ABSTRACT
Taraxacum koksaghyz, the Russian dandelion, produces latex in its taproots and is an alternative to Hevea brasiliensis as a source for rubber. Studying the inheritance of rubber content and yield as well as breeding of T. koksaghyz could strongly benefit from haploid or doubled haploid techniques. Therefore, this study aimed at establishing the conditions to produce haploid and/or double haploid T. koksaghyz plants. This study focused on gynogenesis because analysis of microsporogenesis showed that very small (young) inflorescences already contain mature pollen and that the development of the microspores is not synchronous in different flowers in one inflorescence. Therefore, a surface disinfection protocol, culture media, and culture conditions were established resulting in shoot formation from ovules. Diploid and triploid Taraxacum officinale were also included in the experiments. Depending on the genotype, 0 to 2.6% of the ovules regenerated shoots via callus. These shoots could be rooted and acclimatized to greenhouse conditions without losses. Eleven plants from ovule cultures were analysed for their ploidy level and compared genetically to the donor material using simple sequence repeats (SSRs) to confirm their origin from either haploid or diploid/triploid donor tissues. All regenerated plants had the same ploidy level and were heterozygous at the same loci as their respective donor plants, and thus originated from somatic tissue.
Key words: Flow cytometry, gynogenesis, haploids, microsatellite markers, ovule culture, Russian dandelion.
There is a need to find alternatives to Hevea brasiliensis as a source for rubber, which is harvested in the form of latex. In 1934, Microcyclus ulei, the cause of South American Leaf Blight (SALB) on rubber trees, wiped-out rubber plantations in Southeast Asia and Brazil on a large-scale (Le Guen et al., 2007). However, due to economic reasons, rubber plantations were replaced by oil palm plantations in numerous countries, especially in Malaysia, because labor costs for the laborious latex harvesting were high and the economy of these countries was shifting to an industrial base. Synthetic rubber from petroleum as a substitute for latex is a non-renewable resource, and thus is not a sustainable alternative to natural rubber (Van Beilen and Poirier, 2007). An important limitation of H. brasiliensis rubber is the IgE-mediated latex allergy caused by proteins in the latex (Mooibroek and Cornish, 2000). The aforementioned factors led to the search for alternative sources of rubber to Hevea. Alternative plants may not only secure a sustainable supply, but possibly could also provide hypoallergenic rubber. Two plant species that have received considerable attention as alternative sources of natural rubber are the Mexican shrub, guayule (Parthenium argentatum Grey) and dandelions (Taraxacum species) (Van Beilen and Poirier, 2007). Taraxacum spp. might fit better into the demands of agricultural production than guayule because it could be readily sown and removed in response to market needs; it is fast growing, and produces a large amount of biomass (Van Beilen and Poirier, 2007).
Latex produced from Taraxacum koksaghyz (Russian dandelion) is similar to that of H. brasiliensis in terms of the composition and performance (Cornish et al., 2012). This similarity includes latex, but also the rubber-particle bound proteins that cross-react with type I latex allergy. Therefore, this rubber is a supplement to H. brasiliensis rubber (Cornish et al., 2012). Valuable side products from Russian dandelion processing may include inulin, which is the major storage sugar of dandelions (up to 40% of dry root weight), and could be used directly in non-food applications or fermented for bio-ethanol production (Buranov and Elmuradov, 2010).
The genus Taraxacum is composed of many species and is in the Asteraceae (Kirschner et al., 2016). They are native to Eurasia and North and South America. The genus is widely distributed in the temperate regions of both the Northern and Southern hemisphere (Martonfiova, 2011). Taraxacum spp. also are invasive plants in some parts of the world (Luo and Cardina, 2012). Their ability to germinate in variable, often harsh, environments allow for the establishment of plants in new habitats (Luo and Cardina, 2012). Taraxacum, consisting of about 2800 species, is divided into more than 60 sections (Zeisek et al., 2015). Current taxonomy of the genus is mainly based on morphological characters rather than phylogenetic evidence. Taxonomy is very complicated due to the existence of sexual as well as apomictic reproduction, and species commonly hybridize creating an array of microspecies (Kirschner et al., 2016). Taraxacum is a short day, perennial plant with a basal rosette of jagged, irregularly-lobed or nearly smooth-margined, saw-toothed or deeply cut leaves. The species have a long, thick, fleshy taproot (Artschwager and McGuire, 1943). The haploid chromosome number in the genus Taraxacum is x=8. Species in the genus Taraxacum represent polyploids or diploid sexually-reproducing species (Martonfiova, 2011). The most common polyploidy is triploid (De Kovel and de Jong, 2000). In most species, diploid plants co-exist with polyploid apomicts and in some areas only polyploid apomicts are found (Kirschner and Stepanek, 1996; Kirschner and Stepanek, 1997). The widespread species Taraxacum officinale is the most common triploid apomictic species in which the egg grows into the embryo and the central cell forms the endosperm without fertilization (Van Baarlen et al., 2001). Taraxacum koksaghyz is a diploid, sexual species (Kirschner et al., 2013). Diploid Taraxacum spp. (2x=16) reproduce sexually and are mostly self-incompatible (Morita et al., 1989; Martonfiova, 2011). The self-incompatibility system in several Taraxacum spp. is sporophytically controlled by multiple S-alleles similar to other Asteraceae genera (Okabe, 1956). Additionally, self-incompatibility is not always complete. For example, self-fertility in T. koksaghyz occurred at the end of the growing season or could be induced by a mentor effect of incompatible pollen of triploid Taraxacum spp. (Morita et al., 1989). T. koksaghyz (Russian dandelion) plants have 25 to 50 leaves arranged in one or more basal rosettes and are usually obovate and glaucous grey-green in colour, without sharp teeth (Krotov, 1945; Kirschner et al., 2013). Most plants have several flower heads, and a single stalk bears a single yellow flower. Each inflorescence has 100 to 300 complete florets (Artschwager and McGuire, 1943). T. koksaghyz was first discovered in Kazakhstan in 1931 and cultivated extensively in the Soviet Union (Kirschner et al., 2013). Due to natural rubber shortages during World War II, several other countries, such as the United States of America (USA), the United Kingdom (UK), Germany, Sweden and Spain independently started growing Russian dandelion for latex (Van Beilen and Poirier, 2007).
Although alternative sources of natural rubber were investigated in the past, recent advancements at the molecular level with genomics, metabolomics, and proteomics as well as at the agronomic level, with marker-assisted breeding have not been much used (Van Beilen and Poirier, 2007). Insights into rubber biosynthesis in Taraxacum have been gained recently (metabolic engineering of inulin pathway), which may lay the basis for genetically engineered rubber-enriched plants (Stolze et al., 2017). The primary breeding objectives of Taraxacum are to increase the rubber content and the biomass yield of the roots. To achieve these breeding aims, knowledge on the inheritance of several traits and linked molecular markers are needed. For both, haploids or double haploids will be an important tool. The double haploids will accelerate breeding programs for dandelions by producing homozygous materials, and produce plant material that helps in understanding rubber biosynthesis and related physiological traits. The only attempt to generate haploids from Taraxacum spp. was using anther culture and this was not successful (Zavesky, 2010).
The objective of this study was to establish the conditions to produce haploid and/or double haploid T. koksaghyz plants via gynogenesis. In detail, the study aimed at (i) establishing a surface disinfection protocol, culture media, and culture conditions suitable for shoot formation from ovules and (ii) analysing regenerated plants from ovule cultures for their ploidy level and their simple sequence repeats (SSRs) patterns to confirm their origin from either haploid or diploid/triploid donor tissues.
T. koksaghyz and T. officinale plants that were used for experiments were provided by Dr. Eickmeyer (ESKUSA GmbH, Steinach, Germany) and will be referred hereafter to as donor plants (Table 1).

Donor plant growing conditions
Plants used in the experiments were cultivated fromFebruary 2015 to June 2016 in a greenhouse under controlled conditions at the Faculty of Natural Science, Leibniz Universität, Hannover, Germany. Seeds were sown into a 1:1:1 mixture of topsoil: peat substrate (Einheitserde Typ P): sand. The seed-derived plantlets were potted into 2 l containers filled with standard medium Einheitserde Typ P (Einheitserdewerk Gebr. Patzer, Sinntal-Altengronau, Germany). Plants were transferred to a greenhouse at 8°C (maximum 13°C, ventilation set point: 21°C, 65 to 70% relative humidity) under natural light conditions for eight weeks for vernalisation. Thereafter, plants were maintained in the greenhouse under a day/night temperature of 16/10°C (ventilation set point: 21°C) at 16 h day length under natural light conditions that was supplemented with 25 klux light from high pressure sodium lamps (PHILIPS, SON-T APIA-AGRO 400W).
Fluorescence microscopy
To find the suitable stages of microsporogenesis for possible androgenesis approaches, the microspore development was analyzed depending on the pedicel length. Inflorescences of T. koksaghyz and T. officinale with pedicel lengths ranging from 0.5 to 30 cm were collected in February 2015. After dissection of the flowers, anthers were excised using fine forceps, collected in 1.5 ml Eppendorf tubes, and fixed in 2:1 99% ethanol and 90% lactic acid for 12 to 24 h. The fixing solution was discarded and replaced with 4', 6-diamidino-2-phenylindole (DAPI) solution (CyStain UV Precise P kit-staining buffer, Münster, Germany) to stain the nuclei in the microspores. The Eppendorf tubes were subjected to vacuum for 10 min. Single anthers were placed on a microscopic slide in a drop of DAPI solution, squashed and observed under a fluorescence microscope (AxioScope A1 equipped with filter set 2, excitation 365 nm, beam splitter 395 nm, emission 420 nm; Zeiss, Göttingen, Germany) for the stage of microsporogenesis.
Plant tissue culture media
Because microspores in an appropriate stage could not be identified in the investigated florets, the following work focused on gynogenesis. The ovule culture inoculation medium contained MS (Murashige and Skoog, 1962) salts, 2% (w/v) sucrose, 0.8% (w/v) Plant Agar (Duchefa Biochemie, The Netherlands) and was supplemented with 8.87 µM benzyl aminopurine (BAP), 9.29 µM kinetin and 2.85 µM indole acetic acid (IAA) (medium A) modified after Sitbon (1981) and Wang et al. (2014). In addition, the following variations of plant growth regulator (PGR) concentrations were tested: medium B was augmented with 1.11 µM BAP, 1.16 µM kinetin, and 1.43 µM IAA; and medium C did not contain any PGRs. Shoots obtained from the ovule cultures were transferred to shoot culture medium (Murashige, 1974) containing 23.2 µM kinetin and 2.85 µM IAA. Shoots were rooted on medium (Murashige, 1974) amended with 28.5 μM IAA. For all media, the pH was adjusted to 5.8 before autoclaving at 121°C, 1 bar for 20 min. Autoclaved media were stored at 4°C.
Ovule preparation and culture
Prior to the preparation of ovules, disease-free closed flower buds 1 to 2 days before anthesis (Figure 1) were harvested. Surface disinfection was done by immersion in 2% (v/v) sodium hypochlorite (NaOCl) and few drops of Tween 20 for 10 min, followed by three rinses in sterile, deionized water (1, 2 and 5 min) under aseptic conditions. In February, March, and October, 2015, ovules were dissected by opening the bud/flower heads and separating them into single florets using a stereo microscope (Zeiss Stemi 2000 C; Zeiss, Göttingen, Germany) (Figure 2a). A small cut was made in the basal part of the ovary and ovules were squeezed out (Figure 2b, c, and d). Ten ovules were placed in one Petri dish (6-cm diameter) containing 12 ml medium A. Petri dishes were sealed with Parafilm (Bemis, USA) and incubated at 24°C in darkness for eight weeks.

After the 8-week induction phase, developing calluses and shoots were transferred to 250-ml plastic vessels containing 80 ml shoot culture medium and subcultured after four weeks if necessary. When regenerated plantlets had reached a height of 1 to 5 cm, the basal callus was excised, and the shoots were cultured on rooting medium for 4 weeks resulting in a total culture period of 16 weeks. Eight weeks after preparation, all cultures were transferred to a 16 h photoperiod (provided by cool white fluorescent lamps (30 µmol m-2s, Philips Fluorescent MASTER TL-D Super 80 58W/830, Belgium)). When the roots were well developed, plantlets were transferred to the greenhouse. Green plants (3 fully developed leaves, plant height around 8 cm, root length of 5 cm) were washed carefully and transferred to a potting mix containing white sphagnum peat and perlite (N:P:K - 12:14:24; Steckmedium Klasmann-Deilmann GmbH, Geeste, Germany) in 11-cm diameter pots and maintained under 96% humidity at 22°C. After gradually reducing the humidity, acclimatized plants were transferred to the same greenhouse that was described earlier for the donor plant culture.
Evaluation of ovule development and statistical analyses
After 8 weeks of culture, ovules were evaluated and classified using the following five classes of development: (1) enlarged (smooth surface), (2) with callus (rough surface), (3) with shoots, (4) not enlarged ovules, and (5) dead (brown). The number of ovules in the respective classes was expressed as percentage of the total number of non-contaminated ovules. For statistical analysis, the data were divided into enlarged ovules with callus and shoots, and dead or non-responsive ovules. Data were processed by analysis of variance, least square means and Tukey test (α=0.05) using the R statistical package version 2.15.1.
Ploidy level determination by flow cytometry
Nuclei were extracted and stained using CyStain UV precise P kit (Partec, Münster, Germany) according to the manufacturer’s protocol and used about 0.5 cm2 of young leaf material. The ploidy level was determined with a Partec Cyflow® ploidy analyzer (Partec, Münster, Germany). The signal amplification used for all the flow cytometric analyses (gain) was 447 V, the lower level threshold was 0.3 and sample speed was set to 0.4 µl/s. A minimum of 2000 nuclei in
the main peak was analyzed in each measurement. Regenerant and donor plant materials were mixed and co-chopped in buffer.
Molecular characterization of the regenerants by SSR markers
Regenerated plants were compared to the respective donors for their SSR marker patterns. DNA was extracted using the method of Stein et al. (2001) and quantified using a Nanodrop spectrophotometer (Nanodrop 2000c, PeQLab, Biotechnologie GmbH, Erlangen, Germany). Stocks containing 2 ng DNA/µl and all DNA was stored at -20°C until used. Polymerase chain reactions (PCR) were performed according to Trigiano et al. (2012) except that 2 ng of genomic DNA and 10 µM primer stocks were used. Seven primer pairs for di- and tri-nucleotide motifs were developed from genomic sequencing of T. koksaghyz (Table 2), and synthesized by Integrative DNA Technologies (Corralville, IA, USA). Water instead of DNA was used as a negative control for amplification. Reactions were subjected to a touchdown thermal cycler program as described in Trigiano et al. (2012). The size of the PCR products was determined on the QIAxcel Capillary Electrophoresis System (Qiagen, Valencia, California) using an internal 25 to 500-bp size standard. SSR primers that revealed heterozygous donor loci were used to evaluate the SSR profiles of regenerated plants by recording the bp of the amplified fragments.

Microspore stage determination
Microsporogenesis in flowers from T. koksaghyz and T. officinale was examined using DAPI staining of nuclei to identify inflorescence developmental stages that contain uninucleate microspores suitable for an androgenetic approach. Multiple developmental stages of micro-sporogenesis from different anthers within one bud size were observed, indicating asynchronous develop-ment within an individual inflorescence. In T. koksaghyz (EK14-2x), an inflorescence with a pedicel length of 8 mm contained pollen mother cells before meiosis as well as tetrad stages before cytokinesis. In slightly more mature flower heads with a pedicel length of 1.4 cm, only fully developed pollen grains were observed (data not shown). Consequently, the identification of the desired developmental stage and its correlation with pedicel length was not achieved. Therefore, efforts to establish haploid plants focused on gynogenesis using ovules as explants because they could be easily excised and were available in high numbers (100 to 150 per inflorescence).
Callus induction and shoot regeneration from ovules
Calluses developed from ovules usually within the first four weeks of culture (Figure 3a). They proliferated fast, whereas most of the enlarged ovules did not develop further even after the 8 weeks culture period. Calluses were variable in size and friable. Two to three shoots, which had the same origin (Figure 3b), were regenerated from all calluses obtained in the ovule culture experiments. Occasionally, a few roots developed depending on the genotype within the first four weeks. On the shoot culture medium, the shoots continued to grow and after a few days formed new leaves. The contamination rate was 5% in the first phase of the ovule culture and thereafter no further losses occurred. After four weeks of culture in the shooting medium, several well-developed shoots with basal leaf rosettes grew on the surface of the calluses (Figure 3c and d). Rooted plantlets with leaf rosettes of about 5-cm diameter developed on the rooting medium after four to five months from the start of the cultures. After transfer to the greenhouse, the regenerated plants established well during acclimatization with a survival rate of 100%. Eleven independent regenerants from ovule culture were obtained. All the regenerated plantlets developed into adult plants and some flowered. The regenerants from different ovules of one genotype showed diversity in their development stage and leaf shape at the beginning of growth in the greenhouse. With time, however, they had a more similar morphological appearance.

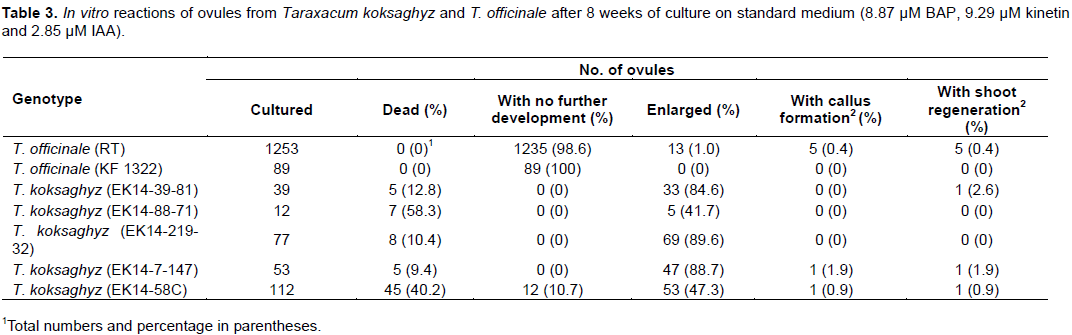
The comparison of different genotypes in ovule culture revealed strong genotypic differences (Table 3). In T. officinale, some of the ovules turned brown within the first 2 weeks of culture and the majority of the ovules did not enlarge during the first 4 weeks of culture. Only 5 out of 1253 ovules (0.4%) of genotype RT formed calluses or shoots. Ovules of T. officinale KF 1322-2x did not produce calluses nor shoots. In all ovule culture experiments, the direct formation of shoots without undergoing callus formation was observed only in one (2.6%) case (T. koksaghyz EK14 - 2x, 39-81). For T. koksaghyz, the majority of ovules enlarged, but only three of five genotypes developed shoots at low frequencies of
0.9 to 2.6%.
Effect of reduced plant growth regulator concentrations on regeneration from ovules
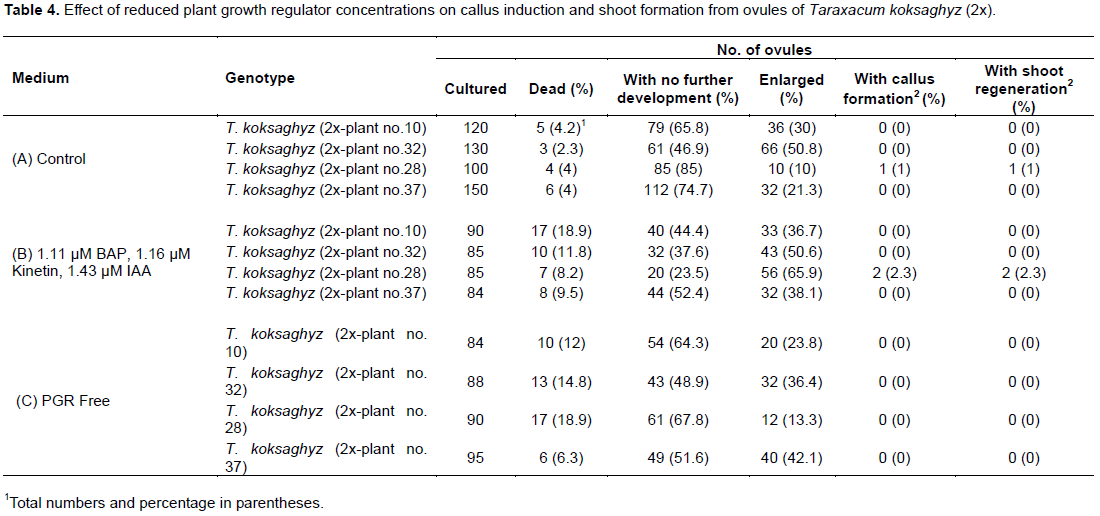
Three different media were tested to optimize PGR concentration for callus and shoot induction with T. koksaghyz (2x). The PGR concentration was reduced compared to the first experiment to prevent callus formation from somatic tissue of the ovule and to activate the haploid cells (egg cell, antipodal, and synergid percentage (47.8%) of enlarged ovules was obtained on medium B when compared with media A and C (around 30%; Table 4). Only one (28) genotype of four tested regenerated shoots on both media A and B. Calluses did not proliferate on medium C, which did not contain PGRs. All calluses that developed on media A and B formed shoots that were ultimately rooted.
Determining the cytological and genetic constitution of regenerants
The regenerated plants were analyzed by flow cytometry to determine their ploidy and by SSR cells) to shoot development. The highest markers in order to compare their genetic constitution to the donor plants.
Flow cytometric analysis of the regenerants
Flow cytometric analysis was conducted employing the co-chopping technique to determine the ploidy level of the regenerants in direct comparison to the donor plants. This seemed necessary because differences in peak positions of the diploid T. koksaghyz plants were observed (Table 5). The triploid T. officinale RT ovule donor plants had a mean fluorescence intensity of 48.78, which was very similar to that determined for the co-chopped regenerants (44.04±6.13).
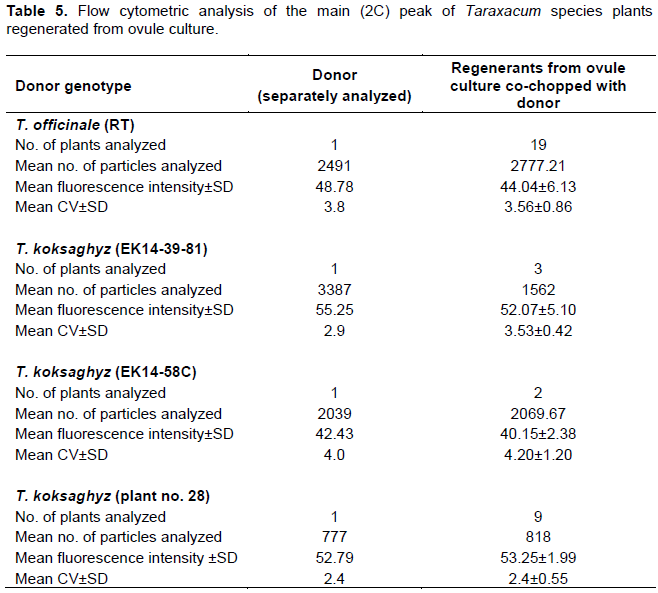
For the ovule donzor T. koksaghyz genotype EK-14-2x, 39-81 alone, a mean fluorescence intensity of 55.25 was detected for the main peak, and for regenerants co-chopped with the donor, just one peak at a fluorescence intensity of 52.07±5.10 was detected (Table 5). Similar values were obtained for T. koksaghyz 28C and its regenerants. Likewise, for the donor T. koksaghyz 58C and its regenerants similar mean fluorescence intensities of 42.43 and 40.15±2.38 were detected, respectively (Figure 4). Thus, the mean fluorescence intensity values for the main peak obtained for all donor plants and their respective regenerants were not significantly different. The ploidy level of all the regenerants can therefore assumed to be the same as in the donor plants. This finding indicated that the cultured shoots either regenerated from somatic tissues of the ovule or spontaneous chromosome doubling had occurred. Therefore, SSR markers were used to determine the genetic constitution regarding the zygosity of the regenerants compared to the donor plants.

SSR analysis of donor plants and regenerants
Donor plants were characterized using seven SSR primer pairs and depending on the genotype, two-to-four heterozygous loci were identified. All regenerated plants examined were also heterozygous at these loci, although there were some minor variations in allele sizes. Therefore, the regenerated plants were not haploid or double haploid, but either diploid (T. koksaghyz) or triploid (T. officinale), and regenerated from somatic cells (Table 6).
Generally, microsporogenesis and pollen formation are precisely timed and choreographed and some typical meiotic stages significantly correlated with the flower bud size, for instance in Eucalyptus species (Yang et al., 2016). However, in the present study, individual florets in inflorescences defined by pedicel length did not contain a uniform population of uninucleate stage microspores. Single florets of varying maturity were present in a single flower head, so that different stages of microsporogenesis were observed within the buds with 8 mm pedicel length in T. koksaghyz (EK 14-2x, 39-81). Consequently, the microspore populations isolated from these anthers were highly heterogeneous. Therefore, anther culture is not feasible in these two species without selecting single florets with appropriate microspore stages of development.
As an alternative haploid technique to androgenesis, the gynogenic response of ovules of T. koksaghyz and T. officinale was studied in the present work. The results indicated that callus induction rates from ovules were low. However, if the bottleneck of callus formation was overcome, shoot regeneration succeeded in all cases (Tables 3 and 4). This is the first report of plant regeneration from ovule culture in Taraxacum. In total, eleven independent regenerants from ovule culture were obtained (for T. officinale RT-3x 5 plants, for T. koksaghyz EK 14-2x 3 plants, and for T. koksaghyz (28) 3 plants). The shoot induction frequency was very low and was similarly reported for Gerbera jamesonii ovules to regenerate haploid shoots (Ahmim and Vieth, 1986). Except for one case (one ovule of genotype EK14-39-81 of T. koksaghyz, Table 3) all the ovules that produced shoots underwent indirect regeneration via a callus phase.
The gynogenic regeneration response in the genotypes of the two Taraxacum spp. was quite low and depended on genotype. Detailed investi-gations are necessary to understand the nature of genetic control of the gynogenic response. Genotypic differences expressed in callus formation, as well as shoot regeneration from ovules, have also been recorded for G. jamesonii (Ahmim and Vieth, 1986; Miyoshi and Asakura, 1996). The gynogenic capacity of additional T. koksaghyz genotypes should be investigated in future studies.
PGR concentration did not significantly affect callus and shoot formation. PGRs play a crucial role in gynogenesis and the excessive application causes vigorous callusing of somatic tissues, which makes it difficult to distinguish the gynogenic derivation of shoots from somatic tissue sources (Yang and Zhou, 1990). Reduced PGR concentrations promoted callus and shoot formation of T. koksaghyz (2x) compared to the medium combination containing higher PGR concentration. Modifications to the PGR composition and variation of further media compounds should be tested in future experiments. Moreover, attention should be paid to the developmental stage and the physiological condition of the donor plants. The temperature and light conditions for donor plants are crucial in many haploid protocols (Dunwell, 1986). Plant age as well seasonal variations (possibly mainly driven by light intensity) could account or the observed differences between experiments for G. jamesonii (Cappadocia et al., 1988; Tosca et al., 1999). The ovule inoculation medium used was based on or modified for the related Asteraceae genera Gerbera and Chrysanthemum (Sitbon, 1981; Wang et al., 2014) and Cichorium intybus (Theiler-Hedtrich and Hunter, 1995). The culture media prepared for shoot multiplication and rooting was the suitable for Taraxacum, as all the calluses regenerated shoots, which rooted, and proliferated easily. In this regard, Taraxacum seems to be quite responsive to the tissue culture conditions. All the regenerants transferred to the greenhouse were acclimatized to ambient greenhouse conditions.
All regenerants from ovule culture produced in this study had the same ploidy level as their donor plants as demonstrated by the flow cytometry. This finding indicated that ovule cultures yielded callus and shoots either derived from somatic tissues of ovules or spontaneously doubled haploid cells. The observed differences between donors and regenerants of two T. koksaghyz genotypes in the position of the 2C peak (channel 42 in EK14-58C versus channel 52 for plant 28) might indicate either differences in secondary metabolite composition in these genotypes that interfered with the fluorescent staining (Bennett et al., 2008) or differences in DNA contents. Chromosome counts and co-chopping with references standards would be needed to clarify this.
As cytological investigations cannot distinguish spontaneously doubled haploids from diploids derived from somatic tissue (Friedt et al., 1997), co-dominant molecular SSR markers were employed to analyze the zygosity of the regenerants. Heterozygous loci of the donor plants were selected to analyze the regenerated plants. If primers of homozygous loci were used, it would be difficult to determine whether the plants were haploid, spontaneous double-haploid or diploid. The advantage of using heterozygous loci is that if the regenerated plants were either haploid or double-haploid, only one allele would be present. However, if the plants were diploid, both alleles of the heterozygous donor plant would be present in the regenerate plants. In all cases, both alleles with minor differences compared to the donor plants were present. In conclusion, the plants regenerated from T. officinale and T. koksaghyz ovules were of somatic origins, perhaps the ovule wall.
To induce the regeneration of haploids, stress treatments to stimulate the gynogenic response could be tested in future experiments. An alternative way to induce gynogenesis is the use of inducer pollen of related species followed by ovule culture such as was done for Chrysanthemum (Wang et al., 2014). Because Taraxacum spp. can be transformed via Agrobacterium tumefaciens (Post et al., 2012), an interesting future approach could be to use CENH3 (centromere-specific histone 3) mediated haploid induction (Britt and Kuppu, 2016). CENH3 determines the position of the centromere and modifications result in severe disturbances of chromosome segregation (Britt and Kuppu, 2016). Thus, lines with modified CENH3 can be used as haploid inducers, because their chromosome set is eliminated during cell division and haploid plants can be produced from seeds.
The authors have not declared any conflict of interests.
REFERENCES
|
Ahmim M, Vieth J (1986). Production de plantes haploides de Gerbera jamesonii par culture in vitro d' ovules. Can. J. Bot. 64:2355-2357.
Crossref
|
|
|
|
Artschwager E, McGuire RC (1943). Contribution to the morphology and anatomy of the Russian dandelion (Taraxacum kok-saghyz). Technical Bulletin No. 83, United States Department of Agriculture, Washington, pp. 1-24.
|
|
|
|
|
Bennett MD, Price HJ, Johnston JS (2008). Anthocyanin inhibits propidium iodide DNA fluorescence in Euphorbia pulcherrima: implications for genome size variation and flow cytometry. Ann. Bot. London 101:777-790.
Crossref
|
|
|
|
|
Britt AB, Kuppu S (2016). Cenh3: An emerging player in haploid induction technology. Front. Plant Sci. 7:357.
Crossref
|
|
|
|
|
Buranov AU, Elmuradov BJ (2010). Extraction and characterization of latex and natural rubber from rubber-bearing plants. J. Agric. Food Chem. 58:734-743.
Crossref
|
|
|
|
|
Cappadocia M, Chrétien L, Laublin G (1988). Production of haploids in Gerbera jamesonii via ovule culture: influence of fall versus spring sampling on callus formation and shoot regeneration. Can. J. Bot. 66(6):1107- 1110.
Crossref
|
|
|
|
|
Cornish K, Xie W, Slutsky, JL, Kamenik RS, Ohlemacher, CJ, Kostyal D, Collins-Silva J, Shintani DK, Kleinhenz M, Michel F, Hamilton RG (2012). Alternative 72 natural rubber sources to Hevea natural rubber-quality and allergenicity. Proceedings of the 18 th Technical Meeting of the Rubber Divisions of the American Chemical Society, Cincinnati, Ohio.
|
|
|
|
|
De Kovel CGF, De Jong GJ (2000). Selection on apomictic lineages of Taraxacum at establishment in a mixed sexual-apomictic population. J. Evol. Biol. 13:561-568.
Crossref
|
|
|
|
|
Dunwell J (1986). Pollen, ovule and embryo culture as tools in plant breeding. In: Withers LA, Anderson PG (eds.): Plant tissue culture and its agricultural applications. London: Butterworths; pp. 375-404.
Crossref
|
|
|
|
|
Friedt W, Nurhidayah T, Röcher T, Köhler H, Bergmann R, Horn R (1997). Haploid production and application of molecular methods in sunflower (Helianthus annuus L.). In: Jain SM, Sopory SK, Veilleux RE (eds.): In vitro haploid production in higher plants. Netherlands: Springer; pp. 17-35.
Crossref
|
|
|
|
|
Kirschner J, ŠtÄ›pánek J (1996). Modes of speciation and evolution of the sections in Taraxacum. Folia Geobot. 31:415-426.
Crossref
|
|
|
|
|
Kirschner J, ŠtÄ›pánek J (1997). A nomenclatural checklist of supraspecific names in Taraxacum. Taxon 46:87-98.
Crossref
|
|
|
|
|
Kirschner J, ŠtÄ›pánek J, ÄŒerný T, De Heer P, van Dijk PJ (2013). Available ex situ germplasm of the potential rubber crop Taraxacum koksaghyz belongs to a poor rubber producer, T. brevicorniculatum (Compositae- Crepidinae). Genet. Res. Crop. Evol. 60(2):455-471.
Crossref
|
|
|
|
|
Kirschner J, Oplaat C, Verhoeven KJF, Zeisekl V, Uhlemann I, TravniÄek, Räsänen J, Wilschut R, Stepanek J (2016). Identification of oligoclonal agamospermous microspecies: taxonomic specialists versus microsatellites. Preslia 88:1-17.
|
|
|
|
|
Krotov G (1945). A review of literature on Taraxacum kok-saghyz ROD. Bot. Rev. 8:417-461.
Crossref
|
|
|
|
|
Le Guen V, Garcia D, Mattos CRR, Doare F, Lepinasse D, Seguin M (2007). Bypassing of a polygenic Microcyclus ulei resistance in rubber tree analysed by QTL detection. New Phytol. 173:335-345.
Crossref
|
|
|
|
|
Luo J, Cardina J (2012). Germination patterns and implications for invasiveness in three Taraxacum (Asteraceae) species. Weed Res. 52:112-121.
Crossref
|
|
|
|
|
Martonfiova L (2011). Comparison of breeding behavior of Taraxacum sect. Ruderalia and Taraxacum sect. Erythrosperma (Asteraceae). Thaiszia J. Bot. 21:177-184.
|
|
|
|
|
Miyoshi K, Asakura N (1996). Callus induction, regeneration of haploid plants and chromosome doubling in ovule cultures of pot gerbera (Gerbera jamesonii). Plant Cell Rep. 16:1-5.
Crossref
|
|
|
|
|
Mooibroek H, Cornish K (2000). Alternative sources of natural rubber. Appl. Microbiol. Biotechnol. 53:355-365.
Crossref
|
|
|
|
|
Morita T, Menken SBJ, Strek AA (1989). Hybridization between European and Asian dandelions (Taraxacum section Ruderalia and section Mongolica). New Phytol. 114:519-529.
Crossref
|
|
|
|
|
Murashige T (1974). Plant propagation through tissue cultures. Ann. Rev. Plant Physiol. 25:135-166.
Crossref
|
|
|
|
|
Murashige T, Skoog F (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15:473-497.
Crossref
|
|
|
|
|
Okabe S (1956). On the nature of incompatibility alleles in Taraxacum elatum x T. longe-appendiculatum (Self-incompatibility studies in Taraxacum 1). Bot. Mag. Tokyo 69:592-597.
Crossref
|
|
|
|
|
Post J, van Deenen N, Fricke J, Kowalski N, Wurbs D, Schaller H, Eisenreich W, Huber C, Twyman RM, Prüfer D, Schulze Gronover C (2012). Laticifer specific cis-prenyltransferase silencing affects the rubber, triterpene and inulin content of Taraxacum brevicorniculatum. Plant Physiol. 158:1406-1417.
Crossref
|
|
|
|
|
Sitbon M (1981). Production of haploid Gerbera jamesonii plants by in vitro culture of unfertilized ovules. Agronomie 1(9):807-812.
Crossref
|
|
|
|
|
Stein N, Herren G, Keller B (2001). A new DNA extraction method for high- throughput marker analysis in a large genome species such Triticum aestivum. Plant Breed.120:354-356.
Crossref
|
|
|
|
|
Stolze A, Wanke A, van Deenen N, Geyer R, Prüfer D, Gronover SC (2017). Development of rubber-enriched dandelion varieties by metabolic engineering of the inulin pathway. Plant Biotechnol. J. 15(6):740-753.
Crossref
|
|
|
|
|
Theiler-Hedtrich R, Hunter CS (1995). Regeneration of dihaploid chicory (Cichorium intybus L.var. foliosum Hegi) via microspore culture. Plant Breed. 114:18-23.
Crossref
|
|
|
|
|
Tosca A, Arcara L, Frangi P (1999). Effect of genotype and season on gynogenesis efficiency in Gerbera. Plant Cell Tiss. Org. Cult. 59:77-80.
Crossref
|
|
|
|
|
Trigiano RN, Wadl PA, Dean D, Hadziabdic D, Scheffler BE, Runge, F, Telle S, Thines M, Ristaino J, Spring O (2012). Ten polymorphic microsatellite loci from Peronospora tabacina, the cause of blue mold of tobacco. Mycologia 104(3):633-640.
Crossref
|
|
|
|
|
Van Baarlen P, de Jong, JH, van Dijk PJ (2001). Comparative cyto-embryological investigations of sexual and apomictic dandelions (Taraxacum) and their apomictic hybrids. Sex. Plant Reprod. 15(1):31-38.
Crossref
|
|
|
|
|
Van Beilen JB, Poirier Y (2007). Guayule and Russian dandelion as alternative sources of natural rubber. Crit. Rev. Biotechnol. 27:217-231.
Crossref
|
|
|
|
|
Wang H, Dong B, Jiang J, Fang W, Guan Z, Liao Y, Chen S (2014). Characterization of in vitro haploid and double haploid Chrysanthemum morifolium plants via unfertilized ovule culture for phenotypical traits and DNA methylation pattern, Front. Plant Sci. 5(738):1-10.
|
|
|
|
|
Yang HY, Zhou C (1990). In vitro gynogenesis. In: Bhojwani SS (ed.): Plant tissue culture: applications & limitations. The Netherlands: Elsevier Science, pp. 242-258.
Crossref
|
|
|
|
|
Yang J, Lan J, Yao P, Huang Z, Kang X (2016). Comparative microsporogenesis and flower development in Eucalyptus urophylla x E. grandis. J. For. Res. 27(2):257-263.
Crossref
|
|
|
|
|
Zavesky L (2010). Somatic embryogenesis in dandelions (Taraxacum) using anther culture. In: Kaiser BJ (ed.): Pollen structure, types and effects. New York: Nova Science Publishers, pp. 317-330.
|
|
|
|
|
Zeisek V, ŠtÄ›panek J, Amini Rad M (2015). Microsatellite variations, sexual reproduction and taxonomic revision of Taraxacum sect. Dioszegia: relationships on a large spatial scale. Preslia 87:55-85.
|
|