ABSTRACT
Previous studies have shown Costus afer to possess ethno-medical and ethno-veterinary properties, however, the link between C. afer and reproductive activities are not clear. The aim of this research was to assess the impact of C. afer leaf on semen quality characteristics and testosterone levels of adult buck rabbits. Eighteen adult crossbred New Zealand and Chinchilla breeds were divided into three treatments (A, B and C) (n=6) in a completely randomized design (CRD) for 12 weeks. The results showed significant (P<0.05) decrease in sperm motility, sperm count, and normal morphology due to treatment effect. Sperm abnormalities, dead cells, sluggishly motile, and pus cells were significantly (P<0.05) increased in treated group. The treatment had no significant impact (P>0.05) on parameters like appearance (normal), semen volume, and viscosity between the groups. Serum testosterone increased significantly (P < 0.05) in treated groups (C: 21.0 ng/mL; B: 11.6 ng/mL) than control (A: 11.0 ng/mL), respectively. These results indicate that C. afer has a negative fertility impact on buck rabbits.
Key words: Costus afer, semen, testosterone, rabbit, buck.
In recent times, the use of herbal medicines has gradually acquired a more vital therapeutic role to replace the synthetic once for animals and humans due to increased incidence of drug resistance (Olowosulu and Ibrahim, 2006). In Africa particularly in Nigeria, several plants have been identified to have medicinal and nutritional importance (Egba et al., 2014). The diverse African herbal plants afford the trado-medical practitioner best opportunities in the selection of herbs for various human and animal diseases (John, 2004). In folk and traditional medicine practices, several herbal plants and/or their extracts have been used to enhance fertility in male and female animals (Vasudeva and Sharma, 2007; Singh and Singh, 2009) in their unaltered form. Some of the herbs and/or its extracts have been reported to improve libido, sexual behaviour, mating performance and spermatogenesis (Tomova et al., 1981; Chauhan et al., 2007), while others balance the levels of hormone such as testosterone, luteinizing hormone, and follicle stimulating hormones (Koumanov et al., 1982) in hypothalamic-pituitary gonadal axis (reproductive axis) of male and female animals (Gamache and Acworth, 1998; Asuquo et al., 2013). Costus afer (Costaceae) is an important indigenous West Africa herbal plants with unique medicinal properties commonly used throughout its area of distribution.
It is tropical perennial herbaceous rhizomatous plants found in the forest belt of Africa (Burkili, 1985; Edeoga and Okoli, 2000). Experimental evidences have shown that C. afer possess important bioactive and medicinal potentials (Etukudo, 2003; Akpan et al., 2012; Ukpabi et al., 2012). Several studies have revealed its ethno-veterinary and ethno-biotic importance such as antimicrobial properties (Akpan et al., 2012; Ijioma et al., 2014), hypoglycemic effect (Momoh et al., 2011), treatment of stomach aches and rheumatic pains (Etukudo, 2003), treatment of hepatic oxidative stress and toxicity (Ukpabi et al., 2012), anti-diabetic (Soladoye and Oyesika, 2008), treatment of eye inflammation, headache, oedema, and fever (Omokhua, 2011). This is due to its numerous phytochemical properties such as steroids, flavonoids, alkaloids phynols, saponins, and phenols (Oliver-Bever, 1986; Tchamgoue et al., 2015; Ukpabi et al., 2012). Previously, tropical ethno-medical and ethno veterinary plants and other products with propensity to limit and/or enhance reproductive function in animals have been reported (Yahaya and Ajuogu, 2014; Yahaya et al., 2015). Therefore, this study was designed to assess the impact of C. afar on the fertility status (semen quality characteristics and testosterone levels) in buck rabbits.
All experimental procedures involving animals and the ethical issues of University of Port Harcourt were strictly followed. The experiment was done at the Research and Demonstration Farm of Faculty of Agriculture, University of Port Harcourt, Rivers State. Eighteen adult cross breed of New Zealand and Chinchilla bucks with an average weight of 1.5 to 2.0 kg were assigned randomly to three groups (A, B, and C) (n=6) and were further subdivided into three replicates (n=2) per group in a completely randomized design (CRD). The study lasted for 12 weeks. The C. afer leaves were harvested fresh and fed along with growers mash ad libitum to the experimental animals (buck rabbits) as follows: Treatment group A (control group), concentrate feed only; Group B, concentrate feed with C. afer; treatment group C, fresh C. afer only. All the groups were fed on ad libitum bases.
Prior to the treatment exposure, blood samples were collected from each animal through the ear vein in all the treatment groups using sterile syringes and hypodermic needles and decanted into well labelled sterile sample bottles for preliminary analysis of serum testosterone levels. Similarly, at the termination of the study, blood samples were also collected from all the bucks in all the treatments groups using the sample procedure mentioned earlier and were used to assess serum testosterone levels using commercial test kits BioCheck, Inc, 323 Vintage Park Dr Foster City CA 94404. The protocol was as described by the manufacturer. Semen samples were collected from the bucks in all the groups with the aid of artificial vagina (AV) using the method described by Herbert and Adejumo (1995) and modified method of Ajuogu and Ajayi (2010).
The samples were collected twice at the beginning of the study and at the expiration of the study. Group C could not mount due to exhortation; as a result, no semen was collected. The semen quality characteristics were assessed by microscopic examination. Data obtained from testosterone and semen samples were analysed using the analysis of variance (ANOVA). According to Steel and Torrie (1980), means with significant differences were separated using Duncan’s Multiple Range Test (DMRT, 1955).
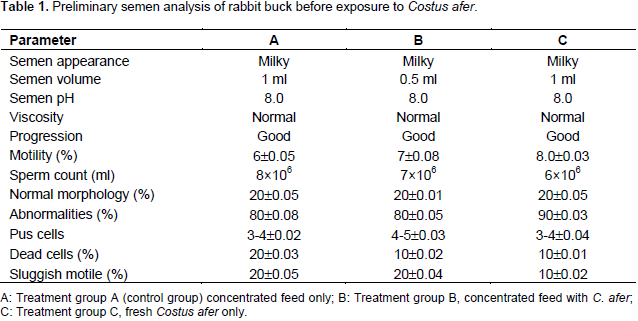
Preliminary semen analysis of buck rabbit before exposure to C. afer
The preliminary semen analysis result of rabbit buck exposed to C. afer is presented in Table 1. The results revealed no significant changes (P>0.05) on the semen quality parameters measured. The semen volumes are 1 ml each in treatments A and C, while in treatment B, it was 0.5 ml. The pH was 8.0 in treatment (A, B and C), respectively. The viscosity and progression of the semen in all the treatments (A, B and C) were both good and normal.
Preliminary semen analysis of rabbit buck
The statistical analysis of semen quality assessment parameters revealed no significant difference between the treatment groups. Sperm motility was 80, 70, and 60% for treatments C, B and A. Sperm count was 8×106, 7×106 and 6×l06 ml-1 for treatments A, B and C, respectively (Table 1). The pH, viscosity and progression were 8.0, normal, and good in all the treatments. The semen appearance was milky in all the groups. For normal morphology, pus cells, was not significantly different between control and treatment groups. Also, Table 2 shows the preliminary semen analysis of rabbit buck before being exposed to C. afer. From the results, serum testosterone of the bucks in all the groups showed no significant difference (P>0.05) between the treatment groups. The results of the effect of C. afer on semen characteristics of buck rabbit are shown in Table 3. There was no record of the semen characteristics of buck in treatment C because the bucks were so weak that they could not mount. The statistical analysis of the motility and sperm count was significantly different (P<0.05) between the treatment group.

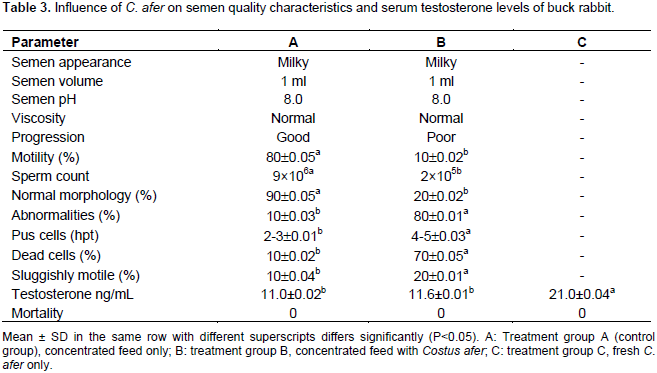
Treatment group A (80%, 9×106) was significantly better (P<0.05) in semen motility and sperm count than group B 10% (2×105). Other semen parameters: semen appearance (normal), semen volume (1 ml) and viscosity (normal) were the same for both groups. Progression was good in treatment group A and poor in group B. Normal morphology was significantly (P<0.05) better in treatment group A (90%) than group B (20%). Effect on semen abnormalities was significantly (P<0.05) higher in group B (80%) than in group A (20%). Pus cells were more in group B (4 to 5 hpt) than group A (2 to 3). Dead cells were significantly (P<0.05) more in group B (70%) than group A (10%). Percentage of sluggish motile sperm cells was significantly higher in group B (20%) than group A (10%). Serum testosterone was significantly (P < 0.05) higher in treatment group C (21.0 Mg/mol) than in groups B (11.6 Mg/mol) and A (11.0 Mg/mol), respectively. No mortality was recorded in all the groups.
The semen status of rabbits is a mixture of spermatozoa suspended in a liquid medium secreted at different locations by the epididymis and at various glands. Its analysis or evaluations provide index for prediction of fertility status in animals (Quintero-Moreno et al., 2007; Ajayi et al., 2011). C. afer is one of the several tropical medicinal plants used against various ailments. The effect of the treatments on semen pH, appearance and volume is within the normal range according to International Rabbit Reproduction Group (2005). The treatment dependent depressed influence (P> 0.05) of C. afer on sperm motility, sperm count and morphology between the treatment groups although within normal levels of fertilizable semen status of rabbit bucks (International Rabbits Reproduction Group 2005), may indicate that C. afer have a negative influence on sperm fertility index (motility, sperm count, and morphology). The reduction of sperm count, mal-formation of spermatozoa, and insufficient motility have been reported as the leading cause of fertility failure or infertility in male (Chauhan and Dixit, 2008).
This result is similar to the findings of Rao and Alice (2001) who reported the antifertility effect of Phylantus amarus at dosage level of 100 mg/kg body weight of male and female mice which was attributed to changes in hormonal profile that regulates reproductive function in animals. Similarly, Adedapo et al. (2003) reported different degrees of testicular degeneration and reduction in diameter of seminiferous tubular of rats treated with extracts of P. amarus. On the contrary, Arhoghro et al. (2014) reported that C. afer leaf extract can be applied for the enhancement of immune system and treatment of serum cholesterol concentration which is a vital precursor in steroidal hormonal secretion. This was evidenced on the significant impact it had on testosterone concentration recorded in treatment group C (21.0) and this may be suggestive of the fact that its reproductive enhancement activity could be on tissues such as testes and prostate as well as promoting secondary sexual characteristics as it is major role of testosterone in determining fertility status in animals.
However, C. afer treatment in this study was observed to increase serum testosterone levels (C: 21.0 nmol/L). This is in line with previous studies that reported increased testosterone profile in animals treated with herbal plants (Chen et al., 1993). Testosterone is a steroid hormone secreted from Leydig cells of the testes in males and it play critical role in the reproductive function and promotion of secondary sex characteristics such as muscle growth and strength, bone mass, and growth of body hairs (Zouboulis and Degitz, 2004). Chen et al. (1993) observed that Chinese yam increases testosterone levels in animals. Yahaya and Ajuogu (2014) exposed adult rabbits to Laguclaria racemosa leaves (white mangrove) and revealed significant increase in testosterone production. It is therefore suggested that graded levels of the test plant be investigated to determine the levels at which it affects other areas of male reproduction such as sexual behavior, reproductive hormonal profile, etc.
The authors have not declared any conflict of interests.
REFERENCES
|
Adedapo AA, Abatan MO, Akinloye AK, Idowu SO, Olorunsogo OO (2003). Morphometric and histopathological studies on the effects of some chromatographic fractions of Phyllanthus amarus and Euphorbia hirta on the male reproductive organs of rats. J. Vet. Sci. 4:181-185.
|
|
|
|
Ajayi FO, Agaviezor BO, Ajuogu PK (2011). Semen characteristics of three strains of local cock in the humid tropical environment of Nigeria. Int. J. Anim. Vet. Adv. 3(3):125-127.
|
|
|
|
|
Ajuogu PK, Ajayi FO (2010). Breeding responses of New Zealand white does to artificial insemination under humid tropical environment. Anim. Prod. Res. Adva. 6(1):46-48.
|
|
|
|
|
Akpan MM, Odeomena CS, Nwachukwu CN, Danladi B (2012). Antimicrobial assessment of ethanolic extract of Costus afer Leaves. Asian J. Plant Sci. Res. 2(3):335-341.
|
|
|
|
|
Arhoghro EM, Berezi EP, Prohp TP (2014). Phytochemical Constituents and Effect of Combined Ethanolic Leaf Extract of Costus afer and Cleome Rutidosperma on Lipid Profile and Some Hematological Parameters in Westar Rats. Int. J. Curr. Microbiol. App. Sci. 3(5):673-679.
|
|
|
|
|
Asuquo OR, Oko OO, Brownson ES, Umoetuk GB, Utin IS (2013). Effects of ethanolic leaf extract of Spondias mombin on the pituitary gonadal axis of female Wistar rats. Asian Pacific J. Reprod. 2(3):169-173.
Crossref
|
|
|
|
|
Burkil HM (1985). The useful plants of West Tropical Africa. Daizials, JM Royal Botanical Gardens Kew2 ed. Families A-I. 1:135-191.
|
|
|
|
|
Chauhan NS, Dixit VK (2008). Spermatogenic activity of rhizomes of Curculigo orchioides Gaertn in male rats. Int. J. Appl. Res. Nat. Product 1(2):26-31.
|
|
|
|
|
Chauhan NS, Rao CV, Dixit VK (2007). Effect of Curculigo orchioides rhizomes on sexual behaviour of male rats. Fitoterapia 78:530-534.
Crossref
|
|
|
|
|
Chen JY, Zhang YY, Wu Q (1993). Effect of jingui shenqi pills on sex hormone in aged rats. Zhongguo Zhong. Yao Za Zhi 18:619-640.
|
|
|
|
|
Duncan DB (1955) Multiple Range and Multiple F-test Biometric ewe. Acta Vet. Scand. 16:145.
|
|
|
|
|
Edeoga HO, Okoli BE (2000) Chromosome numbers of Costus lucrnusianus (costaeae) in Nigeria Folia. Geobotanica 35:315-318.
Crossref
|
|
|
|
|
Egba SI, Sunday GI, Anaduaka EG (2014). IOSR J. Pharm. Biol. Sci. 9(3):61-64
|
|
|
|
|
Etukudo I (2003). Ethnobotany, Conventional and Traditional uses of Plants (Vol. 1 1st edition). Verdict Press, Uyo, Nigeria. pp. 257- 262.
|
|
|
|
|
Gamache PH, Acworth IN (1998). Analysis of phytoestrogens and polyphenols in plasma, tissue and urine using HPLC with coulometric array detection. Proc. Soc. Exp. Biol. Med. 217:274-280.
Crossref
|
|
|
|
|
Herbert U, Adejumo DO (1995). Construction and evaluation of an artificial vagina for collecting rabbit semen. Delta Agric. 2:99-108.
|
|
|
|
|
Ijioma SN, Okafor AI, Ndukuba PI, Akomas SC (2014). Hypoglycemic, hematologic and hypolipidemic activity of Jatropha tanjorensis ethanol leaf extract in alloxan induced diabetic rats. Annals Biol. Res. 5(9):15-19.
|
|
|
|
|
International Rabbit Reproduction Group (2005). Guidelines for the handling of rabbit bucks and semen World Rabbit Sci. 13:147-164.
Crossref
|
|
|
|
|
John AO (2004). Evaluation of the analgesic, anti-inflammatory and anti-diabetic properties of Sclerolarya birea (A. Rich) Hochst. Stembark aqueous extract in Mice and Rats. Phytother. Res. 18:601.
Crossref
|
|
|
|
|
Koumanov F, Bozadjieva E, Andreeva M, Platonva E, Ankov V (1982). Clinical trial of Clinical trial of Tribestan. Exp. Med. 1:2-4.
|
|
|
|
|
Momoh S, Yusuf OW, Adamu MM, Agwu COC, Atanu FO (2011). Evaluation of the Phytochemical Composition and Hypoglycaemic Activity of Methanolic Leaves Extract of Costus afer in Albino Rats. British J. Pharm. Res. 1(1):1-8.
Crossref
|
|
|
|
|
Oliver-Bever B (1986). Medicinal plants in Tropical West Africa. Cambridge. pp. 198-199.
Crossref
|
|
|
|
|
Olowosulu AK, Ibrahim YKE (2006). Studies on the antimicrobial screening of Aqueous extracts of five plants used in Folk medicine in Nigeria. West Afr. J. Biol. Sci. 3(5):21-26.
|
|
|
|
|
Omokhua GE (2011). Medicinal and Socio-Cultural Importance of Costus Afer (Ker Grawl) in Nigeria. Int. Multidisci. J. Ethiop. 5(5):22; 282-287
Crossref
|
|
|
|
|
Quintero-Moreno A, Rigaul T, Rodriguez-Gil JE (2007). Multivariate cluster analysis regression procedures as tools to identify motile sperm subpopulations in rabbit semen and to predict semen fertility and litter size. Reprod. Dem. Anim. 42:3l2-3l9
Crossref
|
|
|
|
|
Rao MV, Alice KM, (2001). Contraceptive effects of Phyllanthus amarus in female mice. Phytother. Res. 15:265-267.
Crossref
|
|
|
|
|
Singh A Singh SK (2009). Evaluation of antifertility potential of Brahmi in male mouse. Contraception 79(1):71-79.
Crossref
|
|
|
|
|
Soladoye MO Oyesika OO (2008). Textbook of Medicinal Plants from Nigeria, pp. 628, University of Lagos Press, Nigeria.
|
|
|
|
|
Steel RGD, Torrie JH (1980). Principles and Procedures of Statistics 2nd edi., McGraw Hill Books Co. New York.
|
|
|
|
|
Tchamgoue AD, Tchokouaha LRY, Tarkang PA, Kuiate J, Agbor GA (2015). Costus afer Possesses Carbohydrate Hydrolyzing Enzymes Inhibitory Activity and Antioxidant Capacity In Vitro. Evidence-Based Complementary and Alternative Medicine, 10.
Crossref
|
|
|
|
|
Tomova M, Gjulemetova R, Zarkova S, Peeva S, Pangarova T, Simova M (1981). Steroidal saponins from Tribulus terrestris L. with a stimulating action on the sexual functions. Int. Conf. Chem. Biotechnol. Biol. Act. Nat. Prod. (Proc) 3:298-302.
|
|
|
|
|
Ukpabi CF, Agbafor KN, Ndukwe OK, Agwu A, Nwachukwu SN (2012). Phytochemical composition of Costus afer Extract and its alleviation of carbon tetrachloride – induced hepatic oxidative stress and toxicity. Int. J. Modern Botany 2(5):120-126.
Crossref
|
|
|
|
|
Vasudeva N, Sharma SK (2007). Post-coital antifertility activity of Hibiscus rosa sinensis Linn. Roots. Oxford J. 5(1):91-94.
|
|
|
|
|
Yahaya MA, Ajuogu PK (2014). Response of Rabbits Testosterone and Estrogen Status to Graded levels of White Mangrove Plant (Langucularia racemosa). Int. J. Sci. Nat. 5(2):196-198.
|
|
|
|
|
Yahaya MA, Wekhe SN Ukpai1 OO Ajuogu PK, Ndor L (2015). Response of rabbit's testosterone and estrogen status to graded levels of white mangrove plant (langucularia racemosa). Global J. Animal Scientific Res. 3(2):487-490.
|
|
|
|
|
Zouboulis CC, Degitz K (2004). Androgen action on human skin -- from basic research to clinical significance. Exp. Dermatol. 13(4):5-10.
Crossref
|
|