Propionibacterium acnes is an anaerobic, Gram positive bacterium that belongs to the normal microflora.
P. acnes play an important role in the pathogenesis of various skin infections and diseases (Purchiaroni et al., 2013, Leheste et al., 2017). It lives primarily on, among other things,
fatty acids in
sebum secreted by sebaceous glands in the
follicles (Beylot et al., 2014). This bacterium is largely
commensal and just largely detectable on the skin of healthy preadolescents. Among different types of microbial populations,
P. acnes is the predominant member in the skin areas of back, face and chest and may also be found throughout the
gastrointestinal tract in humans (Aiyelaagbe et al., 2007) and many other animals. Its populations cover 50% of the human skin normal flora; however, the number of populations differs in different parts of the body. It ranges from less than 10 cells/cm
2 on the nose to 107 cells/cm
2 on the human facial skin. Besides, this bacterium may act as a skin pathogenic microorganism, which may lead to different skin diseases like acne vulgaris (Behazadi et al., 2016) which is a chronic inflammatory skin condition classified by the Global Burden of Disease Study as the eighth most prevalent disease worldwide. Acne develops as a result of increased sebum production; hyperkeratinisation, increase in
Propionibacterium acnes, and inflammation (Omer et al., 2017).
The inflammation and over-production of sebum caused by P. acnes can be overcome by topical and systemic antibiotic therapy. However, due to side effects like rashes, swelling, redness, irritation, dizziness and more importantly antibacterial resistance etc., this therapy needs to be replaced by some alternative solutions (JoÅ„czyk-Matysiak et al., 2017). Due to the increasing failure and rapid development of multi resistant bacterial strains of clinically important medical pathogens required, the development of newer antimicrobial agents comes with broadened horizons (Achermann et al., 2014). Therefore, plant based preparations have attracted attention for the treatment of bacterial diseases (Farnsworth, 1966; Julianti et al., 2017). Historically, plants have always remained a source of inspiration for novel drug compounds, as plant derived medicines have made large contributions to human health and well-being. Their role is two-fold in the development of new drugs; they may become the lead for the development of a medicine and a phytomedicine to be used for the treatment of diseases (Gilani et al., 2010). Traditional medicine using plant extracts continues to provide health coverage for over 80% of the world’s population, especially in the developing world. Many herbs comprise remarkable properties and functions on multiple biochemical pathways capable of controlling several organ systems simultaneously.
Nowadays, majority of scientist are intensifying their research on various herbs or combination of herbs. These plants are supposed to have extraordinary, unique, antimicrobial activity. For example different parts of various plants are being used for treatment of skin infections such as acne caused by P. acnes. Herbs are selected and combined for their ability to inhibit microbial growth in various part of the body and support organ systems responsible for detoxification and immune function. Phytoconstituents such as flavonoids, alkaloids, tannins and triterpenoids, obtained from medicinal plants challenges the modern medicine and stimulating opportunity for the expansion of modern chemotherapies against wide range of microorganisms (Gorle and Patill, 2010; Gupta et al., 2008; Altemimi et al., 2017). Less availability and unaffordable cost of new generation antibiotics initiated the search for alternative phytomedicine with claimed antimicrobial activity. The extractable bioactive compounds in medicinal plants are a significant alternative approach to synthetic antibiotics, which could be used as valuables in human disease management. Many herbs with significant antimicrobial activity have been reported in different traditional literatures (Hay and Adriaans 2004; Iwu, 1993; Farhat et al., 2013).
The current work is aimed at showing the step by step evaluation of four antiacne plants on the basis of indigenous literature to find the effective drug source against P. acnes followed by bioactivity guided fractionation. The objective of this work is to find out the most effective anti P. acnes extract amongst the four selected plants (Rubia cordifolia, Tephrosia purpurea, Viola tricolor, Serenoa repens), and to obtain the characterized fraction of the effective extract with highest anti P. acnes potential. Manjistha (R. cordifolia) can be proven to be a best remedy to cure acne, due to its Varnya, Raktashodhak, Vishaghna, Rasayana, Krimighna properties. It is an Ayurvedic herb mentioned in Charaka samhita as varnya and vishaghna and in Sushruta samhita it is categorized as pittasam samana. Manjistha (R. cordifolia) can be proven to be a best remedy to cure acne, due to its Varnya, Raktashodhak, Vishaghna, Rasayana, Krimighna properties (Meena, 2015). Traditionally, the plant and its leaves are used to treat skin diseases, bacterial infections, snake bite, antioxidant, etc. Review of scientific literature suggests that plant extract has anti-cancer, anti-inflammatory and anti-acne potentials. The hydro-alcoholic extract of the leaves has been proven for anti P. acnes activity, with results more prominent in comparison to Clindamycin. In another study the methanolic extract significantly exhibited anti P. acnes activity (Gorle and Patill, 2010).
T. purpurea is the plant which is traditionally used to cure blood related disorders, bronchitis, boils, pimples and bleeding piles (Negi et al., 2015). In Ayurveda, various parts of the plant is used to treat impotency, tumour, pimple, asthma etc (Upadhyay et al., 2010). The leaves and its various extracts have been reported for anti bacterial (Gupta et al., 2008; Nasri et al., 2015), anti-inflammatory, analgesic and antioxidant potentials. The methanolic extract of the whole plant is reported to be effective against gram +ve and gram -ve microbes (Jayaweera, 1982). Viola (V. tricolor) has traditionally been used as a topical home remedy for skin conditions like eczema and acne. In Ayurvedic terms viola is a blood-cleanser herb. The dried aerial parts of the viola are used in natural medicine preparations as it contains a number of beneficial polyphenols, including salicylic acid - a known antimicrobial used in many homeopathic and commercial acne treatment products (ESCOP, 2009 Monographs: the Scientific Foundation for Herbal Medicinal Products). It is contemporarily used as a remedy for various ailments of skin since ancient times.
In Bulgaria it is used for the treatment of cough, skin infections including acnes, dermatitis; and in Italy it is used to treat psoriasis (Witkowska et al., 2005; Nasri et al., 2015). The ethanolic extract of the whole plant displayed significant inhibitory activity against Staphylococcus epidermidis, Staphylococcus aureus, Candida albicans, Bacillus cereus, and P. acnes (Walter et al., 2011). S. repens is traditionally used in several forms for the management of several skin diseases and bacterial infections. Crude S. repens extract was used by European and Americans for at least 200 years for the treatment of asthma, cough, tubercular laryngitis, weakness, bacterial infections, skin diseases, etc. When the Saw palmetto extract was combined with short acting antibiotic (Prulifloxacin), the extract showed increased efficacy of the therapy in chronic bacterial prostatitis tested in 210 patients. The extract has also been reported to possess anti-inflammatory activity (Ray et al., 2013). The objective of this work is to find out the most effective anti P. acnes extract amongst the four selected plants, and to obtain the characterized fraction of the effective extract with highest anti P. acnes potential.
Collection of samples
The plant material was freshly collected in the month of October from their natural habitats. R. cordifolia (leaves), S. repens (leaves) and V. tricolor (whole plant) were collected from Haldwani (Uttarakhand) and authenticated by Dr. S. K. Srivastava, Scientist D/HOO, Botanical Survey of India (B.S.I), Dehradun (Ref. No. BSI/NRC/TECH.(Ident.)/2013-2014/1032). The T. purpurea (leaves) were procured from Tirupati, Andhra Pradesh and authenticated by Dr. K. Madhava Chetty, Associate Professor, Department of Botany, Sri Venkateshwara University, Tirupati, Andhra Pradesh, India (Voucher specimen No. 1246). P. acnes Strain (MTCC 1951) was procured from (MTCC) IMTECH, Chandigarh.
Preparation of extracts and fractions
The plant materials were dried and ground to a coarse powder. The ground plant powder (300 g) was extracted with 80% methanol using a Soxhlet extractor for 48 h. The extract was filtered through Whatman filter paper, concentrated under reduced pressure, transferred to pre weighed China dish and stored in vacuum desiccators until constant weight was obtained. The antimicrobial screening of the extracts was laid down in order to obtain the purified mixture or potent compound with highest anti P. acnes activity. The extracts (R. cordifolia and T. purpurea) with highest anti P. acnes activity were successively partitioned with petroleum ether, ethyl acetate, chloroform, n-butanol and water separately. The fractions of these two potent antiacne extracts were subjected to antimicrobial testing using serial dilution method to ascertain the most effective fraction against P. acnes. The petro-ether fraction of R. cordifolia showed the highest activity. The fraction (100 mg) was then charged into column containing silica gel G as a stationary phase. The column was allowed to run with different mobile phases beginning from ethyl acetate to methanol for the purification of the fraction. This resulted in five different sub-fractions named A, B, C, D, E and F. These sub-fractions were further evaluated for anti P. acnes activity through disk diffusion method. Among them, sub-fraction D showed the most potent activity hence it was further fractionated by sub column which resulted into six sub-fractions (1, 2, 3, 4, 5 and 6) of D sub-fraction. These subfractions 1 to 6 were evaluated for their potential against P. acnes and the most potent sub-fraction 4 was characterized using chemical and TLC fingerprinting profile (Lutterodt et al., 1999).
Preparation of inoculums
Stock cultures were maintained at 4°C on slants of nutrient agar. Active cultures for experiments were prepared by transferring a loop full of cells from the stock cultures to test tubes of nutrient agar medium and were incubated without agitation for 24 h at 37°C. The cultures were diluted with fresh nutrient agar broth to achieve optical densities corresponding to 2.0 × 106 (Cfu) colony forming units for bacteria.
Antimicrobial susceptibility test
All the extracts and fractions were screened against P. acnes (MTCC, 1951), obtained from IMTECH, Chandigarh (India). The disk diffusion method was used to test the antibacterial activity of the plant extracts. Sterilized nutrient agar medium (20 ml) were poured for each bacterium into each sterilized Petri dish. The plates were allowed to solidify for 5 min and inoculums suspension was swabbed uniformly. The entire agar surface of each plate was inoculated with this swab, first in horizontal direction then in vertical direction, which ensures the uniform distribution of organism over the agar surface (Jones, 1996). The filter paper disks (6 mm in diameter) loaded with 1 and 10 mg/ml of solution prepared by using dry extract were placed on the surface of bacteria seeded agar plates; the compound was allowed to diffuse for 5 min and then the plates were incubated at 37°C for 24 h. Apart from this, Hexa-G antibiotic discs constituting six various antibiotics were used as standards. At the end of incubation, inhibition zones formed around the disk were measured with Hi-media zone measuring ruler. These studies were performed in duplicates.
Minimum inhibitory concentration (MIC)
For measurement of MIC, fresh cultures were grown in 5 ml nutrient broth tubes. Tubes were impregnated with 3, 5, 10, 15, 20 and 25 µg/ml of fractions of R. cordifolia. Each of the tube was inoculated with 0.1 ml of freshly growing cultures. Uninoculated tubes were kept as negative control and tube inoculated with bacteria and antibiotic clindamycin was considered as positive control. Tubes were incubated at 37°C with growth of cultures observed at 24 h and after 48 h of incubation. Minimum concentration that shows the minimum growth was taken as MIC (Kaur et al., 2010).
Determination of MIC
The R. cordifolia fractions were thereafter evaluated to determine MIC value. The broth dilution method was adopted by using 0.5% DMSO for diluting the fractions and was further incubated for 48 h.The minimum dilution of the plant fraction as regards killing of the microbes was observed.
Phytochemical analysis of fractions
The plant fractions were phytochemically screened using standard method. The fraction D and sub-fraction-4 were subjected to preliminary phytochemical screening for the detection of various phytoconstituents such as alkaloids, flavonoids, saponins, steroids and terpenoids (Koduru et al., 2006).
TLC finger printing profile
The thin-layer chromatography (TLC) finger printing profile of anti P. acnes fraction-4 was discovered for the identification/standardization purpose of active fraction. The mobile phase system comprised of toluene: ethyl acetate (8:2 v/v), was used.
Antibacterial activity
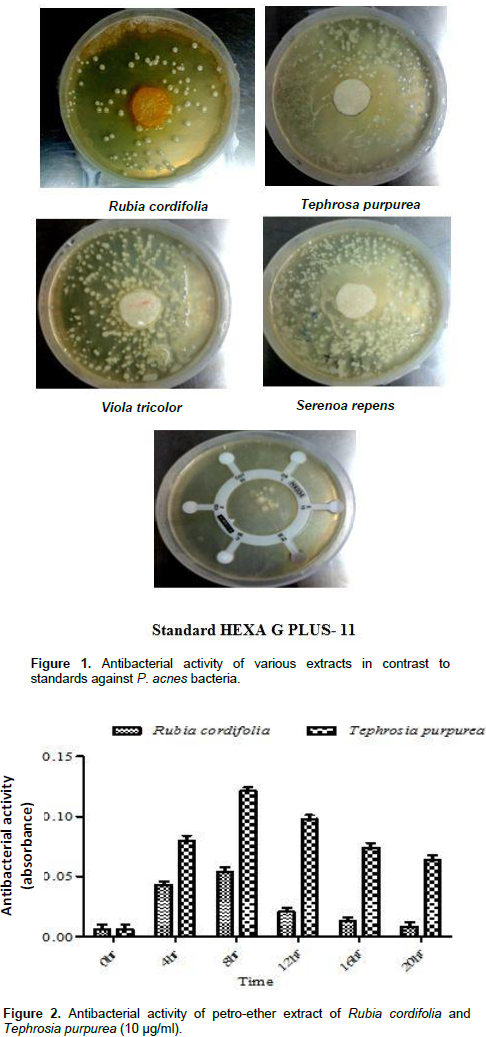
The antibacterial activity was determined by measuring the diameter of zone of inhibition recorded. The extract of the plant R. cordifolia was found to have maximum antibacterial activity in comparison to other three plants, that is, T. purpurea, V. tricolor and S. repens. The results obtained in the evaluation of the antibacterial activity of the different extracts against P. acnes are listed in Figure 1 and Table 1. The extracts had shown zone of inhibition against bacteria P. acnes but methanolic extract of R. cordifolia had shown maximum zone of inhibition against bacteria, that is, 14.0 and 24.0 mm in concentrations 1 and 10 mg/ml, respectively. Petroleum ether fraction showing the most potent antimicrobial activity in different dilutions with time interval of 4 h is listed in Figures 2 and 3. The sub fraction D that had shown highest anti P. acnes activity with zone of inhibition 26.0 and 32.0 mm at concentrations 1 and 10 mg/ml, respectively, are listed in Table 2. The fraction D was further fractionated with the help of sub-column. Six different fractions were collected out and evaluated for antibacterial activity. The results thus obtained were similar to that of fraction D and is listed in Table 3. The results for the phytochemical screening and TLC fingerprinting profile are listed in Tables 4 and 5. The minimum inhibitory concentration of sub fraction -4 was 3 µg/ml is listed in Table 6.




Minimum inhibitory concentration
The MIC of subfraction 4 was observed lowest among all the selected sub fractions, it was found to be 3 µg/ml.
The results obtained in this study indicate a considerable difference in antimicrobial activity between various extracts obtained which was determined by recording diameter of zone of inhibition. The activity of the plant R. cordifolia was more pronounced against the P. acne in comparison to other three plants, that is, T. purpurea, Viola tricolor and S. repens as listed in Figure 1 and Table 1. Till this stage, the anti-bacterial compounds of the plants assayed are not well known; however, the presence of flavonoids and terpenes and a certain degree of antibacterial compounds might be observed. Later, various extracts of R. cordifolia specifically petro-ether, chloroform, ethyl acetate and water were analysed for antibacterial activity and it has been observed that all the extracts had shown zone of inhibition against bacteria P. acnes but petro ether fraction showed the most potent antimicrobial activity in different dilutions with time interval of 4 h as listed in Figures 2 and 3. Furthermore, when petro-ether extract was further purified in a charged column, five sub fractions named as A, B, C, D and E were collected and their antimicrobial activity have been evaluated. The results showed that fraction D has maximum zone of inhibition, that is, 26.0 mm and 32.0 mm in the concentrations of 1 mg/ml and 10 mg/ml respectively. This anti P. acnes activity may attribute to the ability of the fraction to inhibit protein synthesis (Zhou et al., 2012), cell wall or nucleic acid synthesis (Gilani et al., 2010; Kaur et al., 2010).
The fraction D was further purified with the help of sub-column. This purification resulted in the collection of six different fractions which were further evaluated for antibacterial activity. The results thus obtained were similar to that of fraction D listed in Table 3. When fraction-D was sub-fractionated, a decrease in antimicrobial activity has been observed which sucggests that later purification is not required. The higher activity of fraction D may be the result of complexes of components of the plant, relative to that of the sub fractions. Our finding is in agreement with the finding of Witkowska et al. (2015) which reported the higher antibacterial potential of plant extract in the comparison with purified fraction. As the extract may comprise compounds of different polarity in comparison to purified fraction, that might be the reason for suggesting a synergism in antibacterial action between compounds of plants (Witkowska et al., 2005); otherwise, there will be portioning or purification. Moreover, due to the potent fraction (fraction-D) characterized by chemical and chromatographic methods, the anti P. acnes activity is supposed to be due to the presence of anthraquinone and flavones which are chief constituents of the plant (Lee et al., 2006). The above result opens the possibility of finding new clinically effective anti P. acnes drug and could be useful in understanding the relationship between traditional cures and current medicines.
The study concludes that methanolic extract of R. cordifolia leaves has significant anti P. acnes activity. Furthermore, the fractionation of extract enhanced the anti P. acnes potential. The most potent fraction was characterized by chemical and chromatographic methods. The results of qualitative analysis of sub fraction confirmed the presence of anthraquinone and flavones which may be responsible for anti P. acnes activity of the sub-fraction. The above result opens the possibility of finding new clinically effective anti-acne drug and could be useful in understanding the relationship between traditional cures and current medicines.
The authors express sincere thanks to the management and Shri. Parveen Garg, Chairman, ISF College of Pharmacy, Moga, Punjab, India, for providing necessary facilities.