ABSTRACT
Experiments on Senna alata aqueous extract and Hollandia yoghurt were done to determine active compounds responsible for their laxative properties and to further establish sample vulnerability to microbial attack. Phytochemical screening was performed on test substrates to analyze for alkaloids, flavonoids, tannins, saponins, terpenoids and steroids. Escherichia coli (Gram negative), Micrococcus species (Gram positive), Klebsiella pneumoniae (Gram negative), Enterocococcus species (Gram negative) and Salmonella species (Gram negative) were test bacteria while Penicillium species, Trichophyton species, Rhizopus species, Fusarium oxysporium and Aspergillus niger were test fungi used for microbial assays. The results show that alkaloids, tannins, saponins, terpenoids and steroids were found in S. alata while only alkaloids, terpenoid and steroid were found in Hollandia yoghurt. S. alata had activity against Penicillium spp., Trichophyton spp. and Rhizopus spp., while Hollandia yoghurt showed no antifungal activity. Aqueous extract of S. alata and Hollandia yoghurt are however together active against test bacteria of which, K. pneumonia is common.
Key words: Laxative, Senna alata, hollandia yoghurt.
Higher plant products have attracted the attention of microbiologists to search for some phytochemicals that may be linked to their exploitation as antimicrobials as it is felt that such plant products would be biodegradable and safe to human health (Kumar et al., 2008). Similarly, some proprietary food products on market shelves could attract further enquiries on their potency and capacity to improve human health. Senna alata L. has already been recognized as a potent laxative (Ogunti and Elujobi, 1993; Gritsanapan and Mangmeesri, 2009; Sule et al., 2010) and by experience, Hollandia plain sweetened yoghurt drink (www.chihollandia.com - NISISO 9001-2008; NIS ISO 22000-2005; NAFDAC Reg. No. 01-7506) has been found to induce bowel movements. Aqueous extract of S. alata obtained wild from Bowen University campus, Iwo was therefore subjected to biochemical and microbial analysis when compared with Hollandia yoghurt so as to determine their commonalities and differences. This study essentially investigated in vitro activities of extracts of the leaf of S. alata and Hollandia yoghurt on some fungi and bacteria isolates. Several plants and seeds have been used to treat constipation; these includes the mature stem of Neoboutonia velutina Prain which was found to be effective for loperamide-induced constipation in rats (Ateufack et al., 2017) as well as peanut sprout ethanolic extract (Seo et al., 2013). While Zhang et al. (2013) suggested that bowel movement frequency and laxative use have no association with colorectal cancer (CRC), a more recent study by Citronberg et al. (2014) proved that non-fibre laxative use in the treatment of constipation increases CRC while the use of fibre laxative decreases it. S. alata L. has been credited for the treatment of hemorrhoids, constipation, inguinal hernia, intestinal parasites, blennorrhagia, syphilis and diabetes (Abo et al., 1998; Adjanahoun et al., 1991).
The leaves of this plant were reported to be useful as purgative but were especially useful in treating dermatophytosis (Ogunti and Elujobi, 1993). Senna laxative properties were also examined by Izzy et al. (2016) in geriatrics in comparison with sorbitol, lactulose among other laxatives and were discovered to have a higher efficacy together with a very good adverse effect profile. In a study by Owoyale et al. (2005) in Kwara State Nigeria, S. alata was established to have antifungal activities. S. alata (L.) Roxb. is a medicinal plant that its leave extract has long been used as a laxative and antifungal drug (Gritsanapan and Mangmeesri, 2009). The leaves were said to contain anthraquinones with varying content, cultivating locations and harvesting period. Senna is also used for irritable bowel syndrome, hemorrhoids and weight loss. It contains many chemicals called sennosides that irritate the bowel lining and thus causing laxative effect (Therapeutic Research Faculty, 2009). Makinde et al. (2007) already established antifungal potency of alcohol extract of S. alata L. against certain fungi including Trichophyton mentagrophytes, Candida albicans, Aspergillus flavus, Microsporum canis, Blastomyces dermatitidis and also against some bacteria with maximum activity in fractions containing alkaloid salts and base.
Source and identification of S. alata plant
Crude leaves of S. alata plant utilized in this study were obtained from Bowen University, Osun State, Nigeria and identified at the plant taxonomy laboratory, Department of Biological Sciences of the same university.
Test extracts
Extract of S. alata was obtained by soaking freshly washed green leaves in distilled water for three days. The leaves, after 72 h culture showed no growth which is an evidence of no contamination of the aqueous extract. The S. alata extracts (35 mg/mm) were filtered, poured in clean test tubes and stored in a refrigerator at 4°C (set was added as authors’ own experimental measure). Hollandia yoghurt (manufactured by Chi Ltd., Lagos, Nigeria), on the other hand, was sourced and purchased tetra-packed, aseptically stored in the refrigerator at 4°C until used.
Test fungi and bacteria
Five fungi and five bacteria used for this study were obtained from the Microbiology Laboratory of Bowen University. The test fungi were Rhizopus species, Penicillium species, Fusarium species, Aspergillus niger and Trichophyton species and the test bacteria were Escherichia coli, Micrococcus species, Enterococcus species, Klebsiella species and Salmonella species. The choice of these microbes was informed by known potentials and capabilities of causing intestinal tract infections (Joshua et al., 1992; Eistenstein and Zaleznick, 2000). The fungi were obtained by sub-culturing to get pure isolates, before they were inoculated into enriched liquid medium containing ammonium chloride, magnesium sulphate, ferric citrate, dipotassium hydrogen phosphate, starch and yeast in distilled water. The bacteria were confirmed by streaking on Eosin Methylene Blue agar; nutrient agar, thiosulphate citrate bile sucrose (TCBS) agar and Salmonella-Shigella agar producing green metallic sheen; tiny, yellow colonies; mucoid, raised and shiny colonies, and black precipitate, respectively (Isenberg, 2004). Each of the bacteria were inoculated into 5 ml sterile peptone water and incubated at 37°C for 18 to 24 h. Pure isolates were later transferred onto sterile agar slants used for antimicrobial assay of both the aqueous Senna extract and Hollandia yoghurt.
Phytochemical screening
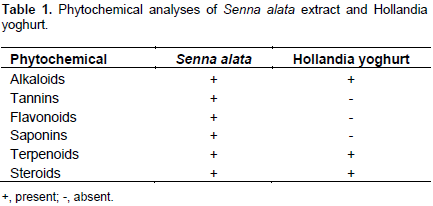
The aqueous test substrates were screened for their phytochemical bases using the standard method of Trease and Evans (1989) and Harborne (1998). The phytochemical components explored were alkaloids, tannins, saponins, flavonoids, terpenoids and steroids. The screening process and results obtained are shown in Table 1.
Antibacterial and antifungal activities
Antimicrobial activities of the S. alata extract and Hollandia yoghurt were determined using filter paper disc method (Jonathan et al., 2008). Whatman number 1 filter paper was cut into discs of 5 mm diameter before sterilization at 121°C for 15 min in an autoclave. After cooling, the sterile discs were soaked in the test extract and yoghurt sample and allowed to dry for about 5 min. Mueller-Hinton agar (MHA) and potato dextrose agar (PDA) were aseptically inoculated with the test bacteria and fungi, respectively; using sterile swab sticks. The sample soaked discs were placed on seeded agar plates and left for about 10 min before incubation at 37°C for 24 h (for bacteria) and 27°C for 72 h (for fungi). Sterile water was used as control. Zones of inhibition were observed and measured metrically after incubation and the results are presented in Table 2.
The results of the phytochemical analyses indicated the presence of alkaloids in Senna extract and the yoghurt which was confirmed by brown precipitate with turbidity; a green-black to blue coloration which indicated the presence of tannins in the Senna extract but not the yoghurt; the formation of stable foam that was taken as evidence of saponins in the Senna extract but not the yoghurt; a yellow coloration was observed for the Senna extract which shows the presence of flavonoid but no reaction for the yoghurt and reaction upper layers turned red and the sulphuric acid layer showed yellow with green fluorescence, indicating the presence of steroids in both the Senna extract and the yoghurt. These are in agreement with the submission of Sule et al. (2010) which revealed alkaloids, carbohydrates, saponins, anthraquinones, steroids and tannins as phytochemicals of S. alata. Egunyomi et al. (2009) already implicated the anthraquinones as acting on the gastro-intestinal tract to increase peristalsis action. By extension, Egunyomi et al. (2009) felt that S. alata may be useful as mild laxative especially in cases where patients complain of constipation. A color change from pink to violet also indicated the presence of terpenoids in both the Senna extract and the yoghurt.
Phytochemical analyses of the Senna extract uncovered important secondary metabolites including tannins, saponins, alkaloids, terpenoids, and steroids, while only the last three are present in Hollandia yoghurt. The commonality of alkaloids, terpenoids, and steroids in both S. alata and Hollandia yoghurt suggested that they may be active laxative constituents which should be further researched. Marked antibacterial activities of Senna extract were recorded for Klebsiella pneumoniae and Micrococcus spp., both having an average of 12 mm inhibition. Hollandia yoghurt was also reactive to Enterococcus spp. (10 mm inhibition) and K. pneumonia (10 mm inhibition), affirming that the two trial samples were active only against K. pneumoniae among the bacteria examined. Hollandia yoghurt showed no antifungal activity, while S. alata extract inhibited Penicillium spp. (5 mm); Rhizopus spp. (8 mm) and Trichophyton spp. (5 mm). This means only bacteria resistance could be established as common to the trial samples and not antifungal resistance. Perhaps, the peristalsis improvement in the alimentary carnal may be attributed to bacteria reticence. The main bacterium implicated in this study is K. pneumoniae with minimum effective inhibition zone of 10 mm. Sule et al. (2011) also reported the antifungal activity of S. alata crude stem bark extract against Trichophyton spp. and other dermatophytes.
The phytochemical analyses revealed the presence of important secondary metabolites including tannins, saponins, alkaloids, terpenoids and steroids while only the last three are present in Hollandia yoghurt. The presence of these bioactive compounds as well as antibacterial properties of S. alata and Hollandia yoghurt provided insight into their usage for relieving constipation. These potentials may have applications against microbial infections and diseases for therapeutic purposes and should be explored in the production of antimicrobial drugs.
The authors have not declared any conflict of interests.
The authors acknowledge the contributions and assistance received from Dr. T. A. Ayanbamiji, a plant taxonomist in the Department of Biological Sciences of Bowen University, Iwo, Osun State, Nigeria.
REFERENCES
|
Abo KA, Adediwura AA, Ibikunle AJ (1998). Biological activities of extracts of Mallotusoppositifolium. Proceeding of the 1st International Workshop on Herbal Medicinal Products, (HMP`98), University of Ibadan, Nigeria. pp. 22-24.
|
|
|
|
Adjanahoun E, Ahyi RA, Ake-Assi L, Elewude JA, Fadoju SD (1991). Traditional Medicine and Pharmacopoeia: Contribution to Ethnobotanical Floristic Studies in Western Nigeria. OAU/STRC, Nigeria. P 420.
|
|
|
|
|
Ateufack G, Kengne NF, Nana YW, Tchoumbou TH, Kamanyi A (2017). Laxative properties of aqueous and methanolic extracts of mature stem bark of Neoboutonia velutina Prain (Euphorbiaceae) in rats. J. Med. Plants Res. 11(5):87-95.
Crossref
|
|
|
|
|
Citronberg J, Kantor ED, Potter JD, White E (2014). A Prospective Study of the Effect of Bowel Movement Frequency, Constipation, and Laxative Use on Colorectal Cancer Risk. Am. J. Gastroenterol. 109:1640-1649.
Crossref
|
|
|
|
|
Gritsanapan W, Mangmeesri P (2009). Standardized Sienna alata leaf extract. J. Health Res. 23(2):59-64.
|
|
|
|
|
Harborne JB (1998). Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis. Chapman and Hall, London. pp. 182-190.
|
|
|
|
|
Isenberg HD (2004). Clinical Microbiology Procedures Handbook, American Society for Microbiology, Washington DC. p. 3.8.1.1-2
|
|
|
|
|
Izzy M, Malieckal A, Little E, Anand S (2016). Review of efficacy and safety of laxatives use in geriatrics. World J. Gastrointest. Pharmacol. Ther. 7(2):334-342.
Crossref
|
|
|
|
|
Jonathan SG, Kigigha LT, Ohimain E (2008). Evaluation of the inhibitory potentials of eight edible Nigerian higher fungi against pathogenic microorganisms. Afr. J. Biomed. Scs.11:195-200.
|
|
|
|
|
Joshua L, Robert ES, Oarks SC (1992). Microbial threats to health in the United States - Front Matter: Emerging Infections. National Academy Press. Washington D.C. P 12.
|
|
|
|
|
Kumar A, Shukla R, Singh P, Prasad CS, Dubey NK (2008). Assessment of Thymus vulgaris L. essential oil as a safe botanical preservative against post harvest fungal infestation of food commodities. Innovative Food Sci. Emerging Technol. 9:575-580.
Crossref
|
|
|
|
|
Makinde AA, Igoli JO, Amal LTA, Shaibu SJ, Garbal A (2007). Antimicrobial activity of Cassia alata. Afr. J. Biotechnol. 6:1509-1510.
|
|
|
|
|
Ogunti EO, Elujobi AA (1993). Laxative activity of Cassia alata. Fitoterapia, 64:437-439.
|
|
|
|
|
Owoyale JA, Olatunji GA, Oguntoye SO (2005). Antifungal and antibacterial activities of an alcoholic extract of Sennaalata Leaves. J. Appl. Sci. Environ. Manage. 9:105-107.
|
|
|
|
|
Seo JY, Kim SS, Kim HJ, Liu KH, Lee HY, Kim JS (2013). Laxative effect of peanut sprout extract. Nutr. Res. Pract. 7(4):262-266.
Crossref
|
|
|
|
|
Sule WF, Okonko IO, Omo-Ogun S, Nwaze J C, Ojezele MO, Ojezele OJ, Alli JA, Soyemi ET, Olaonipekun TO (2011). Phytochemical properties and in vitro antifungal activity of S. alata Linn. Crude stem bark extract. J. Med. Plants Res. 5(2):176-183.
|
|
|
|
|
Therapeutic Research Faculty (2009). Natural medicines comprehensive database Professional.
View
|
|
|
|
|
Trease GE, Evans WC (1989). Phytochemical Screening. In: Textbook of Pharmacognosy, Trease, G.E. and W.C. Evans (Eds.). 10th Edn., Bailiere Tindal Limited, London. P 541.
|
|
|
|
|
White PJ (1978). Tetany and clubbing in patient who ingested large quantities of senna. Letter Lancet. 2: =947
|
|
|
|
|
Zhang X, Wu K, Cho E, Ma J, Chan AT, Gao X, Willett WC, Fuchs CS, Giovannucci EL (2013). Prospective cohort studies of bowel movement frequency and laxative use and colorectal cancer incidence in US women and men. Cancer Causes Control 24(5):1015-1024.
Crossref
|
|