Full Length Research Paper
ABSTRACT
Esophagectomy is a common standard treatment of localized disease. However, local recurrence frequently occurs in patients with advanced esophageal squamous cell carcinoma (ESCC) after operation, resulting in the need for using biomarkers to evaluate recurrence in patients with advanced ESCC during postoperative therapies. This study examined serum matrix metalloproteinase-9 (MMP-9) as a prognosis factor in recurrent patients with advanced ESCC after curative esophagectomy followed by chemotherapy or concurrent radiotherapy. During therapies, patients with recurrent tendencies always have low serum MMP-9 levels compared with those before treatment. For recurrent patients, a difference in recurrence-free survival rate is significant between MMP-9 ≥ 635 ng/mL and MMP-9 < 635 ng/mL before treatment (P < 0.05). Although serum MMP-9 is a negative prognostic factor for patients with recurrence tendency and cannot directly predict recurrence, low serum MMP-9 levels before treatment and after therapies still indicate high recurrence-free survival rate in patients with locally advanced resectable ESCC after chemotherapy or concurrent radiotherapy.
Key words: Matrix metalloproteinase-9, esophageal squamous cell carcinoma, recurrence, chemotherapy, survival.
INTRODUCTION
Esophageal squamous cell carcinoma (ESCC) is a common histological subtype of esophageal cancer that occurs primarily in Eastern Asia and Eastern and Southern Africa (Torre et al., 2015). Chemotherapy or chemoradiotherapy is recommended as an adjuvant therapy following surgery due to metastasis and recurrence frequently occurring in patients with advanced ESCC (Lordick et al., 2016). The prognoses and times to death are similar in patients with node-negative and node-positive superficial ESCC once recurrence occurs (Ozawa et al., 2016). Therefore, patients with ESCC and high risk of recurrence after esophagectomy should receive additional chemoradiotherapy (Xue et al., 2018). Although advance therapies have improved the survival rate, the local recurrence remains high, leading to low cure rate (Brooks?Brunn, 2000). The late emergence of symptoms and the insensitivity and nonspecificity of biomarkers become challenges for prognostic prediction in patients with advanced ESCC (Siegel et al., 2017). Therefore, new sensitive biomarkers for ESCC prognostic prediction are urgent in patients with advanced ESCC after treatments.
Matrix metalloproteinase-9 (MMP-9) is a class of enzymes related to cancer pathogenesis because of its involvement in the degradation of extracellular matrix (Peisker et al., 2017). MMP-9 participates in tumorigenesis, and its high expression indicates poor prognosis in ESCC (Li et al., 2013; Gu et al., 2005). Serum MMP-9 is an effective biomarker in the diagnosis and prognosis of ESCC (?ukaszewicz-Zajac et al., 2009; Mroczko et al., 2008). Esophagectomy is the most common standard treatment for localized ESCC. However, recurrence rate remains high (Alidina et al., 2004). Although surgical resection is a potential mainstay for curable ESCC, locoregional recurrence is up to 23.8 to 58.0% of cases (Miyata et al., 2011; Lu et al., 2013; Guo et al., 2014). Therefore, the correlation of serum MMP-9 with recurrence in patients with locally advanced resectable ESCC should be determined.
This study investigates the prognosis of serum MMP-9 in recurrent patients with advanced ESCC after curative esophagectomy followed by chemotherapy or concurrent radiotherapy and evaluates the potential of serum MMP-9 as a recurrence predictor in patients with locally advanced resectable ESCC.
MATERIALS AND METHODS
Patients
From January 2012 to June 2016, 173 patients (stages III and IV) with advanced ESCC confirmed by histopathology and who underwent R0 resection were enrolled at Jiangsu Cancer Hospital (Nanjing, China). This study was performed in accordance with the Declaration of Helsinki and approved by the Biomedical Research Ethics Committee of Jiangsu Cancer Hospital. All participants provided informed consent.
Treatment modality
At the beginning, patients did not receive any treatment (before treatment). Then, all patients received chemotherapy at four different intervals, with about 1 to 2 months interval. A total of 57 patients received concurrent radiotherapy at the first course of chemotherapy with radiotherapy schemes, such as GTV60-65Gy/28-33f, CTV50-55Gy/28-33f and PTV50-66Gy/28-33f (after therapies). Chemotherapy was consistent with the previous description and included taxane combined with platinum, 5 fluorouracil and its derivatives combined with platinum, and gemcitabine combined with platinum (Ye et al., 2020). Five months after the end of chemotherapy, 57 patients had recurrence at the original lesion or metastasis, including lymph node and distal metastases, and classified as recurrent patients (recurrence patient group). Others were classified as nonrecurrent patients (nonrecurrence patient group). The recurrence patient group received further treatments, including chemotherapy or concurrent radiotherapy, again.
Serum samples and serum MMP-9 detection
Blood samples before and after chemotherapies (that is, the 1st, 2nd, 3rd and 4th cycles of treatment) were collected. The samples of recurrence patients were still collected at recurrence (re-0 cycle) and at course of further treatment (re-1 and re-2 cycle). Samples were stored at -80°C after centrifugation.
Human cytokine/chemokine panel (that is, MMP-9) was purchased from Millipore (CAT no. HMMP2MAG-55K-01; Millipore, USA) and performed in accordance with the manufacturer’s instructions. Serum MMP-9 level was determined using the Luminex FLEXMAP 3D instruments and software supplied by Luminex Corporation (Austin, USA). The preparation of blood samples, setting of detection parameters, and calculation of serum MMP-9 concentrations were conducted in accordance with a previous study (Ma et al., 2017).
Statistical analysis
The concentrations of MMP-9 in serum were presented as mean ± SD. P < 0.05 indicated significance. Differences between two groups were analyzed using unpaired t-test. Comparisons among three or more groups were performed by ANOVA followed by pairwise comparisons by using the Bonferroni post hoc test. The follow-up ended on April 25, 2020, and the overall survival (OS) was calculated. Recurrence-free survival rates were calculated from the date of operation to the date of recurrence via the Kaplan-Meier method, and the significance of comparisons between groups was measured through the log-rank test. Cutoff values for OS and recurrence-free survival of serum MMP-9 were assessed by the receiver operating characteristic (ROC). The area under curve (AUC), sensitivity, and specificity were calculated. Statistical analyses were performed using a commercially available statistical software (that is, GraphPad Prism 5).
RESULTS
Serum MMP-9 decreased in recurrent and nonrecurrent patients with locally advanced resectable ESCC after therapies
The clinical characteristics of 173 patients with locally advanced resectable ESCC are detailed in Table 1. Serum MMP-9 levels were approximately equal between recurrent and nonrecurrent patients before treatment (P > 0.05, Figure 1A). However, serum MMP-9 levels significantly decreased in 116 nonrecurrent and 57 recurrent patients with locally advanced resectable ESCC after therapies compared with those before treatment (P < 0.001, Figure 1B). Furthermore, 57 recurrent patients maintained lower serum MMP-9 levels at recurrence than those before treatment (P < 0.001, Figure 1C). Serum MMP-9 levels did not increase until the third cycle of treatment in recurrent and nonrecurrent patients with locally advanced resectable ESCC after therapies (Figure 2A). Compared with that at recurrence, serum MMP-9 levels no longer decreased in recurrent patients during further treatments (P > 0.001, Figure 2B). No difference in serum MMP-9 level after therapies was observed between recurrent patients treated with chemotherapy and concurrent radiotherapy. However, nonrecurrent patients treated with concurrent radiotherapy had low serum MMP-9 than those treated with chemotherapy (P < 0.05, Figure 2C). Nonrecurrent and recurrent patients treated with concurrent radiotherapy at the 2nd, 3rd, and 4th cycles of treatments had significantly decreased serum MMP-9 levels compared with those before treatment (P < 0.001, Figures 2D and 2E). Nonrecurrent patients treated with chemotherapy had significantly lower serum MMP-9 levels at the 2nd, 3rd and 4th cycles of treatments than those before treatment (P < 0.001), whereas the opposite was observed in recurrent patients (P > 0.05, Figures 2D and E).


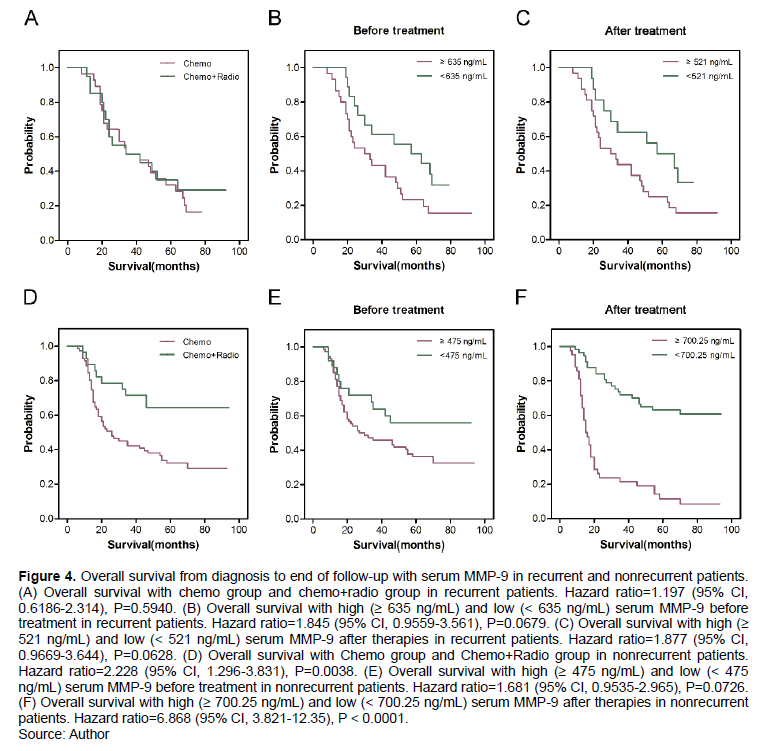
Only non-recurrent patients with low serum MMP-9 had long survival time
The OS values of recurrent and non-recurrent patients were approximately equal (P > 0.05, Figure 3A). However, for recurrent patients, serum MMP-9 ≥ 980 ng/mL at recurrence, MMP-9 ≥ 521 ng/mL after treatment, and MMP-9 ≥ 521 ng/mL after further treatment were not independent factors for OS (P > 0.05; Figure 3B to D). Furthermore, in recurrent patients with locally advanced resectable ESCC, concurrent radiotherapy did not prolong OS compared with chemotherapy (P > 0.05, Figure 4A). Recurrent patients with serum MMP-9 < 635 ng/mL before treatment and MMP-9 < 521 ng/mL after therapies had long OS. However, serum MMP-9 ≥ 635 ng/mL before treatment and ≥ 521 ng/mL after therapies were still not determined as independent factors for OS(P > 0.05, Figures 4B and 4C). However, nonrecurrent patients treated with concurrent radiotherapy had significantly longer OS compared with patients treated with chemotherapy (P < 0.05, Figure 4D). Survival times in nonrecurrent patients with MMP-9 ≥ 475 ng/mL and MMP-9 < 475 ng/mL before treatment were not different (P > 0.05, Figure 4E). However, serum MMP-9 ≥ 700.25 ng/mL after therapies was an independent factor for OS in nonrecurrent patients with locally advanced resectable ESCC. Nonrecurrent patients with low serum MMP-9 (< 700.25 ng/mL) had significantly longer OS than those with high serum MMP-9 (≥ 700.25 ng/mL) after therapies (P < 0.001, Figure 4F).
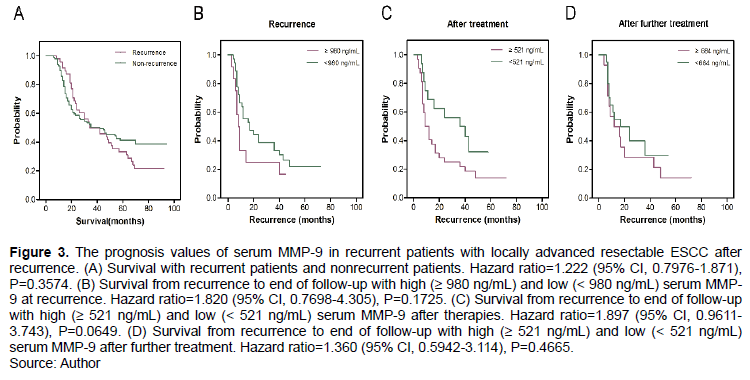
Serum MMP-9 was associated with recurrence-free survival rate in patients with locally advanced resectable ESCC
Among 173 patients with locally advanced resectable ESCC, patients with serum MMP-9 < 577 ng/mL had significantly higher recurrence-free survival rates than those with MMP-9 ≥ 577 ng/mL before treatment (P = 0.0411, Figure 5A). Furthermore, compared with those with MMP-9 ≥ 700.25 ng/mL after therapies, 173 patients with MMP-9 < 700.25 ng/mL had higher recurrence-free survival rates (P < 0.001, Figure 5B). For recurrent patients, the difference in recurrence-free survival rate was significant between MMP-9 ≥ 635 ng/mL and MMP-9 < 635 ng/mL before treatment (P = 0.0483, Figure 5C). However, no significant difference in recurrence-free survival rate was observed between 57 nonrecurrent patients with MMP-9 ≥ 521 ng/mL and MMP-9 < 521 ng/mL after therapies (P = 0.2613, Figure 5D).

DISCUSSION
Radical esophagectomy is regarded as a curative treatment for resectable ESCC in China (Feng et al., 2020). However, many patients develop locoregional recurrence and distant metastasis after surgery (Li et al., 2013; Shen et al., 2017). In this study, although all resectable ESCC patients maintained a low level of serum MMP-9 during therapies including chemotherapy or concurrent radiotherapy, some patients still develop recurrence. It suggests that serum MMP-9 after therapies was not a direct marker of recurrence in resectable ESCC patients treated with chemotherapy or concurrent radiotherapy. However, it does not mean that serum MMP-9 cannot be used as a prognostic factor for patients with resectable ESCC after therapies. After therapies, low serum MMP-9 is beneficial to prolong the survival time of resectable ESCC patients. This result is consistent with the report of Ye et al. (2020) who indicated that serum MMP-9 is a potential prognostic biomarker for response to chemotherapy or concurrent radiotherapy in patients with ESCC. Li et al. (2019) reported that the overexpression of MMP-9 predicts poor prognosis in Kazakh patients with ESCC. In addition, the level of serum MMP-9 before treatment is negatively correlated with the recurrence-free survival rate in patients with resectable ESCC after chemotherapy or concurrent radiotherapy, indicating that for patients with recurrent tendency, high level of serum MMP-9 before treatment is easy to recurrence in a short time.
However, findings about serum MMP-9 in prognosis of esophageal cancer (EC) are controversial. MMP-9 is not a potential biomarker in the prognosis of EC (Mroczko et al., 2008). A meta-analysis indicated that the overexpression of MMP-9 is a potential independent prognosis factor of patients with ESCC in Asia (Zeng et al., 2013). This finding may be because the significance of MMP-9 as a prognostic factor is disaccorded by different patient populations as research subjects. In the present study, these recurrent patients maintain low serum MMP-9 levels during the progress of therapies until recurrence. Although serum MMP-9 < 521 ng/mL after treatment cannot prolong the survival of recurrent patients, serum MMP-9 no longer decreases in recurrent patients who have undergone further treatment after recurrence. The survival time from recurrence to the end of follow-up is not related to serum MMP-9 level at recurrence and after further treatment in recurrent patients with locally advanced resectable ESCC. Low serum MMP-9 before treatment and after therapies cannot prolong the survival time of recurrent patients with locally advanced resectable ESCC, suggesting that serum MMP-9 is a negative prognostic factor for patients with recurrence tendency. However, high MMP-9 levels (≥ 700.25 ng/mL) after therapies are associated with poor prognosis in nonrecurrent patients with locally advanced resectable ESCC.
Esophagectomy is a common standard for the treatment of localized disease. However, local recurrence rate is high in patients with EC after operation (Alidina et al., 2004). Neoadjuvant radiochemotherapy improves the 5-year survival rate in patients with EC (van Hagen et al., 2012; Shapiro et al., 2015). Neoadjuvant chemoradiotherapy followed by surgery prolongs survival time over surgery alone in patients with locally advanced ESCC (Yang et al., 2018). In the present study, 173 patients with advanced ESCC have undergone esophagectomy followed by chemotherapy or concurrent radiotherapy. A total of 57 recurrent patients after cycles of chemotherapy or concurrent radiotherapy are observed. Postoperative nonrecurrent patients who received concurrent radiotherapy have longer survival time than those treated with chemotherapy alone indicating that locally advanced resectable ESCC patients without recurrence obtains benefits from concurrent radiotherapy. Nonrecurrent patients treated with concurrent radiotherapy have maintained lower serum MMP-9 levels than those treated with chemotherapy alone during the progress of treatments. However, serum MMP-9 levels are not significantly low in recurrent patients treated with concurrent radiotherapy compared with those treated with chemotherapy. This finding means that serum MMP-9 is related to survival time and can be used as a potential prognostic factor. Without regard for recurrence, concurrent radiotherapy is a suitable therapeutic regimen for patients with locally advanced resectable ESCC.
This study has limitations. This study is a retrospective analysis based on existing data rather than selecting specific recurrent patients into the group. Therefore, few other risk factors are analyzed when predicting recurrence through serum MMP-9 in patients with locally advanced resectable ESCC. In addition, patients with recurrence at the original lesion or metastasis are classified as recurrence patient group in this study rather than patients with ESCC and local recurrence merely after curative esophagectomy followed by chemotherapy or concurrent radiotherapy.
CONCLUSION
Although serum MMP-9 after therapies is not a direct marker of recurrence, serum MMP-9 level before treatment is negatively associated with recurrence-free survival in locally advanced resectable ESCC patients, suggesting that serum MMP-9 before treatment is a potential risk factor for recurrence in ESCC patients after curative esophagectomy followed by chemotherapy or concurrent radiotherapy.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
ACKNOWLEDGMENTS
The present study was supported by projects of Xuzhou Science and Technology Bureau (KC19183).
REFERENCES
|
Alidina A, Siddiqui T, Burney I, Jafri W, Hussain F, Ahmed M (2004). Esophageal cancer - a review. Journal of Pakistan Medical Association 54(3):136-141. |
|
|
Brooks Brunn JA (2000). Esophageal cancer: An overview. Medsurg Nursing 9(5):248-254. |
|
|
Feng W, Qi Z, Qiu R, Li ZS, Dong SL, Li YK, Hu YP, He M, Wang YX (2020). Risk factors for tumor recurrence in patients with pT3N0M0 thoracic esophageal squamous cell carcinoma after esophagectomy. Journal of International Medical Research 48(12):300060520977403. |
|
|
Gu ZD, Chen KN, Li M, Gu J, Li JY (2005). Clinical significance of matrix metalloproteinase-9 expression in esophageal squamous cell carcinoma. World Journal of Gastroenterology 11(6):871-874. |
|
|
Guo XF, Mao T, Gu ZT, Ji CY, Fang WT, Chen WH (2014). Clinical study on postoperative recurrence in patients with pN0 esophageal squamous cell carcinoma. Journal of Cardiothoracic Surgery 9(1):1-7. |
|
|
Li CL, Zhang FL, Wang YD, Han C, Sun GG, Liu Q, Cheng YJ, Jing SW, Yang CR (2013). Characteristics of recurrence after radical esophagectomy with two-field lymph node dissection for thoracic esophageal cancer. Oncology Letters 5(1):355-359. |
|
|
Li J, Xie Y, Wang X, Jiang C, Yuan X, Zhang A, Liu C, Pang L, Li F, Hu J (2019). Overexpression of VEGF-C and MMP-9 predicts poor prognosis in Kazakh patients with esophageal squamous cell carcinoma. PeerJ 7:e8182. |
|
|
Li Yi, Guo H, Dong D, Wu H, Li E (2013). Expression and prognostic relevance of cyclophilin A and matrix metalloproteinase 9 in esophageal squamous cell carcinoma. Diagnostic Pathology 8(1):1-6. |
|
|
Lordick F, Mariette C, Haustermans K, Obermannová R, Arnold D; ESMO Guidelines Committee (2016). Oesophageal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Annals of Oncology 27(suppl 5):v50-v57. |
|
|
Lu J, Tao H, Song D, Chen C (2013). Recurrence risk model for esophageal cancer after radical surgery. Chinese Journal of Cancer Research 25(5):549-555. |
|
|
?ukaszewicz-Zajac M, Mroczko B, Koz?owski M, Nikli?ski J, Lauda?ski J, Szmitkowski M (2009). Elevated levels of serum metalloproteinase 9 in patients with esophageal squamous cell carcinoma. Polish Archives of Internal Medicine 119(9):558-563. |
|
|
Ma R, Xu H, Wu J, Sharma A, Bai S, Dun B, Jing C, Cao H, Wang Z, She JX, Feng J (2017). Identification of serum proteins and multivariate models for diagnosis and therapeutic monitoring of lung cancer. Oncotarget 8(12):18901-18913. |
|
|
Miyata H, Yamasaki M, Kurokawa Y, Takiguchi S, Nakajima K, Fujiwara Y, Konishi K, Mori M, Doki Y (2011). Survival factors in patients with recurrence after curative resection of esophageal squamous cell carcinomas. Annals of Surgical Oncology 18(12):3353-3361. |
|
|
Mroczko B, Koz?owski M, Groblewska M, ?ukaszewicz M, Nikli?ski J, Jelski W, Lauda?ski J, Chyczewski L, Szmitkowski M (2008). The diagnostic value of the measurement of matrix metalloproteinase 9 (MMP-9), squamous cell cancer antigen (SCC) and carcinoembryonic antigen (CEA) in the sera of esophageal cancer patients. Clinica Chimica Acta 389(1-2):61-66. |
|
|
Ozawa Y, Kamei T, Nakano T, Taniyama Y, Miyagi S, Ohuchi N (2016). Characteristics of Postoperative Recurrence in Lymph NodeNegative Superficial Esophageal Carcinoma. World Journal of Surgery 40(7):1663-1671. |
|
|
Peisker A, Raschke GF, Fahmy MD, Guentsch A, Roshanghias K, Hennings J, Schultze-Mosgau S (2017). Salivary MMP-9 in the detection of oral squamous cell carcinoma. Medicina Oral, Patologia Oral y Cirugia Bucal 22(3):e270-275. |
|
|
Shapiro J, van Lanschot JJB, Hulshof MCCM, van Hagen P, van Berge Henegouwen MI, Wijnhoven BPL, van Laarhoven HWM, Nieuwenhuijzen GAP, Hospers GAP (2015). Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncology 16(9):1090-1098. |
|
|
Shen WB, Gao HM, Zhu SC, Li YM, Li SG, Xu JR (2017). Analysis of postoperative failure in patients with stage pT3N0M0 thoracic esophageal squamous cell carcinoma and consideration of postoperative radiotherapy. Chinese Journal of Radiation Oncology 26(4):394-399 |
|
|
Siegel RL, Miller KD, Jemal A (2017). Cancer statistics, 2017. CA: A Cancer Journal for Clinicians 67(1):7-30. |
|
|
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A (2015). Global cancer statistics, 2012. CA: A Cancer Journal for Clinicians 65(2):87-108. |
|
|
van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, Richel DJ, Nieuwenhuijzen GA, Hospers GA (2012). Preoperative chemoradiotherapy for esophageal or junctional cancer. New England Journal of Medicine 366(22):2074-2084. |
|
|
Xue LY, Qin XM, Liu Y, Liang J, Lin H, Xue XM, Zou SM, Zhang MY, Zhang BH (2018). Clinicopathological parameters predicting recurrence of pT1N0 esophageal squamous cell carcinoma. World Journal of Gastroenterology 24(45):5154-5166. |
|
|
Yang H, Liu H, Chen Y, Zhu C, Fang W, Yu Z, Mao W, Xiang J, Han Y (2018). Neoadjuvant Chemoradiotherapy Followed by Surgery Versus Surgery Alone for Locally Advanced Squamous Cell Carcinoma of the Esophagus (NEOCRTEC5010): A Phase III Multicenter, Randomized, Open-Label Clinical Trial. Journal of Clinical Oncology 36(27):2796-2803. |
|
|
Ye Z, Zhao H, Zhou W, Ye T, Geng C, Li X, Yuan L, Du M, Xu H (2020). Lower Serum Matrix Metalloproteinase?9 in Metastatic Patients with Esophageal Squamous Cell Carcinoma After Concurrent Radiotherapy Was Significant for Prognosis. OncoTargets and Therapy 13:12857-12866. |
|
|
Zeng R, Duan L, Kong Y, Liang Y, Wu X, Wei X, Yang K (2013). Clinicopathological and prognostic role of MMP-9 in esophageal squamous cell carcinoma: a meta-analysis. Chinese Journal of Cancer Research 25(6):637-645. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0