ABSTRACT
Commiphora swynnertonii is among the most commonly used medicinal plants by pastoralist communities especially in northern regions of Tanzania. The effect of resin from this plant on white blood cells (WBC) and haematopoietic organs was studied using albino mice. Sixty adult mice were randomly assigned into four groups (n = 15). G1 acted as control whereas G2, G3 and G4 received oral doses of 50, 100 and 200 mg resin per kg body weight, respectively for 35 days consecutively. Blood samples for differential and total WBC count were collected before treatment and on days 7, 14, 28 and 35 after treatment. Also, three mice from each group were humanely sacrificed before treatment, on day 14 and 35 after treatment. Sternum, liver and spleen samples from sacrificed mice were collected for assessment of any effects of the resin on haematopoietic organs. Results showed that mice in G2 and G4 had a significant increase (P < 0.05) in total WBC counts by day 7 as compared to the control group. This trend was then followed by a gradual decrease towards end of the experiment. No significant changes in total WBC counts were observed in G3 following treatment. The effect of C. swynnertonii resin on differential WBC count was non-specific and insignificant; G1 and G2 mice had their lymphocyte and monocyte counts slightly increasing with time while that of G3 and G4 decreased slightly or remained unchanged. Neutrophils counts decreased significantly in G1 and G2, but there were no significant changes for G3 and G4. Changes in the haematopoietic tissues following exposure to the resin included increased cellularity of sternal bone marrow as compared to spaces occupied by adipocytes. In particular, there were different developmental stages of granulocytes, erythroblasts and all megakaryocytic series. Small patches of erythropoietic series and lymphoblastic cells were observed in the liver and spleen respectively of the mice that received resin. It is concluded that oral administration of C. swynnertonii resin to mice caused a significant but transient increase in total white cell counts as a short-term effect. Prolonged exposure to the resin was associated with changes in the haematopoietic system such as increased cellularity of bone marrow and erythropoietic patches in liver and spleen.
Key words: Commiphora swynnertonii, resin extract, WBC count, haematopoietic tissue, albino mice.
The use of plants for treatment of various diseases affecting humans and animals is a common and popular practice in many developing countries (Idowu et al., 2009). Commiphora swynnertonii, a tropical shrub belonging to Burseraceae family and widely distributed in Africa, is among such plants. Different parts of the plant have been used by pastoralists for treatment of bacterial and fungal infections (e.g., tuberculosis, abscesses, dysentery, gastrointestinal ulcers, wounds, ringworms and candidiosis), control of ecto- and endo-parasites (Kaoneka et al., 2007), and rheumatism (Minja, 1999). Studies by Bakari et al. (2012, 2013) demonstrated that resin extracts from C. swynnertonii had significant biological activities against Newcastle virus, bacteria (Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa) fungi (Candida albicans and Actinomyces pyogenes), and protozoa (Coccidia spp.) using in- vitro and vivo test systems. It was further observed that the C. swynnertonii resin affected some hematological and biochemical parameters during in vivo trials using chickens, guinea pigs and rabbits. For instance, Bakari et al. (2015) found that oral administration of the resin extract was associated with an increase in white blood cell (WBC) counts, particularly monocytes and lymphocytes, in growing chickens, that is, immuno-potentiating effect. This observation is interesting because drugs or supplements with immuno-potentiating effects are vital in patients or individuals with immune-compromising conditions such as acquired immune disease syndrome (AIDS), tuberculosis (TB), cancers and many others. Extracts from other medicinal plants such as Commiphora mukul and Commiphora molmol have been reported to increase the WBC count in mice (Abdallah et al., 2009; El-Naggar, 2011). The effect of C. swynnertonii resin extracts on the immune system in animals has not yet been reported.
The current study was therefore designed to investigate the effect of oral administration of C. swynnertonii resin on peripheral white blood cell counts and major haematopoietic organs (bone marrow, spleen and liver) in chickens.
Source of test resin and preparation
C. swynnertonii resin was collected from the northern Tanzania District of Simanjiro in Manyara Region located at 4°0′0″ S, 36°30′0″ E; 1,360 m above sea level. To harvest the resin, a thin band of bark was removed near the base of the tree and an incision was made at a depth of about half the thickness of the bark to allow resin to ooze from the cortex and was collected in an airtight container. Preparations involved soaking of the resin in 99.8% ethanol followed by separation using a rotary evaporator. The resulting crude extracts were then stored at 4°C in air-tight bottles until used in this experiment.
Experimental design and resin administration
Forty albino mice aged between 2 and 3 weeks old and ranging between 100 and 130 g were divided into four groups (n = 15) and were caged separately. The mice were maintained on broiler mash as basal feed with ad libitum drinking water. The resin was diluted in distilled water to make a stock solution of 50 mg per mL. The control group (G1), received an oral placebo (distilled water). The remaining groups (G2 - G4) received varying oral doses of resin using a 5 mL graded syringe as shown in Table 1. The resin was administered for 35 consecutive days. Before and after treatment with resin, all mice in all groups were closely monitored throughout the experiment for any sign of toxicity that could be associated with the resin.
Sampling and preparation
Determination of white blood cells counts
Three mice from each group were humanely sacrificed, on days 0, 7, 14, 21 and 35, by placing the mice in an induction chamber containing chloroform-soaked cotton wool until they were fully anaesthetized. Then, blood samples were obtained through heart-puncture using EDTA vacutainer tubes. The blood samples were used to prepare thin blood smears for total white blood cells (WBC) counts. For determination of differential WBC counts, blood was diluted using glacial acetic acid at a ratio of 1:20 and then counted using Neubauer chamber.
Assessment of haematopoietic organs
Spleen, liver and bones from sternum and femoral epiphysis of three mice from each group sacrificed on days 0, 14 and 35 were collected and fixed in formaldehyde. The spleen and liver were processed and stained with hematoxyline and eosin (H&E) for histological examination. The bones were decalcified in formic acid to expose the bone marrow before squash smears were made and stained using Giemsa method for examination of haematopoietic cells. Haematopoiesis was assessed quantitatively by examining density of cellularity and mitosis in the histological sections. Bone marrow was assessed by evaluating/comparing haematopoietic and adipocytes areas as follows: + means that 50% of the haematopoietic area is covered by haematopoietic cells and 50% by adipocytes; ++ means that 60% of the haematopoietic area is covered by haematopoietic cells, 40% adipocytes; +++ means that greater than 70% of the haematopoietic area is covered by haematopoietic cells and a small area is covered by adipocytes.
Statistical analysis
The data were subjected to ANOVA using Microsoft Excel 2007. The Student’s t-test (at 95% confidence interval and significance level of 5%) was used to compare the effect of different resin doses among treatment groups. Mean differences with P ≤ 0.05 were considered to be significantly different.
Behavioral changes
This study aimed at determining the effect of resin from C. swynnertonii on white blood cell count (immune system) and haematopoietic organs in mice. Neither behavioral changes nor signs of toxicity were observed following the oral administration of resin.
White blood cell counts
Total white blood cell counts of mice following oral administration of the resin are presented in Figure 1. An increase in total WBC count was only observed in G2 and G4 and it was only significant (P<0.05) on day 7 post-treatment. On the other hand, the levels in G3 remained below that of control group and by the end of experiment, there was no significant difference among the four groups. This suggests that the resin had a positive but only a transient effect on the number of WBC in the mice.
Differential WBC counts are presented in Table 2. Results indicated that administration of CS resin did not cause any significant changes in differential WBC counts and did not reflect the treatment. Comparison of either total or differential WBC counts between control and resin-treated groups at the end of experiment (on day 35) revealed no significant differences.
Haematopoietic organs
Bone marrow
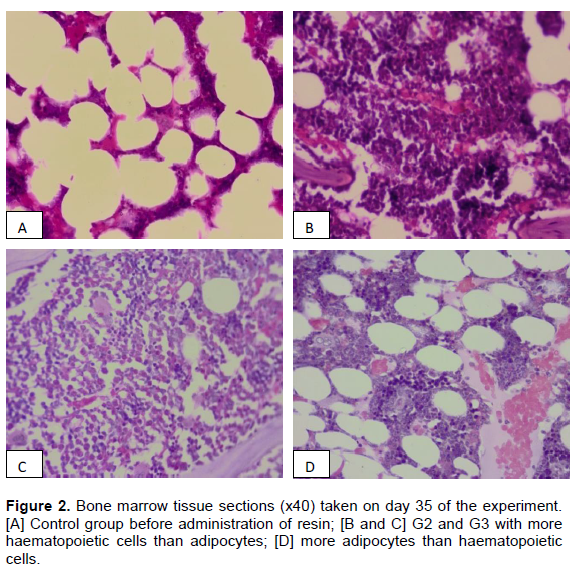
Before the oral administration of resin, haematopoietic cell stimulation was very low and the bone marrow was not active in all the groups. At this stage, bone marrow was occupied by 50% of the adipocytes and less that 50% of haematopoietic cells (Figure 2A). Following administration of the resin, cellularity in the bone marrow increased in a dose-dependent manner with doses not exceeding 100 mg resin per kg body weight (Figure 2B and C). At the lowest dose of 50 mg (G2), more than 60% was covered by haematopoietic cells and the remaining area was covered by adipocytes. In the group that received 100 mg/kg (G3), the bone marrow was very active and the general cellularity was far above 70% with metamyelocytes, megakaryocytic and granulocytic cells being clearly visible in the histological sections. Bone marrow sections from G4 had more adipocytes vacuoles than those in G2 and G3. Figures 2A to C demonstrates the dose-dependent changes in cellularity of sternal bone marrow tissue sampled on day 35 of the experiment.
Liver and spleen
The effect of the resin on haematopoietic activity of the liver had a very similar trend with that of the bone marrow. Prior to the administration of the resin, liver sections showed normal hepatocytes with no evidence of haematopoietic activity (Figure 3A). Administration of the resin induced haematopoiesis as evidenced by presence of dividing polychromatophilic cells (Figure 3B). This tendency was more pronounced in G3 mice, which received 100 mg/kg. However, in mice treated with 200 mg/kg (G4), degeneration of hepatocytes was observed as whitish patches between cells (Figure 3D). In the spleen, increased numbers of lymphoblastic cells were observed as compared to those not treated with resin.
This study aimed at determining the effect of resin from C. swynnertonii on white blood cell count (immune system) and haematopoietic organs in mice. Absence of behavioral changes following the oral administration of varying doses of resin to mice was an indication that the tested doses were mild and well tolerated by the animals. Signs of toxicity including general body weakness and diarrhea were observed in mice that received oral doses higher that 200 mg/kg body weight of C. molmol (Abdallah et al., 2009).
Leukocytes (WBC) proliferation has been used as reliable indicator to make variety of clinical decisions for both the appropriateness of treatment and for surgical intervention. Also, WBCs are major cellular component of the immune system and hence their response to proliferate is critical factor in evaluating the effectiveness of the immune system response. The current study has observed a significant but only a transient increase in total WBC counts following administration of the resin. Furthermore, the changes in WBC counts were not dose dependant. These findings are somehow contrary to those reported in similar studies using other Commiphora spp. Abdallah et al. (2009) found no significant increase in the WBC numbers following administration of C. molmol oleo-gum resins in rat, whereas El-Naggar (2011) observed a significant increase in WBC counts in rats treated with myrrh from C. molmol. Studies by Haffor (2010) revealed that C. molmol activated proliferation and differentiation pathway for all types of leukocytes during effective phase of specific immune responses. Significantly increased total WBC counts were reported in chickens that received higher dose of C. swynnertonii (Bakari et al., 2013), suggesting that low dosage used in the current study could be the reason behind the contrasting results.
Decreased WBC count following administration of high doses of Commiphora extract has also been reported (Akinnuga et al., 2011; Ajali, 2004). This tendency has been associated with saponins, which are predominant bioactive constituents found in resin extract. Saponins are steroids or triterpenoid glycosides, common in a large number of plant products. They are glycosides with distinctive foaming characteristics and are natural detergents found in certain plants (Ajali, 2004). Saponins consist of a sugar moiety usually containing glucose, galactose, glucoronic acid, xylose, rhamnose or methylpentose, glycosidically linked to a hydrophobic aglycone (sapogenin) which may be triterpenoid or steroid in nature (Bachram et al., 2006; Unakalamba et al., 2013). A study done by Akinnuga et al. (2011) reported similar findings but for different plant species, Gongronema latifolium, that the presence of saponin in leaf extract of the plant significantly reduced all blood cells by suppressing the haematopoiesis system.
Despite the insignificant increase in WBC counts amongst groups, histological examination of bone marrow, liver and spleen from mice in this study revealed changes consistent with stimulation of haematopoietic system. Resin-treated mice had higher numbers of myeloblasts, myelocytes and metamyelocytes of granulocytes, erythropoietic and megakaryocytic series as well as lymphoblastic cells in the spleen. This suggests that prolonged exposure to resin beyond the current experimental period could greatly improve/potentiate the immune system.
It is concluded that oral administration of Commiphora swynnertonii resin to experimental mice transiently increased WBC counts and activated/stimulated the haematopoietic tissues. These findings imply that moderate levels of CS can be incorporated in animal feeds to improve cellular immunity.
The authors have not declared any conflict of interests.
REFERENCES
|
Abdallah EM, Khalid HE, Al-Khalifa SK (2009). Toxicological assessment of the oleo-gum resins of Commiphora molmol and Boswellia papyrifera in mice. J. Med. Plants Res. 3(6):526-532.
|
|
|
|
Ajali U (2004). Chemistry of Bio-compounds. First Edition. Rhyce Kerex Publishers, Enugu, Nigeria.
|
|
|
|
|
Akinnuga AM, Bamidele O, Ekechi P and Adeniyi OS (2011). Effects of an Ethanolic Leaf Extract of Gongronema latifolium on Haematological Some Parameters in Mice. Afric. J. Biomed. Res. 14:153 -156.
|
|
|
|
|
Bachram C, Sutherland M, Heisher I, Hebestreet P, Melzig MF, Fuchs H (2006). The saponin-mediated enhanced uptake of targeted saponin-based drugs in strongly dependent on the saponin structure. Exp. Biol. Med. 231:412-420.
|
|
|
|
|
Bakari GG, Max RA, Mdegela RH, Phiri EC, Mtambo MM (2012). Antiviral activity of crude extracts from Commiphora swynnertonii against Newcastle disease virus in vivo. J. Trop. Anim. Health Prod. 44(7):1389-93.
Crossref
|
|
|
|
|
Bakari GG, Max RA, Phiri ECJ and Mtambo MMA (2013). Effect of resinous extract from Commiphora swynnertonii (Burrt) on experimental coccidial infection in chickens. Trop. Anim. Health Prod. 45 (2): 455-459.
Crossref
|
|
|
|
|
Bakari GG, Max RA, Mdegela, RH, Pereka AE, Phiri ECJ and Mtambo MMA (2015). Effect of resinous extract from Commiphora swynnertonii (Burrt) on various physiological parameters in chickens. J. Medic.Plants Res. 9(13):462-470.
Crossref
|
|
|
|
|
El-Naggar SA (2011). Lack of the Beneficial Effects of Mirazid (Commiphora molmol) When administered with Chemotherapeutic Agents on Ehrlich Ascetic Carcinoma Bearing Mice. Adv. Biol. Res. 5(4):193-199.
|
|
|
|
|
Haffor AS (2010). Effect of Commiphora molmol on leukocytes proliferation in relation to histological alterations before and during healing from injury. Saudi J. Biol. Sci. 17(2):139-146.
Crossref
|
|
|
|
|
Idowu A, Taiwo Bola, Oboh O and Peniel N (2009). Properties of aqueous extracts of Phyllasntus amarus (Schum and Thonn) and Xylopia aethiopica (Dunal) in albino mice. Ethno-Med, 3(2):99-103.
|
|
|
|
|
Kaoneka B, Mollel M and Lyatuu F (2007). Leaf essential oils composition and tick repellency activity of Commiphora swynnertonii. J. Biol. Res. 8:213-216.
|
|
|
|
|
Minja MMJ (1999). The Maasai wonder plants. Paper presented at the people and Plants training workshop held at Tropical Pesticide Research Institute, Arusha Tanzania 15-18th March 1999.
|
|
|
|
|
Unakalamba BC, Ozougwu JC and Ejere VC (2013). Preliminary evaluation of the haematological effects of Picralima nitida saponin extracts on Rattus novergicus. Intl. J. Bio. Biol. Sci. 2(2):28-32.
|
|