Full Length Research Paper
ABSTRACT
Lippia multiflora is a plant whose leaves and flowers are used as a food in Benin, so it is important to determine the nutritional properties of leaves and flowers of L. multiflora. Biochemical analyses for the determination of macronutrients, mineral salts and vitamins A and C on leaves and flowers of this leaf vegetable were done. The result indicates that the contents in leaves and flowers are, respectively: 5.44± 1.27 and 6.37± 1.61% for proteins, 13.17± 2.79 and 2.1 ± 0.54% for lipids, 0.46±0.09 and 12.53± 2.38% for carbohydrates, 19.65± 1.05 and 23.65± 0.46%, for fiber, 570±110.99 and 283.868± 26.64 mg/100 g for phosphor, 2872± 371.13 and 2755.83± 427.7 mg/100g for calcium, 683.5±113.35 and 2755.83± 427.7 mg/100 g for magnesium, 2009.83± 167.12 and 1722± 94.55 mg/100 g for potassium, 57.25± 14.97 and 46.75± 6.36 mg/100 g for iron, 64.47±10.73 and 40.52 ± 8.47 mg/100 g for vitamin A, 3511.5±200.03 and 2778.83±444.66 mg/100 g for vitamin C. In conclusion, leaves and flowers of L. multiflora are rich in nutrients and could constitute for this population an important food supplement.
Key words: Lippia multiflora, nutritional properties, food supplement, Benin.
INTRODUCTION
Leaf vegetables constitute excellent contribution for diversification of human food (Agbankpé et al., 2014). Indeed, they play an important role in food regimes of all world population, especially in Africa, Asia and Oceania, where they assure essential part of nutritional and medicinal needs (Batawila et al., 2005; Vodouhè and Dansi, 2012; Akakpo and Achigan-Dako, 2019). These leaf vegetables contain micronutrients (vitamins, minerals) that contribute to human well-being (Food and Agriculture Organization, 1988; Rubaihayo, 1992; Achikanu et al., 2021). They are foods with high nutritional value because they contain carotenes (provitamin A), various B vitamins (thiamin, riboflavin, niacin), folic and folates acid, vitamin C, minerals and proteins (Stevels, 1990; Maundu, 2005; Soro et al., 2012, Melse-Boonstra, 2020). Recent nutritional studies carried out in Benin on some leafy vegetables showed that Crassocephalum rubens and Crassocephalum crepidioides are rich in nutrients and minerals and are very good sources of vitamin C (Adjatin et al., 2013). Vernonia amygdalina, Crateva adansonii and Sesamum radiatum are also rich in nutrients and mineral salts (Agbankpé et al., 2015). An ethnobotanical survey in Benin on LFTs revealed 187 plant species including Lippia multiflora which proved to be of major interest (Dansi et al., 2012; Djengue et al., 2017). L. multiflora is used in Benin like a nutritious food in addition to its medicinal value and possessing antibiotic, antidiabetic, anti-malarial, anti-diarrheal, antidiuretic properties and treats indigestion problems indigestion, headache and toothache, chicken pox, ulcer, fever, stress, hemorrhoids, anemia, dysentery, and epilepsy. It is also used as an aphrodisiac and laxative and is without risk of toxicity for consumers (Djengue et al., 2017).
In spite of its medicinal importance, its nutritional value is not known in Benin. Therefore, the aim of this study was to determine the nutritional properties of L. multiflora in order to stimulate interest in its use by raising awareness among the populations on its nutritional importance as a food. Specifically, it was about:
(1) Assessing macronutrients content (water, lipids, carbohydrates, proteins, ash and fiber) of leaves and flowers;
(2) Determinate mineral salts content and vitamins A and C of leaves and flowers.
MATERIALS AND METHODS
Samples collect and preparation
The samples (leaves and flowers) of L. multiflora were collected from six districts (Bantè, Dassa Zoumè, Djidja, Glazoué, Savalou and Savè) of central Benin. To prepare the samples, leaves and flowers of L. multiflora were washed thoroughly under running tap water followed by sterile distilled water, cut into smaller pieces and dried under shade for 9 days. The dried plant parts were ground using electric blending machine and the powdery samples obtained were sieved using two sieves of 0.2 mm (mesh size) and stored in air tight sterile containers until needed. Chemical analyses were done on the powders of leaves and flowers for assessment of the following constituents: water, proteins, lipids, carbohydrates, fiber, ashes, mineral components (calcium, copper, iron, magnesium, manganese, phosphor, potassium, and sodium) and of vitamin A and C (Senga et al., 2013).
Determination of macronutrients content
Chemical analysis was carried out on powdered materials of leaves and flowers of L. multiflora. Analyses were made for: water, crude proteins, crude lipids, ashes, fibers and carbohydrates (Adjatin et al., 2013; Nair et al., 2012; Senga et al., 2013; Salma 2020). Crude protein was determined by using the Kjeldahl method (Nair et al., 2012; Salma, 2020). Water and crude lipids were determined according to the procedure of Association of Official Analytical Chemist (AOAC, 1990). The total crude fiber content of powders was determined using method described by Diallo et al. (2015). The percentage was calculated based on the dry weight. Ashes were determined after incineration in a muffle furnace following Bangash et al. (2011). Fehling's method was adopted to detect the presence of carbohydrates in leaves and flowers of leaf vegetable (EPSIC, 1999).
Determination of mineral composition
Mineral composition of samples was determined according to methods recommended by Association of Official Analytical Chemists (AOAC, 1990), Badau et al. (2013), and Kamal et al. (2021). The samples were incinerated in the oven at a temperature of 550°C for 3 h. The samples of Lippia multifora were each digested using a mixture of concentrated Nitric (HNO3), perchloric (HClO4) and sulphuric (H2SO4) acids in the ratio 9:2:1 (v/v), respectively (Nair et al., 2012). Copper (Cu), iron (Fe), zinc (Zn), sodium (Na), potassium (K), calcium (Ca) and magnesium (Mg) and manganese (Mn) were determined by Atomic Absorption Spectrophotometer (AAS) (PerkinElmer A Analyst 700, England). Phosphorus contents of the samples were determined using Flame photometer as specified in Alinnor and Oze (2011).
Dosage of vitamins
Dosage of provitamin A and Vitamin C
Provitamin A was assayed according to AOAC method (AOAC, 2005). The assay was performed with 1 mL of trifluoroacetic acid, using a Biomate 3 spectrophotometer at 620 nm. Vitamin C was assayed by the Camag TLC Scanner III densitometer according to the method of Nair et al. (2012).
Statistical analysis
ANOVA was performed to compare the 2 factors (zone and parts) and the interaction. Level of significance was set at 5% (p< 0.05). Quantitative data were processed using XSLTAT version 2015 and CATS 1.2softwares.
RESULTS AND DISCUSSION
Proximal composition
Figures 1 and 2 show variations of proximal compositions of leaves and flowers of six areas (Bante, Dassa-Zoume, Djidja, Glazoue, Savalou and Save). Fiber content is between 18.525 and 20.93% for leaves and 23.15 and 24.22% for flowers. Ash content is between 12.46 and 15.49% for leaves and 10.65 and 12.51% for flowers. That of carbohydrates is between 0.4 and 0.62% for leaves and 8.8 and 15.25% for flowers. Lipids content is between 0.9 and 1.7% for leaves and 1.3 and 2.7% for flowers. That of proteins is between 3.92 and 6.75% for leaves and 3.84 and 8.8% for flowers. Finally, the water content is between 2 and 8.5% for leaves and 7.96 and 11.54% for flowers. We note that there is a variation of these parameters in leaves and flowers of one area to another. This variation could be explained by phenology or plant development stage. These results are similar to those reported in Ivory Coast by Kane et al. (2010) which have demonstrated there is a variation of these parameters on different types of soil.
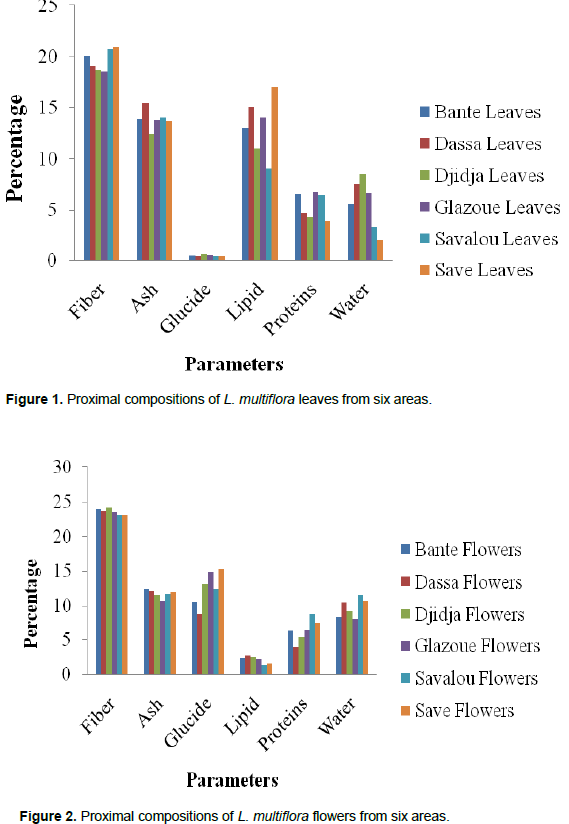
Table 1 describes the significance of these different values. From these results, no significant difference (p>0.05) was observed about water, lipid, carbohydrates, fiber, ash and sodium content by area. However significant difference (p<0.05) was shown with protein content. In leaves and flowers of the same area, a significant difference (p<0.05) was observed with fiber, ash, carbohydrates, protein, lipid and water. But the difference in protein content was not significant (p<0.05). This significant difference could be due to used organs. Furthermore, Dougnon et al. (2012) showed that there is a significant difference in terms of proximal composition between Solanum macrocarpon leaves and fruits. With regard to interaction zone-part, the difference is significant (p<0.05) for fibers, carbohydrates, proteins, lipids and water content but is not significant (p>0.05) for ash content.
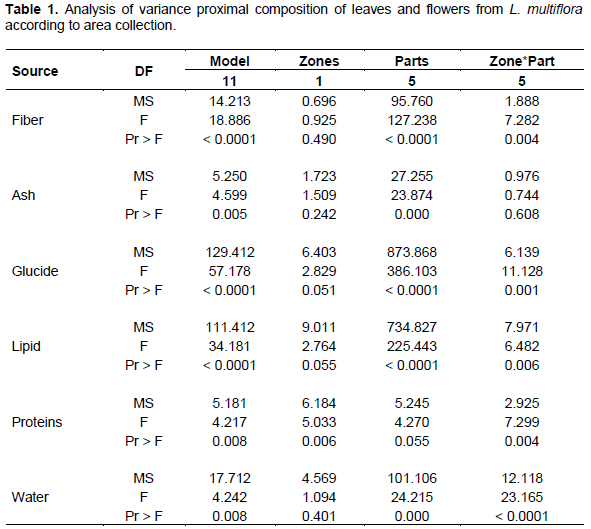
Results on proximal composition revealed that leaves and fibers were most abundant followed by ash, lipids, water, proteins and carbohydrates (Table 2). In flowers, fibers are also most abundant followed by carbohydrates, ash, water, proteins and the least abundant elements are lipids. Thus, flower was richer in fiber, carbohydrates, protein and water than leaf while leaf was richer in ash and lipids.
The water contents of leaves and flowers of L. multiflora were 5.58 and 9.68%, respectively. These values were respectively less than 12 and 17% reported by Kane et al. (2010) and 20.36 and 36.82% according to Ekissi et al. (2011) in Ivory Coast. In addition, Ekissi et al. (2011) reported a water content of less than 19.86% in the flowers of this plant. This difference may be related to phenology. Moreover, these values were also lower than those reported by Adjatin et al. (2013) on C. crepidioides and C. rubens, Yameogo et al. (2011) on Moringa oleifera (73.90%). The differences observed between the results of this study and those of these authors could be due to the plant species used. The low content in water of leaves and flowers of L. multiflora indicates that this species would not be susceptible to microbial attack during storage and would not also be highly perishable (Pillai and Nair, 2013).
The average protein contents of leaves and flowers of L. multiflora are 5.44 and 6.37% g-1, respectively of dry matter. According to the results reported by Kane et al. (2010) and Ekissi et al. (2011), the protein content of fresh leaves were lower than that of dry leaves with values of (8.05%; 8.75%) and (9.63%; 11.21%). In the same plant, the protein content in flowers was also lower (12.95%) according to Ekissi et al. (2011). These results are similar to those reported by Itoua et al. (2015) on Phytolacca dodecandra and different from those reported by Kouame et al. (2015) on Myrianthus arboreus with a protein content of 57.02%. Food plants whose protein content is more than 12% of caloric value are considered to be good sources of protein.
The total lipid contents in leaves and in dried flowers of L. multiflora were 1.32 and 2.1%, respectively. These values are lower than those reported by Adjatin et al. (2013) on C. crepidioides (3.45%) and C. rubens (2.75%), and Kouame et al. (2015) on leaves of M. arboreus (5.8%). A diet providing 1 to 2% of caloric energy as fat may be sufficient for humans because excess in fat consumption leads to cardiovascular disorders such as arteriosclerosis, cancer, and aging (Antia et al., 2006).
Fiber content in leaves and flowers were 19.65 and 23.65%, respectively. These values are higher than those reported by Adjatin et al. (2013) on C. crepidioides (8.18%) and C. rubens (7.95%), and Sodamade (2013) on V. amygdalina (10.46 mg/100 g). However, these values are lower than those reported by Ejoh et al. (2007) on Gymnantheum amygdalinum (25.47%) known as one of leafy vegetables particularly rich in fiber. L. multiflora is a very good source of dietary fiber and deserves more attention. Fiber is involved in digestion and reduces the risk of cardiovascular disease (Badau et al., 2013).
Studies have shown that increasing fiber intake may help reducing the incidence of some diseases such as diabetes, coronary heart disease, colon cancer, and various digestive disorders (Badau et al., 2013). Consuming fiber also softens the stool and lowers blood cholesterol levels in the body (Pillai and Nair, 2013). Maintaining internal distension for normal peristaltic movement of the gastrointestinal tract is a physiological function provided by crude fibers. However, very high fibers content can cause intestinal irritation and decreased nutrient bioavailability (Pillai and Nair, 2012).
The average ash content was 13.89% for the leaves and 11.76% for the flowers of L. multiflora. These results are similar to those reported by Ekissi et al. (2011). However, Adjatin et al. (2013) and Kouamé et al. (2015) reported higher values on C. crepiodioides (19.02%), C. rubens (19.76%) and M. arboreus (36%) while that Yaméogo et al. (2011) and Andzouana and Mombouli (2012) reported lower values on M. oleifera (11.10%) and Ochthocharis dicellandroides (4.19%). The high ash content is a reflection of the mineral salt content in the food. Further study would be needed to determine the types of mineral elements as they are essential for the functioning of tissues and necessary for daily needs.
The average carbohydrate content of L. multiflora was 0.46% for leaves and 12.53% for flowers. These results are similar to those reported in Ivory Coast by Kane et al. (2010). The leaves of C. crepidiodes (42.22%), C. rubens (43.11%), Talinum triangulare (10.87%) and O. dicellandroides (11.73%) are relatively richer in carbohydrates than those of L. multiflora (Adjatin et al., 2013; Aja et al., 2010; Emebu and Anyika, 2011). Most vegetables are not generally good sources of carbohydrates. Some of them are richer while others contain trace (Andzouana and Mombouli, 2012), which means that L. multiflora is not a good source of carbohydrate.
Mineral and vitamins compositions
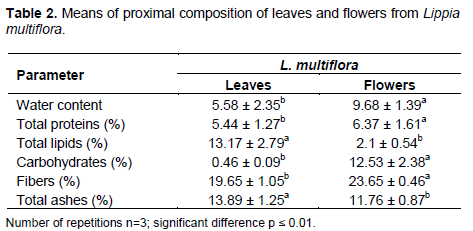
Figures 3 and 4 show variations of mineral and vitamins compositions of leaves and flowers of studied areas. The mineral elements (mg/100 g) in the leaves and flowers from Bantè, Dassa-Zoumè, Djidja, Glazoué, Savalou and Savè was as follows: phosphorus: leaves (from 393.3 to 723.9 mg) and flowers (from 243.07 to 318.4 mg), calcium: leaves (from 2494 to 3270 mg) and flowers (from 1923 to 3270 mg), magnesium: leaves (from 513 to 860 mg) and flowers (from 424 to 620 mg), potassium: leaves (from 1748 to 2246 mg) and flowers (from 1620 to 1910 mg), sodium: leaves (from 49 to 92 mg) and flowers (from 25 to 52 mg), iron: leaves (from 41.85 to 85.92 mg) and flowers (from 39.26 to 56.72 mg), copper: leaves (from 1.86 to 4.8 mg) and flowers (from 1.1 to 1.25 mg) and manganese: leaves (from 5.98 to 9.8 mg) and flowers (from 3.6 to 5.1 mg) (Figures 3 and 4).
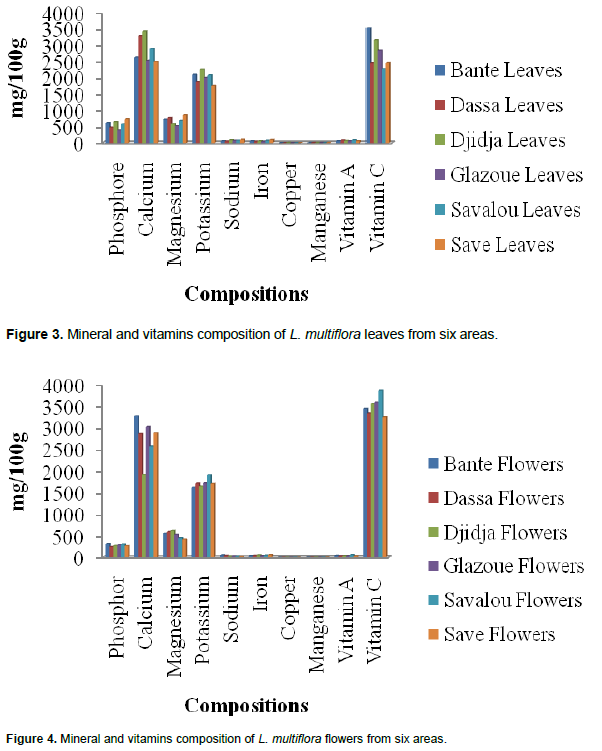
The provitamin A content varied from 54 to 79.6 mg /100 g in leaves and from 32.6 to 56.6 mg/100 g in flowers. Vitamin C content ranges from 2442to 3521 mg/100 g in leaves and from 3248 mg/100 g to 3870 mg/100 g in flowers. This variation could be explained by phenology. These results are similar to those reported in Ivory Coast by Kouassi et al. (2013) on okra varieties. These authors have shown that there is a variation in magnesium, potassium, manganese and sodium contents depending on the growing areas.
The results in Table 3 show that the phosphorus, calcium, magnesium, potassium, iron, copper, manganese, provitamin A and vitamin C contents of leaves and flowers are significantly different between areas (p<0.05). The difference between sodium contents were not significant (p> 0.05). These results differ from those reported in Ivory Coast by Kouassi et al. (2013). These authors have shown that there is no significant difference in magnesium, potassium, and manganese content of okra varieties by growing area. A significant difference (p<0.05) between phosphorus, calcium, magnesium, potassium, sodium, iron, copper, manganese, provitamin A and vitamin C contents in leaves and flowers in the same area was observed. This significant difference could be due to the particular metabolism of these organs. In addition Dougnon et al. (2012) have shown that there is a significant difference in mineral and vitamin content between leaves and fruits of S. macrocarpon. In terms of zone-Organs interaction, the difference is significant (p< 0.05) for phosphorus, calcium, magnesium, potassium, sodium, iron, copper, manganese, provitamin A and vitamin C contents. This justifies that the contents of these minerals and vitamins were specific to the area in which the species were found.
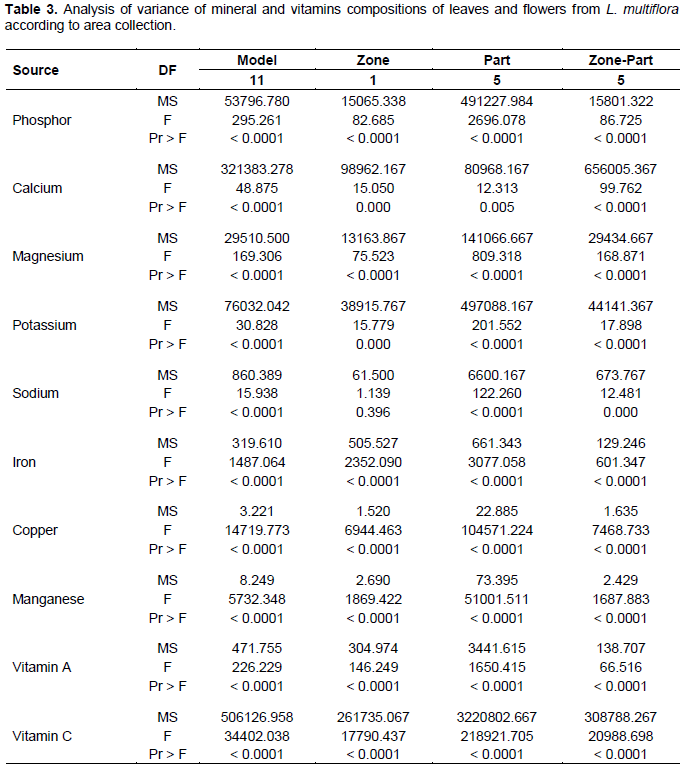
The results presented in Table 4 show that L. multiflora was a leaf vegetable rich in provitamin A, vitamin C, and minerals. The main minerals found in leaves and flowers were calcium (Ca), potassium (K), magnesium (Mg), Iron (Fe), copper (Cu) and manganese (Mn). Sodium (Na) and phosphorus (P) were found in low quantity. Leaves were richer in provitamin A and minerals than the flowers. However, flowers were richer in vitamin C.
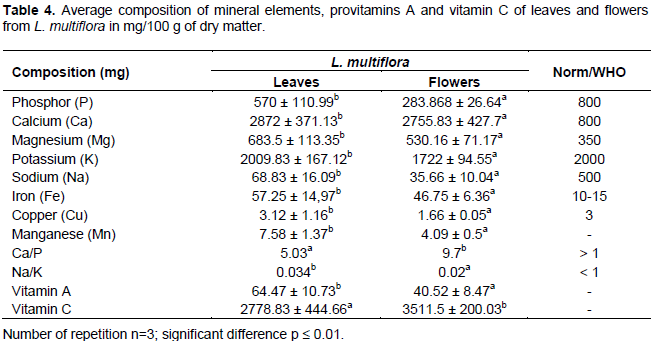
Calcium contents of leaves and flowers of L. multiflora were 2872 and 2755.83 mg/100 g, respectively. These values ??are higher than those reported by Agbankpé et al. (2015) on V. amygdalina (1180 mg/100 g), C. adansonii (2400 mg/100 g) and S. radiatum (1520 mg/100 g).
These values ??are also higher than those reported on C. crepidioides (1012 mg/100 g) by Adjatin et al. (2013) and lower than that of C. rubens (3845.88 mg/100 g) reported by the same authors. This leafy vegetable could then be considered as a potential source of calcium. Calcium is the most abundant mineral in body and is involved in blood clotting, muscle contraction, neurological function, bone and tooth formation (Senga et al., 2013). It is also an important factor in enzymatic metabolic processes (Karau et al., 2012).
The average phosphorus contents were 570 and 283.868 mg/100 g in leaves and flowers of L. multiflora, respectively. According to results reported by Agbankpé et al. (2015) as well as those reported by Adjatin et al. (2013), these contents are lower than those of V. amygdalina (873 mg/100 g), C. adansonii (693 mg/100 g), S. radiatum (650 mg/100 g), C. crepidiodes (1039.2 mg/100 g) and C. rubens (1409 mg/100 g). The recommended daily intake of phosphorus for adults and children is 800 mg/day (Pillai and Nair, 2013). Phosphorus associated with calcium, helps strengthen bones and teeth, especially in children and nursing mothers (Andzouana and Mombouli, 2012). The phosphorus content obtained from leaves and flowers of L. multiflora is below the recommended norm. Therefore, L. multiflora is not a good source of phosphorus.
The average contents of potassium in leaves and flowers were 2009.83 and 1722 mg/100 g, respectively. These values ??are lower than those reported by Agbankpé et al. (2015) on V. amygdalina (4820 mg/100 g), C. adansonii (2810 mg/100 g) and Adjatin et al. (2012) on C. crepidioides (2291.86 mg/100 g) and C. rubens (4469.91 mg/100 g). Potassium is important in regulating heart rate, body water balance and neurotransmission (Alinnor and Oze, 2011). A high quantity of potassium in body increases iron use (Nair et al., 2012) and is beneficial for people taking diuretics to control high blood pressure (Nair et al., 2012).
The average magnesium content in leaves and flowers were 683.5 and 530.16 mg/100 g respectively. These values ??are higher than those reported by Agbankpé et al. (2015) on C. adansonii (670 mg/100 g) and S. radiatum (510 mg/100 g) as well as those reported by Adjatin et al. (2012) on C. crepidioides (336.46 mg/100 g) and C. rubens (434.13 mg/100 g). According to the results reported by Agbankpé et al. (2015), these values ??are lower than that of V. amygdalina (900 mg/100 g). The recommended dietary allowance for magnesium is 350 mg/100 g for adults and 170 mg/100 g for children. From these results, it emerges that L. multiflora is rich in magnesium and could meet the daily needs of adults and children. Magnesium is known to prevent cardiomyopathy, muscle degeneration, growth retardation, alopecia (premature hair loss), dermatitis, immune system dysfunction, gonad atrophy, impaired spermatogenesis, birth defects and coagulation disorders (Andzouana and Monbouli, 2012). According to Alinnor and Oze (2011), magnesium plays an essential role in calcium metabolism and in bone formation and is also involved in the prevention of diseases related to the circulatory system. It helps regulate blood pressure and insulin secretion.
Sodium is an important mineral that helps regulate blood flow and maintain electron potential in body tissues (Alinnor and Oze, 2011). The sodium content average in leaves and flowers were 68.83 and 35.66 mg/100 g, respectively. These contents are much lower than those reported by Adjatin et al. (2013) on C. crepidioides (2291.86 mg/100 g) and C. rubens (2921.04 mg/100 g). They are higher than those reported by Sanoussi et al. (2016) on Ipomoea Batatas (29 and 34 mg/100 g).
The average iron content was 57.25 mg/100 g in leaves and 46.75 mg/100 g in flowers of L. multiflora (Table 4). This content is much higher than that recommended by WHO, which is 10 to 15 mg/day (Senga et al., 2013).
According to Andzouana and Monbouli (2012), iron as a trace element plays many biochemical roles and is a fundamental element in the metabolism of almost all living organisms. In humans, iron is an essential constituent of several types of proteins and enzymes (Andzouana and Monbouli, 2012). It is important for the normal functioning of the central nervous system (Alinnor and Oze, 2011) and facilitates the oxidation of carbohydrates, proteins and lipids. Iron is necessary for formation of hemoglobin contained in blood and is an important component in diet of pregnant women, nursing mothers, infants and the elderly who often suffer from anemia and other illnesses related to blood (Alinnor and Oze, 2011).
The average copper content was 3.12 mg/100 g in leaves and 1.66 mg/100 g in flowers. Copper content in leaves is higher than that reported by Adjatin et al. (2013) on C. crepidioides (1.4 mg/100 g) and C. rubens (2.6 mg/100 g). Copper is necessary for enzymes production and electrons transport in body (Alinnor and Oze, 2011).
The average manganese content was 7.58 mg/100 g in leaves and 4.09 mg/100 g in flowers. These values ??are lower than those reported by Adjatin et al. (2013) on C. crepidioides (7.7 mg/100 g) and C. rubens (8.22 mg/100 g). Manganese is a trace element that plays an important role in synthesis of bones, amino acids and metabolism of carbohydrates. It contributes to the proper functioning of thyroid gland and serves to heal inflammation and sprains (Erikson et al., 2005). Manganese deficiency can lead to problems with infertility, diabetes, and joint pain. It is an antioxidant which protects body against damage caused by free radicals. Despite its essential character, the accumulation of manganese in blood is toxic to central nervous system (Sidoryk and Aschner, 2013).
The value of Ca/P ratio was, respectively 5.03 and 9.7 for leaves and flowers. According to Adeyeye and Aye (2005) and Alinnor and Oze (2011), a Ca/P ratio greater than 2 helps to increase the absorption of calcium in small intestine. Diet is considered good if Ca/P ratio is greater than 1 and poor if the ratio is less than 0.5. Therefore, L. multiflora appears to be a good source of food. Furthermore, Na/K ratio of leaves and flowers were, respectively 0.034 and 0.02. Alinnor and Oze (2011) reported that Na/K ratio promotes blood pressure control and a Na/K ratio less than 1 lowers blood pressure. So L. multiflora could be used as a food to treat or prevent blood pressure problems.
The results of Table 4 have shown that the average of provitamin A content was 64.47 mg/100 g in leaves and 40.52 mg/100 g in flowers. These values are higher than those reported by Millogo-Koné et al. (2010) on M. oleifera leaves (39 mg/100 g) and lower than those reported by Tchiégang and Aissatou (2004) on Hibiscus cannabinus (60 mg/100 g) and Cerathotheca sesamoïdes (90 mg/100 g). Vitamin A is involved in the synthesis of bones, teeth, hair and reproduction (Wardlaw and Kessel, 2002). It is mainly involved in the processes of vision maintenance, regulation of gene expression and cell differentiation (Herrero et al., 2012). According to Vanisha et al. (2008), the vitamin A requirements for an adult weighing 70 kg are 1000 μg/d which means that L. multiflora could be considered as a potential source of provitamin A.
The average vitamin C content was respectively 2778.83 and 3511.5 mg/100 g in leaves and flowers of L. multiflora. These values ??are much higher than those reported by Millogo-Koné et al. (2010) on M. oleifera (210 mg/100 g), C. crepidioides (9.17 mg/100 g) and Adjatin et al. (2013) on C. rubens (3.60 mg/100 g). According to Tchiégang and Aissatou (2004), these values are less than 4590 mg/100 g in T. triangulare and 4920 mg/100 g in Vigna unguiculata. Daily vitamin C requirements vary between 40 and 90 mg/day (Vanisha et al., 2008). So this leafy vegetable would be a very good source of vitamin C. Vitamin C plays an important role in maintaining good health and preventing disease. It has immuno-stimulating, anti-allergic and antioxidant effects and protects the cardiovascular system and eyes. Vitamin C is necessary for the synthesis of collagen, the intercellular substance that provides the structure of muscles, vascular tissues, bones, tendons and ligaments (Olayinka et al., 2012).
CONCLUSION
The results of this work provided data on the nutritional composition of leaves and flowers of L. multiflora in Benin. Leaves and flowers of L. multiflora are rich in fiber, ash, calcium, magnesium, potassium, copper and especially iron, provitamin A and vitamin C. So this plant is a good source of nutrients. So it is important to use it in improving the health status of populations and in the fight against malnutrition.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests
REFERENCES
|
Achikanu CE, Akpata E, Uwa J (2021). Level of minerals in ten leafy vegetables eaten in Enugu State Nigeria. Special Journal of Public Health, Nutrition, and Dietetics 1(2):1-10. |
|
|
Agbankpé AJ, Dougnon TVB, Bankole S, Yèhouénou B, Yedomonhan H, Legonou M et Dougnon TJ (2014). Etude ethnobotanique des légumes feuilles thérapeutiques utilisés dans le traitement des diarrhées au sud-Bénin (Afrique de l'Ouest). International Journal of Biological and Chemical Sciences 8(4):1784-1795. |
|
|
Agbankpé AJ, Bankolé SH, Dougnon TJ, Yèhouénou B, Hounmanou YMG, Baba-Moussa LS (2015). Comparison of nutritional values of Vernonia amygdalina, Crateva adansonii and Sesamum radiatum: Three main vegetables used in traditional medicine for the treatment of bacterial diarrhoea in southern Benin (West Africa). Food and Public Health 5(4):144-149. |
|
|
Adjatin A, Dansi A, Badoussi E, Sanoussi AF, Dansi M, Azokpota P, Ahissou H, akouegninou A, Akpagana K, Sanni A (2013). Proximale, mineral and vitamine C composition of vegetable Gbolo (Crassocephalum rubens and C. crepidioides) in Benin. International Journal of Biological and Chemical Sciences 7(1):319-331. |
|
|
Aja PM, Okaka ANC, Ibiam UA, Uraku AJ, Onu PN (2010). Proximate analysis of Talinum triangulare (water leaf) leaves and its softening principle. Pakistan Journal of Nutrition 9(6):524-526. |
|
|
Akakpo Amandine DM, Achigan-Dako Enoch G (2019). Nutraceutical Uses of Traditional Leafy Vegetables and Transmission of Local Knowledge from Parents to Children in Southern Benin. Agronomy 9(12):805. |
|
|
Alinnor IJ, Oze R (2011). Chemical evaluation of the nutritive value of Pentaclethra macrophylla Benth (African Oil Bean) Seeds. Pakistan Journal of Nutrition 10(4):355-359. |
|
|
Antia BS, Akpan EJ, Okon PA, Umoren IU (2006). Nutritive and Anti-Nutritive valuation of Sweet Potatoes (Ipomoea batatas) Leaves. Pakistan Journal of Nutrition 5:166-168. |
|
|
Association of Official Analytical Chemists (AOAC) (1990). Official methods of Analysis (15th Edition): Helrich K. Ed.; Association of Official Analytical Chemists, Washington D.C. |
|
|
Association of Official Analytical Chemists (AOAC) (2005). Official method of analysis of the Association of official Analytical Chemist. 5th ad. AOAC Press Arlington, Virginia, USA. |
|
|
Andzouana M, Mombouli JB (2012). Assessment of the chemical and phytochemical constituents of the leaves of a wild vegetable Ochthocharis dicellandroides (Gilg). Pakistan Journal of Nutrition 11(1):94-99. |
|
|
Badau MH, Abba HZ, Agbara GI, Yusuf AA (2013). Proximate composition, mineral content and acceptability of granulated maize dumpling (Dambu Masara) with varying proportions of ingredients. Global Advanced Research Journal of Agricultural Science 2(1):320-329. |
|
|
Batawila K, Akpavi S, Wala K, Kanda M, Vodouhe R, Akpagana K (2005). Diversité et gestion des légumes de cueillette au Togo. African Journal of Food Agriculture Nutrition and Development 21 p. |
|
|
Dansi A, Vodouhè R, Azokpota P, Yedomonhan H, Assogba P, Adjatin A, Loko YL, Dossou-Aminon I, Akpagana K (2012). Diversity of the Neglected and Underutilized Crop species of importance in Benin. The Scientific World Journal pp. 1-19. |
|
|
Djengue HW, Dansi A, Adjatin A, Dossou-Aminon I, Dansi M and Sanni A (2017). Ethnobotanical Investigation of Lippia multiflora Moldenke, a Local Aromatic Leafy Vegetable under Domestication in Benin. International Journal of Current Research in Biosciences and Plant Biology 4(5):44-51. |
|
|
Diallo KS, Koné KY, Soro D, Assidjo NE, Yao KB, Gnakry D (2015). Caractérisation biochimique et fonctionnelle des graines de sept cultivars de voandzu (Vigna subterranea L., Fabaceae), cultivés en Côte d'Ivoire. European Scientific Journal 11(27):288-304. |
|
|
Dougnon TV, Bankolé HS, Johnson RS, Klotoé JR, Fernand GD, Assogba GF (2012). Phytochemical screening, nutritional and toxicological analyses of leaves and fruits of Solanum macrocarpon Linn (Solanaceae) in Cotonou (Benin). Food and Nutrition Sciences 3(11):1595-1603. |
|
|
Ekissi Alice Christine, Konan Amoin Georgette, Yao-Kouamé Albert, Bassirou bonfoh and Kati-Coulibaly Séraphin (2011). Evaluation of the chemical constituents of savannah tea (Lippia multiflora) leaves. Journal of Applied Biosciences 42:2854-2858. |
|
|
Ejoh RA, Nkonga DV, Innocent G, Moses MC (2007). Nutritional components of some non-conventional leafy vegetables consumed in Cameroon. Pakistan Journal of Nutrition 6(6):712-717. |
|
|
Emebu PK, Anyika JU (2011). Proximate and mineral composition of kale (Brassica oleracea) grown in Delta State, Nigeria. Pakistan Journal of Nutrition 10(2):190-194. |
|
|
EPSIC (1999). Nutriments et énergie. Menu de la chimie: démonstrations disponibles et Travaux Pratiques (TP), Chapitre 7 pp. 1-6. |
|
|
Erikson KM, Syversen T, Aschner JL, Aschner M (2005). Interactions between excessive manganese exposures and dietary iron-deficiency in neuro-degeneration. Environmental Toxicology and Pharmacology 19(3):415-421. |
|
|
Food and Agriculture Organization (FAO) (1988). Traditional food plants. Food and Nutrition Paper 42:1-3. |
|
|
Herrero M, Cifuentes A, Banez E (2012). Comprehensive sampling and sample preparation, Elsevier, J. Pawliszyn Editor, Amsterdam3. |
|
|
Itoua OYS, Elenga M, Moutsamboté JM, Mananga V, Mbemba F (2015). Évaluation de la consommation et de la composition nutritionnelle des légumes-feuilles de Phytolacca dodecandra consommés par les populations originaires des districts d'Owando et de Makoua. Journal of Animal and Plant Sciences 27(1):4207-4218. |
|
|
Kamal B, Farid M, Abdessamad BM, Marianne S, Marie-Laure F, Mohamed B, Hana SC, Ahmed E (2021). Proximate Composition, Amino Acid Profile, and Mineral Content of Four Sheep Meats Reared Extensively in Morocco: A Comparative Study. The Scientific World Journal 6633774, 11 pages. |
|
|
Kane F, Yao-kouame A, Konan AA, N'guessan AK (2010). Dosage de quelques composantes biochimiques des feuilles de Lippia multiflora (verbénacée) à deux stades de développement et qualité des infusions, en fonction de la dose d'urée. Agronomie Africaine 22(3):227-235. |
|
|
Karau GM, Njagi NM, Machocho AK, Wangai LN (2012). Phytonutrient, mineral composition and in vitro antioxidant activity of leaf and stem bark powders of Pappea capensis (L.). Pakistan Journal of Nutrition 11(2):123-132. |
|
|
Kouame NM, Soro K, Mangara A, Diarrassouba N, Coulibaly AV, Boraud N, Kama M (2015). Étude physico-chimique de sept (7) plantes spontanées alimentaires du centre-ouest de la Côte d'Ivoire. Journal of Applied Biosciences 90(1):8450-8463. |
|
|
Kouassi JB, Massara CC, Daniel ES, Tiahou GG, Djohan FY (2013). Détermination des teneurs en Magnésium, Potassium, Manganèse et Sodium de deux variétés de gombo. Journal of Applied Biosciences 67:5219-5227. |
|
|
Maundu P (2005). Les légumes-feuilles traditionnels. |
|
|
Melse-Boonstra Alida (2020). Bioavailability of Micronutrients From Nutrient-Dense Whole Foods: Zooming in on Dairy, Vegetables, and Fruits. Frontiers in Nutrition 7:101. |
|
|
Millogo-Koné H, Kini BF, Yougbaré Z, Yaro MB, Sawadogo M (2014). Etudes de la phytochimie et de l'activité antimicrobienne in vitro des feuilles de Moringa oleifera (Moringaceae). Pharmacopée et Médecine Traditionnelle Africaine P 16. |
|
|
Nair AG, Pradeesh S, Devi CM, Mini I, Swapna TS (2012). Diplazium esculentum: A Wild Nutrient-Rich Leafy Vegetable from Western Ghats. Prospects in Bioscience 1:293-301. |
|
|
Olayinka OO, Kareem AM, Ariyo IB, Omotugba SK, Oyebanji AO (2012). Antioxidant contents (vitamin C) of raw and blanched different fresh vegetable samples. Food and Nutrition Sciences 3:18-21. |
|
|
Pillai LS, Nair BR (2013). Proximate composition, Mineral elements and Anti-nutritional factors in Cleome viscosa L. and Cleome burmanni W. & A. (Cleomaceae). International Journal of Pharmacy and Pharmaceutical Sciences 5(1):384-387. |
|
|
Rubaihayo EB (1992). The diversity and potential use of local vegetables in Uganda. In: Guarino L (Ed.). The First National Plant Genetic Resources Workshop: Conservation and Utilization. IPGRI, Kenya, pp. 109-114. |
|
|
Salma S (2020). Nutritional and functional properties of Moringa oleifera. Metabolism Open 8 (100061). |
|
|
Sanoussi AF, Adjatin A, Dansi A, Adebowale A, Sanni LO, Sanni A (2016). Mineral composition of tenelites sweet potato (Ipomoea Batatas L. Lam.) Landraces of Benin. International Journal of Current Microbiology and Applied Sciences 5(1):103-115. |
|
|
Senga KP, Opota OD, Tamba VA, Tona LG, Kambu KO, Covaci A (2013). Chemical composition and nutritive value study of the seed oil of Adenanthera pavonina L. (Fabaceae) growing in Democratic Republic of Congo. International Journal of Pharmtech Research 5(1):205-216. |
|
|
Sidoryk-Wegrzynowicz M, Aschner M (2013). Manganese toxicity in the central nervous system: the glutamine/glutamate-γ-aminobutyric acid cycle. Journal of Internal Medicine 273(5):466-477. |
|
|
Soro LC, Ocho-Anin AAL, Kouadio KKA, Kouamé C (2012). Evaluation de la composition nutritionnelle des légumes feuilles. Journal of Applied Biosciences 51:3567-3573. |
|
|
Sodamade A (2013). Proximale analysis, mineral content, amino acid composition and functional properties of Vernonia amygdalina vegetable leaf protein concentrates. Greener Journal of Agricultural Sciences 3(3):204-210. |
|
|
Stevels JMC (1990). Legumes traditionnels du Cameroun, une etude agro-botanique. Agricultural University, Wageningen, the Netherlands Papers N° 90, 262 p. |
|
|
Tchiégang C, Aissatou K (2004). Données ethnonutritionnelles et caractéristiques physico-chimiques des légumes-feuilles consommés dans la savane de l'Adamaoua (Cameroun). Tropicultura 22(1):11-18. |
|
|
Vanisha S, Ambiar N, Shilpa P (2008). Standardization and organoleptic evaluation of Drumstick (Moringa oleifera) leaves incorporated into traditional Indian recipes. Trees for life Journal 3(2):1-7. |
|
|
Vodouhè R, Dansi A (2012). The "Bringing into Cultivation" Phase of the plant domestication process and its contributions to In Situ conservation of genetic resources in Benin. The Scientific World Journal, pp. 1-13. |
|
|
Wardlaw GM, Kessel M (2002). Perspective in nutrition (5thed). Mc graw-hill, Boston pp. 271-274. |
|
|
Yaméogo CW, Bengaly MD, Savadogo A, Nikiema PA, Traore SA (2011). Determination of chemical composition and nutritional values of Moringa oleifera leaves. Pakistan Journal of Nutrition 10(3):264-268. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0