i.d., 1.0 mm film thickness). Then, 0.03 mL of the essential oil was injected through a Hamilton syringe into the GC with a cover of 1.0 mL. Hydrogen was used as the drag gas at a flow rate of 5 mL/min and a pressure of 20 psi. The temperature of the GC oven was programmed at 70 to 210°C using a heating ramp at a rate of 2.5°C/min, and the injector and detector temperatures (FID) were maintained at 230°C. The GC-MS analysis with a DSQ MS screen was performed on a Thermo Electron Corporation equipment, Waltham, MA, USA, using a BP-5 (30 × 0.25 × 0.25 mm) capillary column. Helium was used as the drag gas at a flow rate of 1 mL/min and 1:20 split. The temperature of the column was programmed to range from 65 to 210°C using a heating ramp at a rate of 3°C/min. Mass spectra were obtained in the range of 40 to 650 amu, operating at 70 V, and the source was maintained at 200°C (Aguiar et al., 2015).
Identification of compounds
The components present in the essential oil were identified through the computerized comparison of the mass spectrum obtained with those contained in the NIST library database of the mass spectrum of GC-MS, according to the methodology adopted by Moronkola et al. (2017).
Statistical analyses
Analysis of variance was performed by the Tukey’s test (P<0.05). Statistical analyses were performed using SISVAR 4.6 (Ferreira, 2001), and the graphs were produced using SIGMA PLOT 11.0 (Systat Software, Inc. San Jose, USA).
Phytochemical studies have shown that essential oils can be obtained from dried and fresh leaves, as well as from stems and fruits; however, the leaves comprise the part of the plant with the highest yield in extraction according to the literature on S. guianensis (Aguiar et al., 2015); therefore, the leaves were chosen as the object of this study. The extraction of the essential oil from the plant was performed by the hydrodistillation process using the Clevenger apparatus. The leaves were collected during the first hours of the day to avoid loss of volatile material and were immediately sent to the laboratory for extraction.
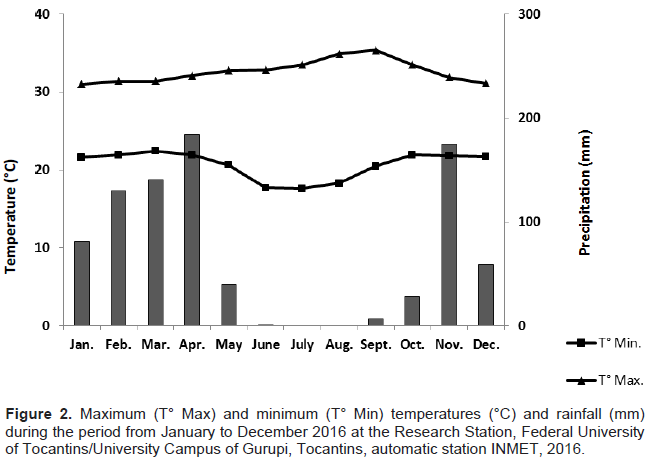
The percentage values found in this study were calculated from the weight of the oil obtained by the weight of the leaves in natura (w/w). The seasons of autumn (April-June) and winter (July-September) are characterized by a drought period in the Cerrado biome with greater temperature variation between the day and night period, whereas the spring and summer seasons present higher values of precipitation and smaller temperature variations along the day, as observed in Figure 2.
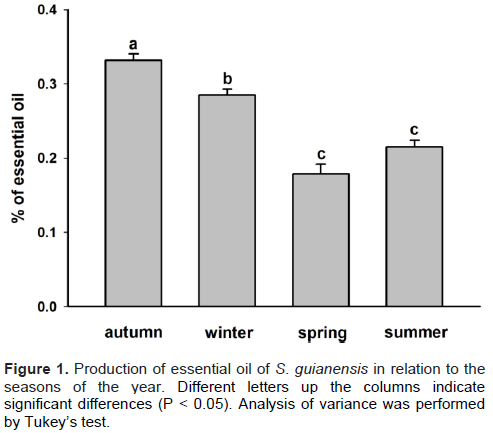
The extraction yields in autumn and winter were 0.33 and 0.29% (w/w), respectively. In the spring and summer seasons, the amount of oil obtained from the leaves of S. guianensis was lower, with 0.18 and 0.22% (w/w), respectively (Figure 1). These results are consistent with the data found in the literature on the yield of oil extraction from S. guianensis leaves, with values ranging from 0.10 to 0.61% (w/w), in terms of quantity and seasonal variation, as shown in Figure 1 (Castellani et al. 2006; Valentini et al., 2010b). Castellani et al. (2006) also obtained higher levels of essential oil in autumn and lower in the spring for leaves of S. guianensis.
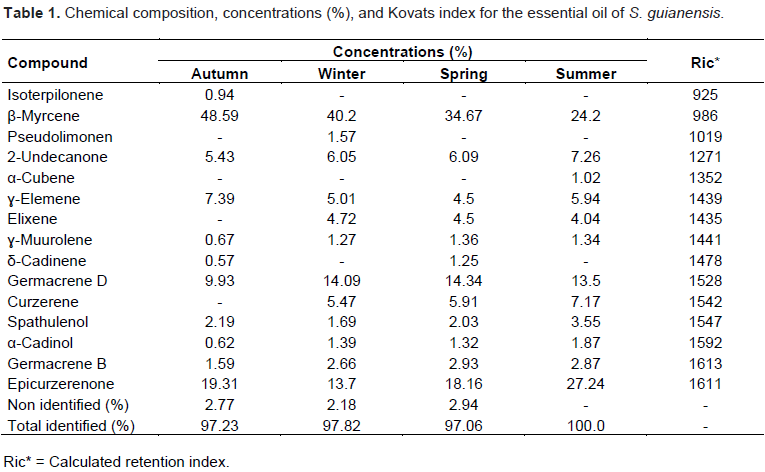
Analyses of the chemical composition were carried out using samples collected during the four seasons. Figure 3 presents the chromatograms referring to the composition using GC/MS, which enabled the determination of the chemical constituents present in the essential oil of S. guianensis. Table 1 shows the composition values of the essential oil of S. guianensis in the four seasons of the year, with 97.23% of the sample collected in autumn, 97.82% in winter, 97.06% in spring, and 100.00% in summer. The data obtained from the qualitative and quantitative determinations are presented in Table 1.
Autumn was the period with the highest yield of the essential oil, a period characterized by the end of the rains and the beginning of the dry season. Water stress during this period favors the production of some metabolites, such as the terpenoids that are the primary components of the essential oils (Castellani et al., 2006; Yang et al., 2014). Such metabolites are contained in secretory cells, epidermal cells, cavities, channels, or glandular trichomes (Yang et al., 2014).
Liu et al. (2011) clarified that the greatest accumulation of metabolites is attributed to the closure of plant stomata in the prolonged drought period. The effect of water stress can be observed in other studies, such as that of Jaleel (2008), where Catharanthus roseus L. produced more ajmalicine under stress conditions. Valentini et al. (2010b) studied S. guianensis in Minas Gerais, Brazil and found that in the dry season, the production of the essential oil reached 0.61%, whereas in the rainy season, it was about 0.10% (w/w).
The oil obtained in the autumn season had the following major compounds: β-myrcene (48.59%), epicurzerenone (19.31%), germacrene D (9.93%), É£-elemene (7.39%), and 2-undecanone (5.43%). β-Myrcene was the component that mostly contributed to the increase in oil production. In the other seasons, it was observed that the concentration of oil obtained was directly proportional to the concentration of this component (Table 1), evidencing that the period of water stress favors its production.
The availability of water changes the production of metabolites, and stress positively stimulates the production of some metabolites such as cyanogenic glycosides, glucosinolates, anthocyanins, alkaloids, and some terpenoids (Liu et al., 2011). Samples collected during winter showed the following chemical composition: β-myrcene (40.20%), germacrene D (14.09%), epicurzerenone (13.70%), 2-undecanone (6.05%), curzerene (5.47%), and É£-elemene (5.01%), as described in Table 1. This season is also characterized by drought, promoting water stress.
Samples collected during the seasons of spring and summer showed inferior yields compared to those collected during the other two seasons (Figure 1), periods characterized by high precipitation, as shown in Figure 2. Spring was the season with a lower yield, a period characterized by fruiting and sprouting of the plant. This result was consistent with the results for other plants (Ganzera et al., 2008). Valentini et al. (2010a) concluded that the amount of oil of S. guianensis remains constant throughout the year, and during the fruiting period, the oil content reduces in the leaves and branches; however, it is compensated by the essential oil present in the fruits.
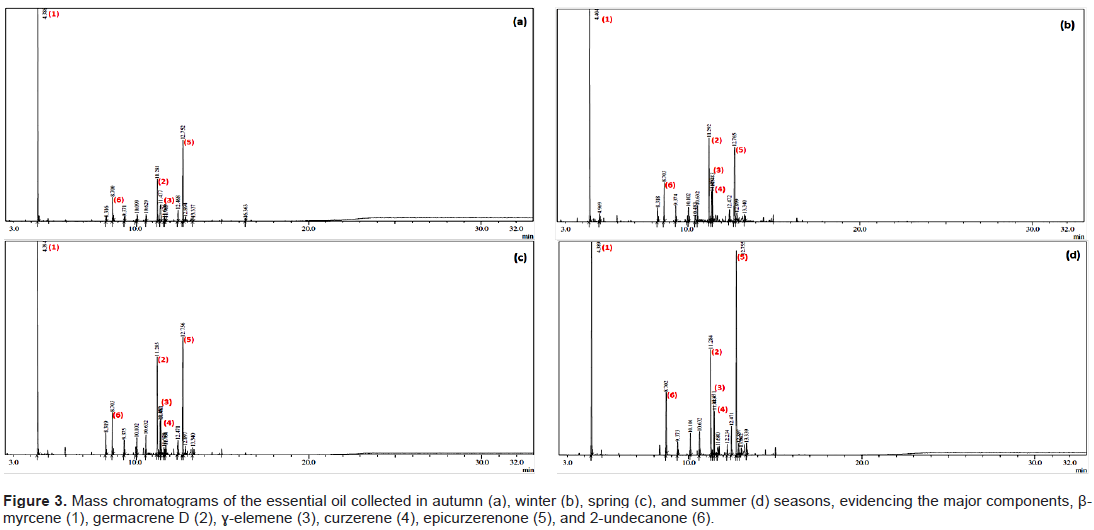
Samples collected during the spring season showed the following major composition: β-myrcene (34.67%), germacrene D (14.34%), epicurzerenone (18.16%), 2-undecanone (6.09%), curzerene (5.91%), and É£-elemene (5.05%), as shown in Table 1. Samples collected during summer showed the same major compounds, that is, β-myrcene (24.20%), germacrene D (13.50%), epicurzerenone (27.24%), 2-undecanone (7.26%), curzerene (7.17%), and É£-elemene (5.94%), as shown in Table 1. Although the spring and summer seasons had higher radiation rates, which is one of the factors that increase the production of essential oils, it is noted that water stress plays a major role in the production of essential oils for this species.
The fact that these plants are shrubs and groves that develop in the interior of the forest in an environment with low radiation is less relevant, differing from other medicinal plants that present higher production of essential oils in periods with higher radiation (Liu et al., 2011). The production of essential oils in the spring season did not show significant changes compared to the production in the summer season; however, it was observed that the content of β-myrcene in the summer (24.20%) was inferior when compared to that in the spring (34.67%), as shown in Table 1. The difference in the concentration of β-myrcene is compensated by the content of epicurzerenone that had yields of 27.24 and 18.16% in the summer and spring seasons, respectively (Table 1).
The concentration of epicurzerenone in the summer (27.24%) is superior when compared to that in the other seasons, with 19.31% in autumn and 13.70% in winter, as depicted in Table 1. These data show that the relevant factor for the production of this secondary metabolite is not water stress but solar radiation. Therefore, a higher composition was found in the summer, even after taking into account the different quantities extracted throughout the year. Liu et al. (2011) concluded that the amount of available radiation and the wavelength type are the factors that alter the composition of the essential oil; the incidence of UV radiation is a factor that contributes to the increased production of certain secondary metabolites.
The composition of the metabolite 2-undecanone presented lower yield in the autumn season (5.43%) and this is due to the amount of oil extracted, which was higher than that in the other seasons. The concentration in the other seasons ranged from 6.05 and 7.26%, as shown in Table 1. In autumn, germacrene D production (9.93%) was lower than that in the other seasons (13.50-14.34%), as shown in Table 1, a period characterized by the appearance of flower buds, evidencing that flowering reduces the production of this metabolite or that this metabolite migrates to the flowers.
For the metabolite curzerene, as similar phenomenon was observed but with greater intensity, this metabolite was not identified in the composition analysis done in autumn. However, the metabolite ɣ-elemene (7.39%) presented a higher composition in autumn, indicating that the flowering period stimulates its production and storage in the leaves, consequently increasing the composition of this metabolite in the essential oil when compared to the composition observed during the other seasons (4.50-5.9%), as depicted in Table 1. Flowering and fruiting periods can also quantitatively and qualitatively alter the extracted oils (Ganzera et al., 2008).
Variations in the production of secondary metabolites, especially in medicinal plants, have been studied for their relevance. The genetic factor is preponderant regarding the chemical composition. However, in the same plant species, the essential oil can vary quantitatively and qualitatively depending on the climate, soil composition, organ from which it was extracted, plant age, vegetative cycle stage, and the technique used for extraction (Simões et al., 2010). These conditions promote changes in the composition and, consequently, in the activity of
the final product.
The essential oils extracted from the leaves of S. guianensis in three Brazilian cities, Belém common Mojú (both in the state of Pará) and Rio Branco, in the state of Acre, have been investigated. Analysis of the chemical composition revealed that in the first municipality, the primary constituents were germaerona (23.2%), germacrene B (8.0%), and atractilona (31.4%); whereas in Mojú, epi-α-bisabolol (25.1%) and espatulenol (15.7%) were identified. The samples from Rio Branco presented the following composition: Espatulenol (22.0%), selin-11-en-4-α-ol (19.4%), β-eudesmol (10.0%), and elemol (10.0%) (Zoghbi et al., 1998). Studies performed in Gurupi-Tocantins, Brazil, found another composition; β-myrcene (79.71%) and 2-undecanone (14.58%) (Aguiar et al., 2015).
The primary constituent, comprising around 90%, of most essential oils are monoterpenes (C10), followed by sesquiterpenes (C15). Several monoterpenes are linked to pollination and sesquiterpenes are most often related to the protection against fungi and bacteria (Simões et al., 2010; Silvia et al., 2011). The essential oils have specific functions in development and cellular respiration and have an important role in primary metabolism. A majority of terpenoids have the function of mediating the relationships between the environment and the plant.
Its production follows two pathways, the cytosolic pathway or the acetate-mevalonate pathway and the plastid pathway or the methylerythritol phosphate pathway, both of which produce isopentenyl diphosphate (IPP) and its isomer dimethylallyl diphosphate (DMAPP).
Monoterpenes such as menthol, geraniol, linalos, and citral are relevant in the perfumes and flavoring industries. Sesquiterpenes such as farnesol, zingiberane, and cariofileno that act in the defense can be used as chemotherapeutic agents and in the treatment of glaucoma (Coteau et al., 2000; Yang et al., 2014).
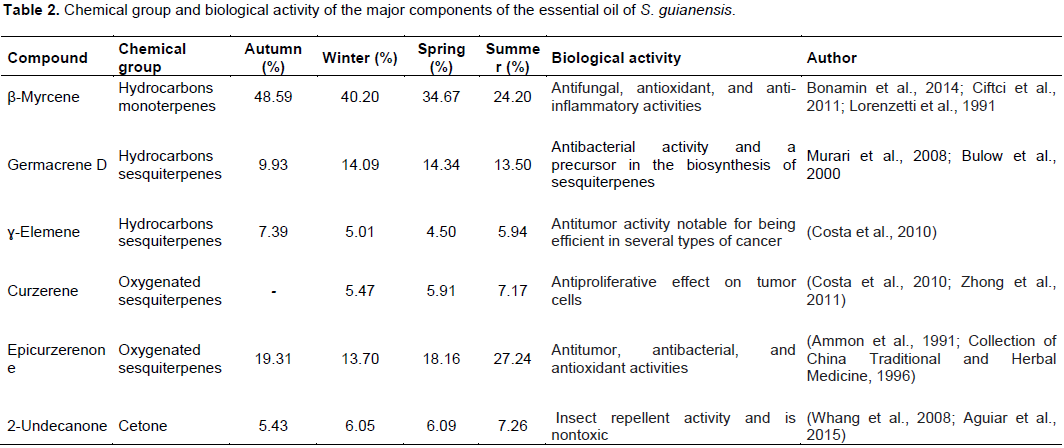
The chemical structures of the major components of S. guianensis essential oil are presented in Figure 4. The biological activities found in the literature for these components are described in Table 2, including the chemical group to which they belong.
β-Myrcene is a hydrocarbon monoterpene that has antifungal, antioxidant, and anti-inflammatory activities (Lorenzetti et al., 1991; Ciftci et al., 2011; Bonamin et al., 2014). Germacrene D is a hydrocarbon sesquiterpene that has antibacterial activity (Murari et al., 2008) and is studied as a precursor in the biosynthesis of sesquiterpenes (Bulow et al., 2000). The metabolite É£-elemene is a sesquiterpene hydrocarbon that has antitumor activity notable for being efficient against several types of cancer (Costa et al., 2010). It has been reported that curzerene (an oxygenated sesquiterpene) has an antiproliferative effect on tumor cells (Costa et al., 2010; Zhong et al., 2011).
Epicurzerenone, another oxygenated sesquiterpene, is a major compound in Curcuma sichuanensis (Zhou et al., 2007), and it has been used in traditional Chinese medicine owing to its antitumor, antibacterial, and antioxidant activities (Ammon et al., 1991; Collection of China Traditional and Herbal Medicine, 1996). 2-Undecanone, a methyl nonyl ketone, is a naturally occurring nontoxic compound with insect repellent activity (Whang et al., 2008; Aguiar et al., 2015).