ABSTRACT
In Africa, cultivation of potato (Solanum tuberosum) represents an important food source and income generation. However, its productivity is constrained by biotic and abiotic stresses. Bacterial wilt caused by Ralstonia solanacearum is an important constraint to the world potato industry. In Kenya, the disease affects 77% of potato farms causing yield losses of up to 100%. Control methods are limited mainly due to the broad diversity and wide spread of its pathogen. Understanding the population structure and geographical distribution of this pathogen is an important starting point in the development of effective control strategies. In this study, R. solanacearum strains affecting potato cultivation in Nakuru county were successfully isolated and characterized. Kelman’s triphenyl tetrazolium chloride media were used to isolate the pathogen and 20 isolates were selected based on their virulence for further characterization and confirmation of their status at the molecular level through polymerase chain reaction using 759/760 primers and sequencing of partial endoglucanase (egl) gene. The phylogenetic assay was done using specific primers and it was found that the phylogenetic diversity was highly heterogeneous, since all the four phylotypes of R. solanacearum were identified. Phylotype I was the most prevalent phylotype and represented 50% of the collection. Based on their ability to utilize sugars and alcohols, all the isolates were grouped as biovar III except 2 (Rs18 and Rs49). The aggressiveness of isolated bacteria was then evaluated using a hypersensitive reaction test on tobacco and their virulence was further confirmed on a susceptible potato variety Shangi under greenhouse conditions. All isolates elicited a reaction in tobacco with different grades. They also showed varying levels of virulence with Rs6 isolate being the most virulent. Taken together, these findings provide baseline information for improvement programs targeting host-based resistance to multiple strains causing bacterial wilt of potato in this region.
Keywords: Bacterial wilt, Biovar, hypersensitive reaction, pathogenicity, phylotype, potato, Ralstonia solanacearum.
Potato (
Solanum tuberosum), a starchy edible tuber belonging to the Solanaceae family is one of the most important food crops in the world (FAO, 2010). The nutritional value of this crop is attributed to its ability to supply high quality proteins, essential amino acids, vitamins, minerals and trace elements to the human diet (Abong et al., 2009; Ahuja et al., 2013). The plant is ranked the fourth staple food crop after rice, wheat and corn with its average global production estimated at 380 million tonnes under production on 20 million hectares of land in 2016 (FAOSTAT, 2018). In Kenya, potato is the second most cultivated crop after maize with an annual production of about 3 million tonnes which is valued at USD 500 million (GIZ-PSDA, 2011; MoALF, 2016). This represents 8 to 15 t/ha in terms of average production relative to cultivated area which is low compared to the expected 30 to 40 t/ha under normal field conditions (Muthoni et al., 2013; Gitari et al., 2018). The low yield is mainly attributed to various challenges ranging from biotic to abiotic stresses. This includes lack of nutrients, pests and high incidences of diseases particularly, bacterial wilt disease caused by
Ralstonia solanacearum which is still the most devastating disease of potato and the Solanaceae family as whole (Kaguongo et al., 2010).
The disease has been found to occur in all the potato growing areas of the country affecting 77% of potato farms and causing yield losses of 50 to 100% (Kaguongo et al., 2010; Muthoni et al., 2012).
Several management practices have been proposed and implemented to control the disease and these include phytosanitary, cultural and chemical methods as well as breeding for resistance. However, these strategies have not been 100% effective in controlling the disease, although in locations where the pathogen is established, a combination of diverse methods have shown some promising results (Champoiseau et al., 2009). The diversity of R. solanacearum species, couple with its wide host-range as well as its persistence in the soil are the most significant impediments to the existing control methods. Diversity is key not only in understanding the phyto-pathological interactions between the host and pathogens but also in developing control measures. Virulence and pathogenicity (Hayward, 1991; Kinyua, 2014), phylotype (Fegan and Prior, 2005; Sagar et al., 2014)and biovar determination based on biochemical properties (Fegan and Prior, 2005)are among strategies employed in identification and characterization of R. solanacearum strains. Recently, phylotype have been improved to sequevar level based on the similarity of a 750-bp fragment of the endoglucanases (egl) gene and so far, 55 sequevars have been identified (Li et al., 2016; Liu et al., 2017).
The current study sought to isolate, characterize and define the population structure of R. solanacearum strains affecting potato cultivation in Nakuru County-Kenya. Here, successful recovery and identification of the pathogen from infected potato plants with further classification of the isolates into respective biovars and phylotypes were reported. It is envisaged that this information will contribute towards an integrated management approach for better control of bacterial wilt disease, resulting in reduced losses and poverty.
Survey site, sample collection and identification of R. solanacearum
Samples entailing plants presenting typical bacterial wilt symptoms (CIP, 2017)were randomly collected from ten farms in three different sub-counties in Nakuru county located within the Great Rift Valley. A total of 10 farms were surveyed for wilting plants in Kuresoi North, Njoro and Mau Narok. Five samples per farm were packaged in collection bags and transported to the Plant Transformation Laboratory at Kenyatta University for isolation and further characterization of pathogen. Prior to isolation, infected samples were first watched with tap water for 5 min, surface sterilized using 2% sodium hypochlorite (NaOCl) and rinsed three times with sterile distilled water. The pathogen isolation was done by plating 0.5 cm of surface the sterile plant tissue on Kelman’s triphenyl tetrazolium chloride (TZC or TTC) medium (Kelman, 1954). The plated tissues were incubated at 28°C in an incubator and monitored until the bacteria colonies were formed. Individual distinct colony from each sample was streaked onto new TZC medium to obtain pure culture. The resulting isolates were given codes based on the collection areas and preserved in 25% (v/v) glycerol solution at -20°C to be used in subsequent experiments. Morphological characteristics were used to classify the isolated bacteria into virulent (based on milky, flat, irregular, fluidal colonies with pink or red color center and whitish margin) and avirulent strains (smaller, off-white and non-fluidal or less fluidal colonies). This was done on TTC medium containing 0.005% TTC according to Kelman (1954). The virulent isolates were selected for further analysis.
Molecular characterization of R. solanacearum
The isolated R. solanacearum were characterized using polymerase chain reaction (PCR) to validate their species, determine their phylotype and infer their evolutionary relationships by sequence analyses of the partial endoglucanase (egl) gene. To extract genomic DNA, bacterial cells retrieved from the glycerol stock cultures were grown on TZC agar medium and a single colony was transferred into nutrient broth. The cultures were then incubated overnight at 28°C in a shaking incubator. DNA was extracted using a DNA extraction kit (Qiagen, USA) according to the manufacturer’s instructions. The DNA quality was checked through gel electrophoresis and quantified using a nanodrop spectrophotometer (Maestrogen, Taiwan). The R. solanacearum species was determined by using universal primer of R. solanacearum species: 759/760 which produces 281 bp amplicons of the species genome, a common region among R. solanacearum (Fegan and Prior, 2005). Identification of the phylotypes was done using a multiplex PCR based on phylotype-specific primers shown in Table 1 as described by Fegan and Prior (2005)and Sagar et al. (2014). A DNA sample was randomly selected from each resulting phylotype, to carry out a PCR targeting the endoglucanase gene shown in Table 1. All PCR amplifications were carried out in a 25 μl reaction mix containing: One Taq 2X Master mix with standard buffer (New England, Biolabs), 0.2 μM of each primer and 2 μl of the 5 μg/μl DNA template. Amplifications were done in an automated thermocycler (Eppendorf AG, 22331 Hamburg, Germany) using the following conditions: initial denaturation at 94°C for 3 min, followed by 35 cycles comprising denaturation at 94°C for 30 s, the annealing temperatures specific to each primer for 30 s and extension at 72°C for 30 s. Final extension was done at 72°C for 5 min. The phylotype of each isolate was determined according to the size of the amplified product (Table 1) after separation on 1.5% agarose gel stained with SYBR® Green and visualized under UV light transilluminator (UVIdoc HD2, Cambridge). The PCR products for sequencing were cleaned using a PCR purification kit (Qiagen, USA) then sequenced using the forward and reverse primers at Inqaba Biotech (Inqaba, South Africa).
Sequence analysis and phylogeny
The sequences obtained from Sanger sequencing platform were retrieved from Inqaba’s server. The primer sequences were removed using Vector NTI Advance (Invitrogen, USA). Resulting sequences were then used to query nucleotide databases at National Center for Biotechnology Information (NCBI, USA) using nucleotide Basic Local Alignment Search Tool (BLASTn). Four best matching results per query sequence were selected based on the highest percentage of identity and low E-values. The selected matches were then retrieved and aligned using default parameters in ClustalW’s tool in MEGA7 software. Phylogenetic tree was constructed using Maximum Likelihood (ML) algorithm based on Jukes-Cantor model with 1000 bootstrap resampling of the data to test the tree topologies (Kumar et al., 2016).
Biovar determination
Isolated R. solanacearum were differentiated into biovars based on their ability to oxidize three disaccharides (maltose, lactose and cellobiose) and three hexose alcohols (mannitol, sorbitol and dulcitol) as previously described by Hayward (1954). Standard biovar test medium (basal medium) was prepared by adding 1.0 g NH4H2PO4, 0.2 g KCl, 0.2 g MgSO4.7H2O, 1.0 g bactopeptone, 3.0 g agar and 80 mg Bromothymol blue into a final volume of 1 L of distilled water (Denny and Hayward, 2001). The pH was adjusted to 7.0 then 10% solutions of cellobiose, lactose, maltose, dulcitol, mannitol and sorbitol were prepared separately and 1 part of each carbon solution was mixed with 9 parts of the basal medium to obtain a final concentration of 1% of the carbohydrate. The medium was autoclaved and allowed to cool before 150 μl of each preparation was dispensed into the 96 wells micro-titration plates. All isolates were inoculated into individual wells with 50 μl of bacterial suspensions adjusted to OD600 = 0.1 (~108 cfu/mL), with two replicates per isolate. The plates were incubated at 28°C and monitored daily for change in pH by a color change (Schaad et al., 2001). The experiment was repeated 3 times.
Determination of R. solanacearum aggressiveness using hypersensitivity reaction test
A total of 20 R. solanacearum isolates were determined as virulent based on Kelman (1954) method. To further analyze the aggressiveness of the 20 isolates, a hypersensitive reaction (HR) test was conducted using tobacco model plant through bacterial infiltration. Briefly, tobacco plants (Nicotiana tabacum) were first grown on autoclaved soil and maintained in a glasshouse at 18 to 22°C with regular watering. The inoculum was prepared by growing bacterial overnight in liquid nutrient broth medium as earlier described then pelleted by centrifugation. The concentration of cell suspension was adjusted to OD600 = 0.1 (~108 cfu/mL) using a spectrophotometer (JENWAY 6300, Dunmow, UK). Fully expanded leaves from 54 days old plant were infiltrated with a suspension of each R. solanacearum isolate using a sterile syringe following the injection technique described by Klement (1963). Sterile distilled water was used as a negative control while an isolate provided by CIP was also included as a positive control. Two leaves per plant from a total of 4 plants were infiltrated with each isolate and HR described as necrotic or yellowing areas in the region surrounding an infection point monitored daily for 2 weeks post infiltration. This was scored as described by Shahbaz et al. 2015). The experiment was repeated 3 times and the data were presented in a graph using graphpad prism version 6 to show the most frequent reaction induced by the identified isolates.
Pathogenicity test of R. solanacearum on potato
To confirm the virulence of the R. solanacearum isolates, pathogenicity test was performed on potato seedlings of variety Shangi by root irrigation method according to Rado et al. (2015).
This variety was chosen based on its agronomic traits and farmers preferences as it the most common potato cultivar cultivated in Nakuru County (Mwaniki et al., 2016; Gitari et al., 2018). Certified potato seeds of this cultivar were purchased from the Agricultural Development Corporation (ADC) in Molo-Kenya and sown in autoclaved potted soil. One tuber was sown per pot and emerging seedlings thinned to leave one plant in each pot. The plants were maintained in a glasshouse with natural light conditions (12 h of light and 12 h of darkness), temperatures of 24 to 28°C and a humidity of 60%. The seedlings with 4 to 6 expanded leaves were then infected with the R. solanacearum isolates. To prepare the inoculum, one R. solanacearum isolate was randomly selected from each for each surveyed areas and grown on TZC plates for 48 h at 28°C. A single colony showing virulence (fluidal, irregular and creamy white with pink at the center) was selected from each culture and transferred into a 10 mL tube containing modified Kelman media (MKM) (French and Elphinstone, 1995)then incubated at 28°C for 24 h on a shaker (180 rpm). The cultures were then pelleted by centrifugation, the bacterial cells suspended in sterile distilled water and their concentration normalized to OD600 = 0.1 (~108 cfu/mL). Plant infection was done using the root irrigation method described by Rado et al. (2015). According to the protocol, the soil layer around the stem was scooped to the side of the plant, the main root wounded by gently scratching with a sterile 1 mL tip and 10 mL of the prepared bacterial suspension poured around the base of the injured plant. The scooped soils were returned to cover the injured area and the plants maintained under the same conditions. Ten plants were then inoculated with each of the four selected isolates. Sterile distilled water was also included as a mock infection to act as a normal control and the experiment was repeated 3 times. Virulence of the isolates was assessed by analyzing bacterial wilt symptoms (CIP, 2017)on infected plants. From the first day when the wilt symptom appeared, scoring was carried out weekly for a month following Timila and Manandhar (2016)method. Plants with visible symptoms (wilted leaves) were recorded as diseased plants (Park et al., 2007). The disease incidence (DI) was calculated following the method by Xue et al. (2009)as DI (%) = [ ∑ (number of diseased plants in this index × disease index) / (total number of plants investigated × the highest disease index)] × 100%. The data were presented as mean of disease incidence (DI) percentage in a disease progression curve using graphpad prism version 6. The pathogen was re-isolated from diseased plants for confirmation.
Isolation, species validation and virulence of the pathogen
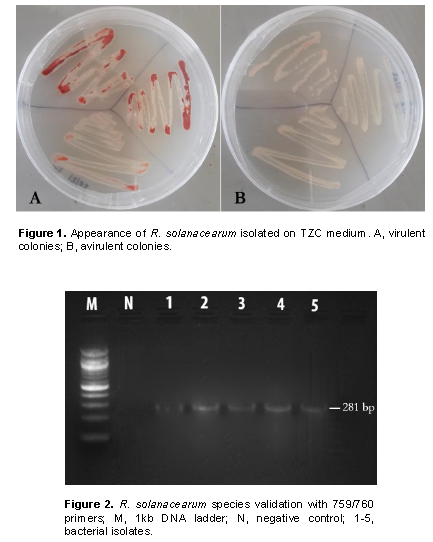
Cultural methods and media used in the current study allowed isolation of 54 isolates from the 3 sub-counties Kuresoi North, Mau Narok and Njoro sampled in Nakuru county. These isolates, suspected to be R. solanacearum, were subjected to various confirmatory biochemical and molecular tests to ascertain their identity. The Kelman’s TZC agar differentiation test gave pink or light red color colonies with characteristic red center and whitish margin for the virulent isolates while the avirulent isolates produced smaller, off-white and non-fluidal or dry colonies on TZC medium after 48 h of incubation (Figure 1). Twenty isolates from those producing fine pink or light red color colonies were randomly selected from the sample areas for further characterization. A PCR analysis on the 20 isolates from those producing fine pink or light red color with whitish merges colonies using 759/760 primers returned a 281 bp amplicons confirming the isolates to belong to R. solanacearum species as shown in the representative gel in Figure 2.
Phylotype analyses
The R. solanacearum strains of potato from Nakuru showed different levels of genetic diversity. The prevalence and distribution of the 4 phylotypes varied throughout the 3 major potato-growing areas in the County. The greatest diversity was found in Kuresoi North. The second highest diversity was found in Njoro, with 3 different phylotypes (I, III and IV) while all strains from Mau Narok were only identified as phylotype I. In addition, it was found that the most prevalent phylotype was phylotype I, which represented 50% of the collection (Table 2).
Characterization of partial endoglucanase (egl) gene sequences
Sequencing of the partial endoglucanase (egl) gene allowed further confirmation of the cultures to belong to R. solanacearum species. The sequences have been deposited in the NCBI database awaiting accession numbers. Multiple sequence alignment and phylogenetic analysis resulted in generation of a phylogenetic tree indicating the evolutionary relationships of the current isolates with previous isolates deposited at NCBI (Figure 3). According to the Maximum Likelihood algorithm, the analyzed R. solanacearum clustered into 3 groups with the isolates from this study falling in groups I and III. Isolate Rs15 was found in group I, with close relation to other R. solanacearum and Ralstonia syzygii the causal agent of banana blood disease. The remaining three isolates were clustered alone in group III (Figure 3).
Differentiation of the identified R. solanacearum into biovars
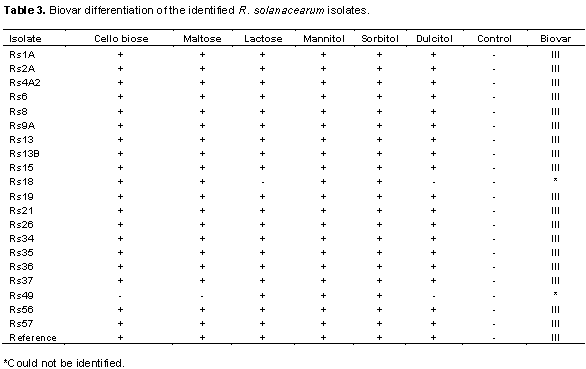
All the 20 isolates of R. solanacearum with the exception of 2 (Rs18 and Rs49 whose biovars were not identified) were able to oxidize the disaccharides and sugar alcohols within 3 to 5 days although at different rates. Their ability to oxidize the substrates was confirmed by a change of the basal media color from blue-green to yellow upon incubation with bacteria isolates. Eighteen isolates which oxidized all substrates were therefore classified as belonging to biovar III. The 2 other isolates Rs18 and Rs49 were not identified due to their inability to oxidize certain substrates (Table 3). Rapid oxidation of mannitol and sorbitol were observed in relative to other substrates. A color change in the test medium containing these substrates was observed by 2 days after culture while in dulcitol, complete color change occurred at 4 days after incubation. All the 3 disaccharides were utilized at a similar rate and resulted in complete color changes at the 4th day. Control plates with sterile distilled water did not show a color change following incubation with all bacterial isolates.
Analysis of the virulence and pathogenicity of R. solanacearum in plants
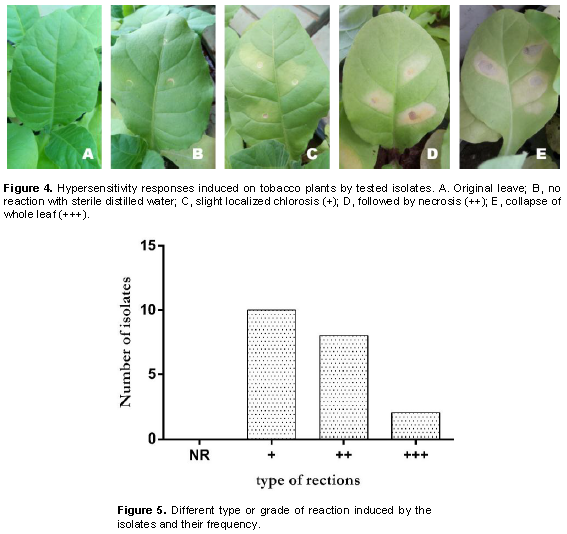
To assess whether there were any differences in virulence and pathogenicity of the identified R. solanacearum isolates, tobacco leaves were infiltrated with bacterial inoculum of the 20 isolates and monitored development of hypersensitivity reactions. It was observed that all the isolates resulted in a hypersensitivity reaction from the infiltrated plants manifested by slight localized chlorosis followed by necrosis, although at different grades (Figure 4). Tobacco leaves infiltrated with sterile water were unaffected. Isolates Rs9A and Rs37 resulted in the highest hypersensitive response in the tobacco leaves and were therefore considered the most virulent (Table 1). On the other hand, most of the isolates were able to induce only the first grade of reaction, slight localized chlorosis (Figure 5).
Four isolates representing the four phylotypes were randomly selected following the HR assay and were used to analyze their aggressiveness on the most cultivated potato cultivar Shangi. Upon infection, disease symptoms characterized by wilting, yellowing of leaves and black streak on the stem were observed from day 8 following infection (Figure 6). The disease progression was monitored weekly for one month and the observed disease incidence (DI) ranged from 0 to 40% with Rs6 isolate (from Kuresoi North) being the fastest and most virulent followed by Rs15 also from the same region. The isolates from Mau Narok and Njoro (Rs1A and Rs35) were weakly aggressive and Rs35 showed no wilt symptoms but resulted in yellowing of leaves and development of black streak on the stem, compared to the control where none of these symptoms were observed (Figure 7).
The findings of this study show successful isolation and characterization of R. solanacearum causing bacterial wilt of potato in Nakuru county of Kenya. Cultural, biochemical and molecular methods were used to characterize the isolates into biovars, phylotypes and further determine their evolutionary relationship with other known R. solanacearum species all over the world. TZC medium allowed successful isolation of R. solanacearum as revealed by the different pigmentation. Apart from selective isolation, this medium has also been implicated in differentiating virulent from avirulent strains with Kelman (1954)reporting that virulent colonies appear white with pink or light red centers and non-virulent colonies appear as small off-white colonies. On this medium, typical virulent bacterial colonies (fluidal, irregular in shape and white with pink or light red centers) were obtained as also report by Champoiseau and Momol (2008)) and Rahman et al. (2010). The isolates were then confirmed to be R. solanacearum species, in line with the work by Fegan and Prior (2005)where they also reported the amplification of 281 bp of the common region of the species genome with 759/760 primers.
The isolated R. solanacearum was further characterized into biovars based on their ability to utilize sugars and/or alcohols. Differentiation of R. solanacearum into biovars based on the utilization of carbohydrates has been previously reported by Hayward (1964). It was found that all isolates from this study successfully oxidized the disaccharides and hexose alcohols and this confirmed their classification as biovar III, relative to what was established by Denny and Hayward (2001). The successful isolation of R. solanacearum which all belong to biovar III confirmed their ability to adapt to several conditions (Denny, 2007).
Moreover, the high virulence of biovar III strains in the region can be justified by their wide host range and compatibility with number of environmental factors favorable for disease appearance such as temperature, rainfall, soil type, inoculum potential, and other soil biological factors such as wilt complexes formed among nematodes (Meloioigyne species) fungi (Fusarium species) and R. solanacearum (Shahbaz et al., 2015).
Cultural and biochemical methods in bacterial identification is often supported by molecular characterization using known sequences to further affirm the categories to which each isolate belong to. In the current study, the phylotypes of each isolate were determined and it was found that all 4 phylotypes of R. solanacearum (I, II, III and IV) were present in the region. Even though, all phylotypes were found from the sampled regions, their distributions were not even. For instance, all the 4 phylotypes were recovered in Kuresoi North, 3 in Njoro and phylotype I was the only phylotype isolated in Mau Narok. The uneven distribution of this pathogen could be due to differences in adaptation to climatic conditions and the competitive fitness advantage of R. solanacearum as earlier reported by Huerta et al. (2015). Phylotype I, exhibited the highest incidence than the others phylotypes. This can be explained by its capacity to infect a wide range of hosts including herbaceous and woody plants (Hayward, 1994). Furthermore, phylotype I is known to be distributed worldwide (Hayward, 1991)and it is reported to be highly recombinogenic (Coupat et al., 2008; Wicker et al., 2012). Several studies have also reported prevalence and distribution of different phylotypes in other African countries. For instance, in Cameroon and Ivory Coast, phylotypes I, II and III have been reported (Mahbou et al., 2009; N’Guessan et al., 2012)while in Ethiopia phylotypes I and II have been identified (Lemessa and Zeller, 2007). Phylotype I was also previously reported in Madagascar and eastern African countries bordering the Indian Ocean including Kenya and South Africa (Wicker et al., 2012; Ravelomanantsoa et al., 2016; Carstensen et al., 2017). This is the first report of phylotype IV in Kenya which could have been introduced in the country through imported potato seed with latent infection (Kaguongo et al., 2010).
Sequencing of the endoglucanase gene from the isolated R. solanacearum confirmed the identity of these bacteria and allowed deciphering of their evolutionary relatedness with other known R. solanacearum species. This gene has previously been used for sequevar determination (Li et al., 2016; Liu et al., 2017), evolutionary dynamics to reveal genetic relationships between R. solanacearum species complexes (RSSC), phylogenetic and statistical analysis of housekeeping, virulence-related and pathogenicity-related genes (Fegan and Prior, 2005; Castillo and Greenberg, 2007).
All R. solanacearum isolates elicited a hypersensitive response from infiltrated tobacco leaves which was a good indicator of their potential virulence. Hypersensitive reaction is a defense mechanism used by plants to prevent the spread of pathogen infection to non-infected parts. It is associated with plant resistance and characterized by rapid and programmed cell death localized in the region surrounding an infected region (Nimchuk et al., 2003). Reports have indicated that most pathogenic bacteria induce hypersensitivity in leaves of tobacco or other non-host plants and this is often used as a prescreen technique in virulence assays (Poussier et al., 2003; Yabuuchi et al., 2006). In R. solanacearum, the hypersensitivity reaction and pathogenicity genes or hrp genes have been implicated in controlling induction of both disease development and hypersensitive reactions with hrp mutants unable to induce symptoms in susceptible host plants (Boucher et al., 2001). The ability of all isolates under this study to induce hypersensitive reaction (HR) could be attributed to presence of the hrp genes in their genome as reported by Boucher et al. (2001).
The virulence of these isolates was determined through pathogenicity tests on a susceptible potato cultivar Shangi. Here, plants were infected with different isolates with a uniform concentration of the pathogen and maintained under the same conditions then evaluated for disease occurrence and severity, as previously reported by Timila and Manandhar (2016). It was observed that the isolates caused disease symptoms on potato plants albeit at different rates. The nature of these symptoms also varied from those observed under field conditions as also reported by Huerta et al. (2015). This could be due to the fact that R. solanacearum pathogenicity is distinctly regulated in early or late stages of infection in response to environmental conditions such as soil, humidity, temperature and texture as well as bacterial population densities (Schell, 2000; Hikichi et al., 2007). It is important to note that R. solanacearum-host interaction occurs through three main stages including root colonization, cortical infection and xylem penetration. These stages are affected by plant structure and metabolism; and these could have played a role in the observed phenotypes upon infection (Vasse et al., 2005).
R. solanacearum strains affecting potato in Nakuru county-Kenya were successfully isolated and characterized using phylogenetic and pathogenetic analyses. It was found that the pathogen is highly diverse with several phylotyes and variable distribution. Phylotype I was found as the most predominant phylotype and widely distributed in the region. Therefore, it should be considered in development of control strategies such as grafting for resistance or propagation programs. The results also demonstrated that biovar III strains have adapted themselves to the more diverse environment of Nakuru than other biovar strains showing severe reaction on tobacco and variable virulence on potato plants. These findings provide vital information on the R. solanacearum strains in this region as well as their associated virulence and distribution which form a basis for breeding programs for potato bacterial wilt resistance and development of control strategies with special emphasis on the improvement of pathogen-targeted and geographically-targeted management practices.
The authors have not declared any conflict of interests.
The authors acknowledge the financial support from the African Union through Pan African University. They are grateful to the Ministry of Agriculture Nakuru department for support during sampling and the International Potato Center (CIP) for providing the reference strain.
REFERENCES
|
Abong GO, Okoth MW, Karuri EG, Kabira JN, Mathooko FM (2009). Nutrient contents of raw and processed products from Kenyan potato cultivars. Journal of Applied Biosciences 16:877-886.
|
|
|
|
Ahuja JKC, Haytowitz DB, Pehrsson PR, Roseland J, Exler J, Khan M, Mille C (2013). Composition of Foods Raw, Processed, Prepared USDA National Nutrient Database for Standard Reference, Release 27 Documentation and User Guide. U.S. Department of Agriculture Agricultural Research Service Beltsville Human Nutrition Research Center Nutrient Data Laboratory, 2(November):1-136.
|
|
|
|
|
Boucher C, Genin S, Arlat M (2001). Current concepts on the pathogenicity of phytopathogenic bacteria. Comptes Rendus de l'Academie Des Sciences. Serie III. Sciences de La Vie 324(10):915–922.
Crossref
|
|
|
|
|
Carstensen GD, Venter SN, Wingfield MJ, Coutinho TA (2017). Two Ralstonia species associated with bacterial wilt of Eucalyptus. Plant Pathology 66(3):393-403.
Crossref
|
|
|
|
|
Castillo JA, Greenberg JT (2007). Evolutionary dynamics of Ralstonia solanacearum. Applied and Environmental Microbiology 73(4):1225-1238.
Crossref
|
|
|
|
|
Champoiseau PG, Jones JB, Allen C (2009). Ralstonia solanacearum race 3 biovar 2 causes tropical losses and temperate anxieties. Plant Health Progress 10:1-10.
Crossref
|
|
|
|
|
Champoiseau PG, Momol TM (2008). Bacterial wilt of tomato. Ralstonia Solanacearum, 12.
|
|
|
|
|
CIP (2017). Strategies for Bacterial Wilt (Ralstonia solanacearum) Management in Potato Field: Farmers' Guide. Centro Internacional.
|
|
|
|
|
Coupat B, Chaumeille-Dole F, Fall S, Prior P, Simonet P, Nesme X, Bertolla F (2008). Natural transformation in the Ralstonia solanacearum species complex: number and size of DNA that can be transferred. FEMS Microbiology Ecology 66(1):14-4.
Crossref
|
|
|
|
|
Denny T (2007). Plant pathogenic Ralstonia species. In Plant-associated bacteria. Springer. pp. 573-644
|
|
|
|
|
Denny TP, Hayward AC (2001). Laboratory guide for identification of plant pathogenic bacteria. American Phytopathological Society.
|
|
|
|
|
Food and Agriculture Organization (FAO) (2010). International year of the potato. Food and Agriculture Organisation of the United Nations, Rome, Italy.
|
|
|
|
|
Food and Agriculture Organization of the United Nations (FAOSTAT) (2018). Food and Agriculture Organization of the United Nations. Retrieved from
View
|
|
|
|
|
Fegan M, Prior P (2005). How complex is the Ralstonia solanacearum species complex. American Phytopathological Society press.
|
|
|
|
|
French ER, Aley E, Elphinstone J (1995). Culture media for Ralstonia solanacearum isolation, identification and maintenance. Fitopatologia 30:126-130.
|
|
|
|
|
Gitari HI, Karanja NN, Gachene CKK, Kamau S, Sharma K, Schulte-Geldermann E (2018). Nitrogen and phosphorous uptake by potato (Solanum tuberosum L.) and their use efficiency under potato-legume intercropping systems. Field Crops Research 222:78-84.
Crossref
|
|
|
|
|
GIZ-PSDA (2011). Potato value chain improving the livelihood of Kenyan farmers by growing profits. Deutsche Gesellschaft Fur Internationale.
|
|
|
|
|
Hayward AC (1991). Biology and epidemiology of bacterial wilt caused by Pseudomonas solanacearum. Annual Review of Phytopathology 29(1):65-87.
Crossref
|
|
|
|
|
Hayward AC (1994). The hosts of Pseudomonas solanacearum. Center for Agricultural and Biosciences International.
|
|
|
|
|
Hayward AC (1964). Characteristics of Pseudomonas solanacearum. Journal of Applied Bacteriology 27(2):265-277.
Crossref
|
|
|
|
|
Hikichi Y, Yoshimochi T, Tsujimoto S, Shinohara R, Nakaho K, Kanda A, Ohnishi K (2007). Global regulation of pathogenicity mechanism of Ralstonia solanacearum. Plant Biotechnology 24(1):149-154.
Crossref
|
|
|
|
|
Huerta AI, Milling A, Allen C (2015). Tropical strains of Ralstonia solanacearum outcompete race 3 biovar 2 strains at lowland tropical temperatures. Applied and Environmental Microbiology, AEM-04123.
Crossref
|
|
|
|
|
Kaguongo WP, Ng'ang'a NM, Muthoka N, Muthami F, Maingi G (2010). Seed potato subsector master plan for Kenya (2009-2014). Seed Potato Study Sponsored by GTZ-PSDA, USAID, CIP and Government of Kenya. Ministry of Agriculture, Kenya, 55 p.
|
|
|
|
|
Kelman A (1954). The relationship of pathogenicity of Pseudomonas solanacearum to colony appearance in a tetrazolium medium. Phytopathology 44(12).
|
|
|
|
|
Kinyua AN (2014). Factors affecting the performance of Small and Medium Enterprises in the Jua kali sector in Nakuru Town, Kenya. Journal of Business and Management 6(1):5-10.
|
|
|
|
|
Klement Z (1963). Rapid detection of the pathogenicity of phytopathogenic pseudomonads. Nature 199(4890):299.
Crossref
|
|
|
|
|
Kumar S, Stecher G, Tamura K (2016). MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution 33(7):1870-1874.
Crossref
|
|
|
|
|
Lemessa, F, Zeller W (2007). Isolation and characterisation of Ralstonia solanacearum strains from Solanaceae crops in Ethiopia. Journal of Basic Microbiology 47(1):40-49.
Crossref
|
|
|
|
|
Li Y, Feng J, Liu H, Wang L, Hsiang T, Li X, Huang J (2016). Genetic diversity and pathogenicity of Ralstonia solanacearum causing tobacco bacterial wilt in China. Plant Disease 100(7):1288-1296.
Crossref
|
|
|
|
|
Liu Y, Wu D, Liu Q, Zhang S, Tang Y, Jiang G, Ding W (2017). The sequevar distribution of Ralstonia solanacearum in tobacco-growing zones of China is structured by elevation. European Journal of Plant Pathology 147(3):541-551.
Crossref
|
|
|
|
|
Mahbou STG, Cellier G, Wicker E, Guilbaud C, Kahane R, Allen C, Prior P (2009). Broad diversity of Ralstonia solanacearum strains in Cameroon. Plant Disease 93(11):1123-1130.
Crossref
|
|
|
|
|
MoALF (2016). The National potato strategies. Ministry of Agriculture, Livestock and Fisheries, Nairobi, Kenya.
|
|
|
|
|
Muthoni JJ, Shimelis H, Melis R (2013). Potato production in Kenya: Farming systems and production constraints. Journal of Agricultural Science 5(5):182
Crossref
|
|
|
|
|
Muthoni J, Shimelis H, Melis R (2012). Management of Bacterial Wilt [Rhalstonia solanacearum Yabuuchi et al., 1995] of Potatoes: Opportunity for Host Resistance in Kenya. Journal of Agricultural Science 4(9):64.
|
|
|
|
|
Mwaniki PK, Birech R, Wagara IN, Kinyua ZM, Freyer B (2016). Distribution, Prevalence and Incidence of Potato Bacterial Wilt in Nakuru County, Kenya. International Journal of Innovative Research and Development 5(1).
|
|
|
|
|
N'Guessan CA, Abo K, Fondio L, Chiroleu F, Lebeau A, Poussier S, Koné D (2012). So near and yet so far: the specific case of Ralstonia solanacearum populations from Cote d'Ivoire in Africa. Phytopathology 102(8):733-740.
Crossref
|
|
|
|
|
Nimchuk Z, Eulgem T, Holt Iii BF, Dangl JL (2003). Recognition and response in the plant immune system. Annual Review of Genetics 37(1):579-609.
Crossref
|
|
|
|
|
Park EJ, Lee SD, Chung EJ, Lee MH, Um HY, Murugaiyan S, Lee SW (2007). MicroTom-A model plant system to study bacterial wilt by Ralstonia solanacearum. The Plant Pathology Journal 23(4):239-244.
Crossref
|
|
|
|
|
Poussier S, Thoquet P, Trigaletâ€Demery D, Barthet S, Meyer D, Arlat M, Trigalet A (2003). Host plantâ€dependent phenotypic reversion of Ralstonia solanacearum from nonâ€pathogenic to pathogenic forms via alterations in the phcA gene. Molecular Microbiology 49(4):991-1003.
Crossref
|
|
|
|
|
Rado R, Andrianarisoa B, Ravelomanantsoa S, Rakotoarimanga N, Rahetlah V, Fienena FR, Andriambeloson O (2015). Biocontrol of potato wilt by selective rhizospheric and endophytic bacteria associated with potato plant. African Journal of Food, Agriculture, Nutrition and Development 15(1):9762-9776.
|
|
|
|
|
Rahman MF, Islam MR, Rahman T, Meah MB (2010). Biochemical characterization of Ralstonia solanacerum causing bacterial wilt of brinjal in Bangladesh. Progressive Agriculture 21(1-2):9-19.
|
|
|
|
|
Ravelomanantsoa S, Robène I, Chiroleu F, Guérin F, Poussier S, Pruvost O, Prior P (2016). A novel multilocus variable number tandem repeat analysis typing scheme for African phylotype III strains of the Ralstonia solanacearum species complex. Peer Journal 4:e1949.
Crossref
|
|
|
|
|
Sagar V, Jeevalatha A, Mian S, Chakrabarti SK, Gurjar MS, Arora RK, Singh BP (2014). Potato bacterial wilt in India caused by strains of phylotype I, II and IV of Ralstonia solanacearum. European Journal of Plant Pathology 138(1):51-65.
Crossref
|
|
|
|
|
Schaad NW, Jones JB, Chun W (2001). Laboratory guide for the identification of plant pathogenic bacteria. American Phytopathological Society (APS Press).
|
|
|
|
|
Schell MA (2000). Control of virulence and pathogenicity genes of Ralstonia solanacearum by an elaborate sensory network. Annual Review of Phytopathology 38(1):263-292.
Crossref
|
|
|
|
|
Shahbaz MU, Mukhtar T, Begum N (2015). Biochemical and serological characterization of Ralstonia solanacearum associated with chilli seeds from Pakistan. International Journal of Agriculture and Biology 17(1):31-40.
|
|
|
|
|
Timila RD, Manandhar S (2016). Biovar differentiation and variation in Virulence of Ralstonia solanacearum Isolates Infecting Solanaceous Vegetables. Journal of Nepal Agricultural Research Council 2:22-26.
Crossref
|
|
|
|
|
Vasse J, Danoun S, Trigalet A, Allen C, Prior P, Hayward A (2005). Microscopic studies of root infection in resistant tomato cultivar Hawaii7996. Bacterial Wilt Disease and the Ralstonia Solanacearum Species Complex pp. 285-291.
|
|
|
|
|
Wicker E, Lefeuvre P, De Cambiaire JC, Lemaire C, Poussier S, Prior P (2012). Contrasting recombination patterns and demographic histories of the plant pathogen Ralstonia solanacearum inferred from MLSA. The ISME Journal 6(5):961.
Crossref
|
|
|
|
|
Xue QY, Chen Y, Li SM, Chen LF, Ding GC, Guo DW, Guo JH (2009). Evaluation of the strains of Acinetobacter and Enterobacter as potential biocontrol agents against Ralstonia wilt of tomato. Biological Control 48(3):252-258.
Crossref
|
|
|
|
|
Yabuuchi (2006). COMMISSION DIRECTIVE 2006/63/CE of 14 July 2006. Official Journal of the European Union. Retrieved from
View
|
|