Aloe vera L. (Aloe barbadensis Mill.) belonging to the family Liliaceae (Xanthorrhoeaceae) is an important perennial, xerophytic, medicinal, succulent herb and the genus comprises about 300 perennial species. Generally, Aloe species are identified by their leaf structure, characteristics, inflorescences and rosettes of succulent leaves (Singh et al., 2013). Aloe can grow in nutritionally poor soil and it has great demand in pharmaceutical and cosmetic industry. The plant has green fleshy leaves covered by a thick cuticle or rind and an inner clear pulp (Williams et al., 2010). Aloe is used externally for the treatment of burns, scalds, skin irritation, sunburn wounds, psoriasis, acne, dermatitis, eczema, and ulcers. The plant has numerous pharmacological values like antioxidant, anti-inflammatory, antidiabetic, anti-microbial, anti-fungal, anti-viral, anti-tumor, antiseptic, anti-aging, and other properties (Paoulomi et al., 2013). Administration of gel extract reduces blood glucose levels, blood urea and glycosylated haemoglobin (Rajasekaran et al., 2005).
In additional to the pharmaceutical applications, Aloe vera is widely used in food and cosmetic industry. In the food industry, it is used as an ingredient for health drinks, desserts and beverages and yogurt (Reynolds, 2004). In addition to the medical applications, Aloe vera has been introduced in cultivation as ornamental and house hold plant. The morphological studies are very important for cultivating the high quality of the Aloe gemplasm. Aloe is generally propagated by suckers arising from the base of the mother plant and natural vegetative propagation of Aloe is very slow. Earlier studies showed that Aloe vera has been sterilized using the mercuric chloride which is harmful for the environment and also has effect on kidney, liver, adrenal and fertility (WHO, 2005). Mercury chloride (HgCl2) is biotransformed into methyl mercury chloride (CH3HgCl) and causes adequate environmental effects by human consumption of contaminated fish, shellfish, and algae (Silva-Pereira, 2005). The alternative method for rapid multiplication of selected genotypes is possible by using sodium hypo-chlorite, which is the main objective of the present study.
Tissue culture of different Aloe spp. was reported by many researchers. Cytokinins are one of the most important growth regulators affecting the shoot proliferation (Garland and Stolz, 1981). This research work which deals with the use of shoot tip and apical meristem for micro propagation have been proposed by Debiasi et al. (2007) and Campestrini et al. (2006). Acclimatization of rooted plantlets in pots containing a mixture of sand, silt and compost under greenhouse conditions with 80 to 90% of moisture is suited for young plants survival (Hirimburegama and Gamage, 1995). Currently, it is necessary to develop efficient regeneration method for Aloe cultivation to meet the high demand, particularly, for plant genetic transformation and cloning techniques (Velcheva et al., 2005). Micro propagation of various accessions of Aloe from different regions in India has not been reported so far.
Several studies have reported about rapid in vitro propagation of Aloe vera (Albanyl, 2006). Regeneration of Aloe vera in nature is very slow due to its male sterility which forms a barrier in rapid propagation. Aloe is an exclusively propagated crop using lateral buds or off shoots produced by donor plant. A single plant usually produces 2 to 3 off shoots in a year which is not sufficient for undertaking commercial cultivation and to meet the industrial demand.The main objective of this study was to develop a rapid, efficient, cost effective, and easy method of micropropagation of Aloe vera at commercial level. In this study, media composition with new combinations and concentrations of different growth regulators for efficient and rapid micro propagation of Aloe vera was standardized using shoot tip explants.
Collection of ex-plant material
This study was carried out on germplasm of Aloe vera (A. barbadensis Miller), an important medicinal and ornamental plant collected from different places such as National Bureau of Plant Genetic Resources (NBPGR), New Delhi and Central Institute of Medicinal and Aromatic Plants (CIMAP), Boduppal, Hyderabad, India. The experimental plant material consists of 12 accessions of Aloe vera (A. barbadensis Miller); among them, 10 accessions were collected from NBPGR, voucher no: NBPGR/ 2011/1771 dated 11-07-2011 from different geographical regions of India from Rajasthan (IC111267, IC111269, IC111271, IC111272), Gujarat (IC111279), Haryana (IC111280), and Delhi (IC471882, IC471883, IC471884, IC471885), respectively and 2 accessions of Aloe CIM-Sheetal (CAL 14) and wild Aloe vera (local) were collected from CIMAP, voucher no: CIMAP/63/6222 dated 20-07-2011. All the plants used in this study were about two years of age. High potential accessions showed the highest incidence in morphological studies which are as shown in Figure 1a to f and the details of 12 accessions grown at Indian Immunologicals Ltd. Shown in Table 1.

All the accessions were examined for a number of morphological parameters; from each accession, 5 mature plants were randomly selected and the observations were recorded and mean data were used for statistical analysis. Morphological data of 12 individuals were observed. The data was transformed into numerical form and was analyzed by Newman-Kuel’s multiple comparisons test. While studying Aloe genus, various characters such as number of leaves, number of suckers, leaf width/breadth (cm), stem length (cm), peduncle length (mm), leaf thickness (density of leaf) (mm), gel fresh weight (g), leaf dry weight (g), biomass (g), root length (cm), and Aloin concentration (%) were considered.
Sterilization of explants
The shoot apices were washed thoroughly under running tap water for 30 min to remove all the adhering dust particles and microbes from the surface. The explants were then washed with 0.1% of liquid detergent (Labolene, France) for another 30 min and then rinsed several times with distilled water to remove detergent. The explants were then treated with 1% of Bavistin (systemic fungicide) and 0.25% of Dithane M-45 for 30 min, and were washed with distilled water. Then, were later treated with ampicilin (systemic bactericide) along with streptomycin for 30 min each to eliminate fungus and bacteria and the fungicide and bactericide were washed out with sterilized distilled water. Under the sterile conditions, explants were rinsed with isopropyl alcohol for 45 s and then 10 explants were treated with 2.5% sodium hypochlorite (10% Clorox) for 30 min. The explants were then thoroughly washed (4 to 5 washings) with sterilized distilled water to remove the traces of sodium hypochlorite and were trimmed to remove extra outer portion of stem discs and carefully cultured in sterile culture bottles containing 40 ml of MS medium under laminar air flow hood (Murashige and Skoog, 1962). After sterilization, shoot apices were directly inoculated into different concentrations of media for shoot elongation (Ahmed et al., 2007).
Culture medium
The basal medium contains Murashige and Skoog (MS) medium salts, vitamins, 3% sucrose and agar. The basal media was supplemented with various concentration and combination of growth regulators: 2, 4-D, 6-benzylaminopurine (BAP), naphthalene acetic acid (NAA), kinetin (KIN), indole-3-butric acid (IBA), and indole-3-acetic acid (IA). Sucrose (3%) was used as carbon source and media were solidified with agar (0.7%). Preparation of media ingredients and their concentrations as per MS medium are shown in Table 2. The medium was adjusted to pH 5.9 prior to autoclaving at 121°C and 15 lbs pressure for 20 min. The reagent grade chemicals were obtained from Fischer Scientifics and Hi Media Laboratories, India. The media sterilization was done before adding growth hormones into it because they are heat sensitive.
Inoculation of explants
After sterilization of explants, they were inoculated in culture bottles aseptically and explants were transferred onto sterile paper with the help of sterile forceps for inoculation under aseptic conditions. The explants were further trimmed and extra outer leaves were removed to make them in suitable sizes (2 to 3 cm). After cutting, explants were vertically inoculated into culture of 500 mL capacity bottles containing MS medium with 0.3 mgL-1 of IAA and 3 mgL-1 of BAP; then the bottles were tightly capped and properly sealed with wrap to avoid entry of external air. The bottles were transferred to growth chamber. All the cultures were kept in the culture room at 28 ± 1°C and at photoperiod of 16 h provided by cool-white fluorescent light and the cultures were observed periodically. Only root cultures were maintained in darkness.
Establishment of shoot cultures
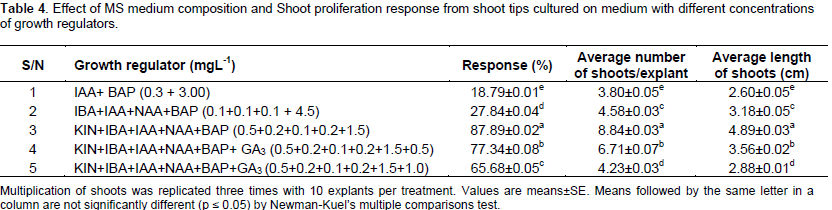
Explants of in vitro regenerated plantlets were used for proliferation of shoot cultures on the basal MS medium supplemented with kinetin (0.5 mgL-1) and BAP (1.0 mgL-1) in combination with NAA (0.1 or 0.2 mgL-1) and with IAA (0.1 mgL-1) and IBA (0.2 mgL-1) for shoot amplification. Shoots were multiplied by repetitive transfer of original explants. Cluster of shoots amplified from initial lateral shoot explants were sub-cultured as it is without separation from the explants on the same regeneration media after one month from initial establishment stage multiplication of shoots were replicated 3 times with 10 explants per treatment. Multi shoots were divided into single micro shoots and transferred to fresh medium every 5 weeks of subculture. The data was recorded after 30 days of culture and only shoots greater than 2 cm were considered for taking data. The percentage of shoot induction, total number of shoots and length of shoots were recorded after 6 weeks of fifth, seventh, and eighth subcultures. The percentage of shoot induction was calculated as the total number of initial explants, which gave response to hormonal treatment per total number of explants multiplied by 100%. After another 6 weeks of incubation, the proliferating cultures were transferred to different media for shoot elongation (Table 4).
Establishment of root cultures
The newly formed shoots were measured in the length of 2 to 3 cm,excised individually from the parent explants and transferred to a rooting media. Two types of rooting medium were used; one is MS basal media with 3 types of hormones NAA, IAA, IBA and the other one is half strength MS media. The data was recorded after 30 days of culture.
Acclimatization
The crucial step of micro propagation is acclimatization of the in vitro obtained plantlets. In vitro regenerated plants of Aloe vera with no morphological abnormalities were transplanted into pots with a survival rate of 100%. After 30 days, the micro propagated plantlets with well-developed roots were successfully acclimatized to ex vitro conditions. The pots (8×6 cm) were kept ready for filling with garden soil, compost and sand in the proportion of 2:1:1, respectively. The plants were then transplanted into the pots, then thoroughly watered and kept under plastic house having 80% humidity and 31°C temperature for 10 days. The plants were watered periodically and upper layer of the soil mulched occasionally whenever necessary, then the plants were shifted to shade house with less humidity and indirect sunlight. After 15 days, the plants were transferred to the soil. The micro propagated plants were morphologically uniform and grew well in the field. In the present study, about 100% of plantlets survived from tissue culture to the experimental plot, whereas Baksha et al. (2005) observed the survival rate of 70%, Dwivedi et al. (2014) observed 83% survival for Indian Aloe and Liao et al. (2004) observed 93% survival for Chinese Aloe.
Statistical analysis
The collected data were subjected to one way analysis of variance (ANOVA) carried out in different accessions of Aloe vera covering various parameters followed by Newman-Kuel’s multiple comparisons test. The ANOVA revealed highly significant differences among various accessions for various characters. The results indicated the presence of adequate amount of genetic variability in the germplasm.
Morphological studies
The evaluation and characterization of germplasm is prerequisite for any breeding programme aimed at improvement of yield. The qualitative as well as quantitative evaluation of germplasm is not only conducive in core collection, but also for its utilization in cultivation and breeding. The assessment of variability existing in the germplasm of accessions is of great interest for conservation of genetic resources and also for broadening of genetic base of species to be exploited by plant breeders. It helps in systematic cultivation of the plant for commercial purposes. The morphological studies for Aloe spp. are very essential characteristics for the medicinally and economically important genus to regularize the commercial and economical production. In the present study, 12 Aloe accessions were studied and the morphological characteristics results are shown in Table 3.

The result shows the occurrence of variation in morphological and biochemical characteristics when compared with the mother plants and their tissue culture generated plants. The extent of variation, however, differs from accession to accession. This could be related to differences in the genotypes of various accessions. Morphological analysis indicates that all the studied characteristics have a significant difference at P < 0.01 among Aloe accessions. Based on the morphology, the collection of accessions of Aloe vera germplasm has been divided into two morphotypes that are small Aloe vera (SAV) (plant size up to 40 cm) and large Aloe vera (LAV) (plant size above 40 cm). The LAV type of accessions shows the highest incidence. Accession IC111272(4), IC471883 (8), IC471882 (7), IC111267(1), and Aloe CIM-Sheetal (11) were found to be the tallest (61 to 67 cm range) as it possessed a distinct stem (calescent) with long inter nodes.
Minimum leaf thickness (18.1 mm) for accession IC111271(3) and minimum wideness (3.9 cm) accession for local Aloe vera (12) was recorded. Leaf dry weight of local Aloe vera (12) was very low (12.20 g) and the highest (23.7 g) was in IC 111269 (2) among other accessions. Gel fresh weight was recorded as 58.52 ml/100 g in IC 111267 (1) with overall mean value of 20.025 as the highest and the lowest was 4.10 g in local Aloe vera (12) though the fresh gel weight was highest in IC 111267(1) and the dry gel weight was recorded as 12.80 g in IC111271 (3) as compared to all other accessions. Lowest aloine concentration recorded about was 0.86% from IC111267 (1), whereas the highest was 1.68% for accession IC111280 (6). The medicinal and cosmetic value of the Aloe depends on the quantity of its biochemical constituents. Presently, an attempt has been made to evaluate the collected Aloe vera germplasm for its quality and yield components. The quality of the germplasm depends on the relative composition of leaves/leaflet. The plants producing higher amount of fresh gel, dry gel, biomass, presence of chemical compounds and lower amount of aloin are consider being of good quality.
The accessions of IC111271, IC111269, IC111267 and Aloe CIM-Sheetal show better performance in vitro and in vivo than the local Aloe vera (wild) obtained from Hyderabad region. Among the present studies of Aloe accessions, the leaf yield per plant ranged between 0.652 kg and 23.2486 kg/plant and dry leaf weight between 12.20 and 23.70 g/kg. Accessions with high yield are IC111271, IC111269, IC111267 and Aloe CIM-Sheetal. The amount of gel (dry) obtained from one plant in one year is estimated to be 2.10 to 12.80 g/plant. High yield of gel can be obtained from accessions IC111271, IC111269 and Aloe CIM-Sheetal. Morphochemical evaluations can provide insight into the genetic structure and diversity within and among varieties from different geographical origins, producers and distributors. Without this information, there is no means of selecting appropriate plant material for the participation in screening programs with a view to introduce the novel varieties for industrial purpose (Radhamadhavi et al., 2012).
In vitro study
In the in vitro study, the shoot initiation, shoot multiplication, rooting and hardening of plantlets for 12 accessions were studied. The surface sterilization process is an important step to avoid any type of endogenous and exogenous contaminants and also standardized media composition for the mass multiplication and fast growth of Aloe vera. In the present investigation, shoot tip was used as an explants cultured on MS supplemented medium with various concentrations and combinations. Establishment of aseptic cultures was difficult, but once a healthy culture was established, there was no further contamination. Under given conditions and over a culture of 60 days, explants from all the treatments produced multiple explants and roots simultaneously. In earlier studies, Aloe vera has been sterilized using the mercuric chloride which is harmful for environment. The method of the present study is easy and new for explants sterilization for in vitro culture of Aloe vera.
The ability of plant cell, tissue and organs to differentiate plants in culture has resulted in widespread applications in propagation and plant breeding. Conventional method of propagation in Aloe vera is through vegetative means (side buds) which is very slow. There is no viable seed setting in the plants. Aloe is exclusively a vegetatively propagated crop where young side branches are used as planting material. Single plant may produce 2 to 3 side shoots per year making availability of planting material in good quantity and quality, a problem. Keeping the aforementioned things in mind, tissue culture studies were undertaken in the Aloe vera plants in this study. A number of protocols have been developed for micropropagation of Aloe plants using a variety of explants like shoot tip, axillary bud, stem cuttings, etc., by various researchers (Hosseini and Parsa, 2007). The method used for clonal propagation is also an important tool for raising pathogen free plants in culture (Walkey, 1980). Tissue cultures of A. barbadensis were established by Sanchez et al. (1988) using vegetative meristems and leaf explants. Shoot tips and auxillary buds are popular explants for micropropagation of Aloe (Aggarwal and Barna, 2004).
Shoot initiation
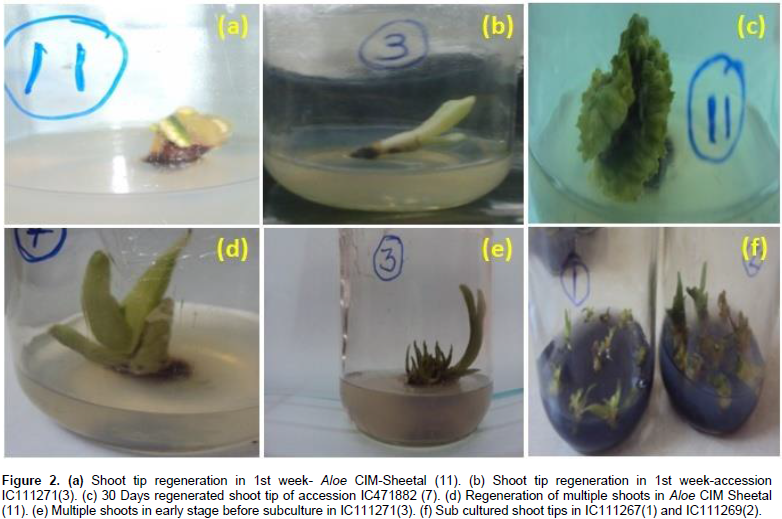
For in vitro clonal propagation of Aloe vera plants, shoot tips were used as explants. Explants were obtained from healthy parent plants of 12 accessions for micro propagation. After sterilization, shoot apices were directly inoculated into various media. These explants were inoculated in wide mouth culture bottles containing MS medium. The media was supplemented with different concentrations of BA alone or in combination with IBA or IAA. The explants cultured in combination of MS basal medium with BA, NAA, KIN and IBA started showing signs of proliferation after 15 days of culturing (Figure 2a to f). Best growth was observed on MS medium supplemented with 1.5 mgL-1 BAP, 0.2 mgL-1 IBA, 0.1 mgL-1 IAA, 0.5 mgL-1 KIN, and 0.5 mgL-1 GA3. New buds appeared from the axils of leaves of shoot explants and bud developed.
Cytokinin level produced a significant response upon the number of explants formed per plant and also showed influence on production of leaf numbers and rooting (Dwivedi et al., 2014). The shoot tip of explants initially produces 2 to 3 shoots within two weeks after inoculation. But in our method, 15 to 35 shoots/cultures were produced from single explant by subsequent 2 to 3 subcultures with the same medium which indicate the high efficiency of this protocol. The average length of shoots per culture was 4.89 ± 0.03 cm. Formation of the roots was best observed in Aloe CIM-Sheetal and IC111271 in the media containing MS basal media with 3 types of hormones NAA 0.2 mgL-1, IAA 0.1 mgL-1 and IBA 0.2 mgL-1 within four weeks after inoculation for rooting. Proliferating shoots obtained from shoot tip explants of Aloe took maximum 6 to 7 weeks from the time of establishment to attain the size (2 to 3 cm) suitable for rooting. The highest percentage of shoots that induced roots (91.12%) was observed in MS medium supplemented with NAA (0.2 mgL-1), IAA (0.1 mgL-1) followed by IBA (0.2 mgL-1).
Shoot multiplication
After initiation of the growth on the explants (30 days culturing), the newly formed shoots were excised individually with the help of sterilized blade and re-cultured on fresh bottles containing the same medium (MS medium with different supplements) to increase the number of shoots. The results obtained in the present study revealed that BAP at concentration of 1.5 mgL-1 provides better shoot multiplication. All the cultures showed shoot proliferation on MS medium with different concentrations. On an average, each explants produced 5 to 8 shoots (Figure 3a to l).The results show that accessions Aloe CIM-Sheetal (Tag no:11), IC 111271 (3), IC 111279 (5), and IC111269 (2) are high potential accessions, IC 471882 (7), IC111267 (1), IC471885 (10) have shown moderate proliferation and IC 111280 (6), IC111280 (8), IC471884 (9), and wild Aloe vera (12) showed poor shoot proliferation and rooting among all accessions for their multiplication ratio of axillary buds, multiple shoots/clumps, and roots were regenerated after sub culturing.

Cytokinins are one of the most important growth regulators affecting the shoot proliferation (Garland and Stolz, 1981). A range of cytokinins BA, kinetin and 2-ip have been used in micropropagation (Bhojwani and Razdan, 1992). BA variations affecting shoot proliferation were also reported by Bhandari et al. (2010), Gantait et al. (2010), Mangal (2009), and Chaudhuri and Mukundan (2001) also reported the use of BA in shoot proliferation of Aloe polyphylla and A. vera, respectively. Some researchers have shown that the presence of both auxin and cytokinin is necessary for shoot proliferation (Rout et al., 2001). An interesting result was observed in explants preparation, sizing and a single mother plant can be multiplied for 30 to 35 explants per mother plant and 8.84 ± 0.03 shoots per explants were obtained on MS medium supplemented with BAP (1.5 mgL-1), KIN (0.5 mgL-1), NAA (0.2 mgL-1), IAA (0.1 mgL-1), and IBA (0.2 mgL-1) in 7 weeks, in comparison with 30 shots from 18 explants obtained from 18 mother plants in 8 weeks and 20 shoots per plant in 8 weeks was reported (Balraj and Neelu, 2009). It was also reported that the enhancement of shoots was observed by using BA and NAA. The clusters of shoots were separated into pieces and each was sub- cultured individually on the same medium periodically. After third subculture, the shoot multiplication rate remainedconstant.
On the other hand, regeneration of shoot buds was moderate (65 to 77%) on a medium containing: 1.5 mgL-1 BAP, 0.5 mgL-1 kinetin, 0.2 mgL-1 of IBA, 0.1 mgL-1 of IAA, 0.2 mgL-1 of NAA, and 1.5 mgL-1 BAP, 0.5 mgL-1 kinetin, 0.2 mgL-1 of IBA, 0.1 mgL-1 of IAA, 0.2 mgL-1 of NAA and 1.0 mgL-1 of GA3, respectively. Comparatively, the lowest number of 18 to 28% adventitious shoots were observed in the medium containing 0.3 mgL-1 of IAA, 3.0 mgL-1 of BAP (IM) and 0.1 mgL-1 NAA, 0.1 mgL-1 of IBA, 0.1 mgL-1 of IAA, and 4.5 mgL-1 BAP. The highest concentration of 4.5 mgL-1 of BAP did not increase shoot proliferation. It was also reported that the highest shoot proliferation in A. vera was found in MS medium containing BA and IBA (Mukesh et al., 2011), where better proliferation occurred on medium containing kinetin instead of BA (Dwivedi, 2014). NAA and IBA are the most commonly used for root induction (Bhojwani and Razdan, 1992). Effect of 0.1 mgL-1 of NAA, 0.5 mgL-1 of IAA, and 0.5 mgL-1 of IBA on MS medium in rooting showed poor response.
For shoot proliferation, growth regulators especially cytokinins (Bhojwani, 1980) are one of the most important factors affecting the response. A range of cytokinin (kinetin, BA, 2-ip and Zeatin) has been used in micro propagation of work. Studies conducted by different researchers have clearly shown that BA is a more effective, reliable and useful cytokinin for shoot proliferation in Aloe vera (Debiasi et al., 2007). IBA (Chaudhuri and Mukundan, 2001) and acetic acid (Mukherjee and Roy, 2008) were also reported to be helpful in shoot proliferation in Aloe. Meyer and Staden (1991) reported auxillary shoot formation using IBA, whereas Roy and Sarkar (1991) obtained shoot on medium containing 2, 4-D with Kinetin. Richwine et al. (1995) reported induction of shoots using Zeatin. Liao et al. (2004) reported that the best medium for micro propagation of Aloe vera was MS + 2 mgL-1 BA + 0.3 mgL-1 NAA. Budhiani (2001) demonstrated that the best initiation and multiplication of shoot was on MS medium supplemented with 0.2 mgL-1 BAP + 0.002 mgL-1 NAA and 2 mgL-1 BAP + 0.002 mgL-1 NAA, respectively.
Hashemabadi and Kaviani (2008) reported that MS with 0.5 mgL-1 BA and 0.5 mgL-1 NAA produced the highest number of shoots. According to Liu (2001), the best medium for shoot proliferation is MS with BA (1.0 mgL-1) and IBA (0.3 mgL-1). Best medium for bud initiation according to Liao et al. (2004) is MS with 2.0 mgL-1 BA + 0.3 mgL-1 NAA with 30 gL-1 sucrose + 0.6 gL-1 PVP (pH 5.8). Hirimburegama and Gamage (1995) cultured the plant on MS medium supplemented with 0.18 mgL-1 IAA and 2.25 mgL-1 BA. Zhou et al. (1999) suggested MS + 6 BAP (3 mgL-1) as the best medium for the induction of buds. Also, in the present study, shoot proliferation occurred in the presence of cytokinin. The different phytohormonal combinations of BAP proved to be more effective. MS medium supplemented with 1.5 mgL-1 BAP + 0.2 mgL-1 IBA + 0.5 mgL-1 KIN + 0.1 mgL-1 IAA + 0.2 mgL-1 NAA + 0.5 mgL-1 GA3 gave (77 to 88%) best shoot proliferation/multiplication. This is in contrast with earlier work by Meyer and Staden (1991) who reported better proliferation in Aloe vera on medium containing kinetin instead of BA. This difference may be due to the difference in the genotype of plant used (Abrie and Staden, 2001).
Rooting
The in vitro raised 3 to 4 cm long shoots were excised individually from the proliferated shoot clumps and cultured on rooting media where MS medium was supplemented with different concentrations of NAA. All the combinations showed induction of roots. Maximum number of roots (2 to 7) per plant was obtained in plantlets cultured on MS + 0.2 mgL-1 of NAA. The plantlets cultured on MS medium supplemented with 2 to 10 mgL-1 NAA showed induction of only one root per shoot. The roots obtained were creamish yellow in color and with/without branching. Newly formed micro roots measuring 2 to 3 cm in length (Figure 4a to d) were excised individually from the parent explants and transferred to rooting media. Two types of rooting media were used; one is MS basal media with 3 types of hormones NAA, IAA, IBA and other half strength MS media (Table 5). Data were recorded after 30 days of culture. The highest root response in A. vera was reported in hormone free medium (Bhandari et al., 2010).


In the current study, healthy rooting was observed in NAA (0.2 mgL-1) and IBA (0.2 mgL-1) medium. Healthy roots (number > 7 and length > 3 cm) were obtained in 8 weeks of time. Hardening is an important step in tissue culture. Rooting response of micro shoots is reported to be controlled by growth regulators in the medium (Abrie and Staden, 2001), basal salt composition (Garland and Stoltz, 1981), genotype (Rines and McCoy, 1981) as well as cultural conditions. NAA and IBA are the most commonly used auxins for root induction (Bhojwani and Razdani, 1992). By use of IBA, many plants such as Lycoperscicon esculentum (Sibi, 1982), Hedychium roxburgii (Tripathi and Bitaillion, 1995) and Carnation (Werker and Leshem, 1987) gave in vitro rooting. Rooting was achieved on MS medium + 0.18 mgL-1 NAA + 0.226 mgL-1 BA (Hirimburegama and Gamage, 1995). Zhou et al. (1999) used supplements NAA (0.3 mgL-1) and IBA (0.3 mgL-1) for rooting and found that NAA was better than IBA in the average number of roots produced and rooting rate. Best rooting was observed by Liao et al. (2004) by using ½ MS + 0.2 mg NAA.
The shoot tips subjected to MS medium supplemented with different concentration of NAA were also examined. Maximum rooting was observed in MS + 0.2 mgL-1 NAA + 0.2 mgL-1 IBA + 0.1 mgL-1 IAA. These results are in agreement with the results of Zhou et al. (1999). However, Natali et al. (1990), Meyer and Staden (1991), and Richwine et al. (1995) reported rooting in hormone free medium. In the present study, no rooting was obtained in hormone free medium even on prolonged waiting. Regeneration of plants from callus may help to induce variability in the Aloe germplasm for future improvement. Over the last years, a number of micropropagation protocols have been developed using a variety of explants like shoot tips (Hashemabadi and Kaviani, 2008), auxillary buds (Hirimburegama and Gamage, 1995), stem cutting and leaf explants and plant regeneration via callus formation in A. barbadensis which occurs at low frequency (Sanchez et al., 1988).
Successful establishment of calli and subsequent plantlet regeneration is reported in Aloe pretoriensis (Groenewald et al., 1975), Aloe saponaria (Yagi et al., 1983) and A. vera (Gui et al., 1990; Roy and Sarkar, 1991). Racchi (1987) used MS medium supplemented with 0.5 mgL-1 2,4-D and 1 mgL-1 kinetin for root explants and 0.2 mgL-1 2,4-D and 1 mgL-1 kinetin for leaf meristems. In A. saponaria, the best results were obtained using root tissue with a combination of 1 ppm indoleacetic acid (IAA) and 0.5 ppm 2,4-D and 2 ppm kinetin (Yagi et al., 1983). However, the occurrence of plant regeneration from these calli was not reported. Gui et al. (1990) used stem segments of A. vera on MS medium with different hormones and successfully regenerated a large number of plantlets via callus. The best results were obtained on the medium with zeatin 2 ppm + 0.5 ppm NAA. Roy and Sarkar (1991) reported that MS basal medium supplemented with 1 mgL-1 2,4-D and 0.2 mgL-1 kinetin gave the best callus induction. A. vera has a great future for tissue engineering applications, because it is a unique plant and appealing for physicochemical and biological properties (Shekh et al., 2017).
The shoot tips were used as explants, cultured on different combinations of auxins and cytokinins and were examined. Among all the combinations, no response was observed in MS basal medium with 2, 4-D (0.5 mgL-1) and kinetin (0.2 mgL-1). It was also revealed that regenerated plants were morphologically similar to the mother (control) plant and that the method of micropropagation used in this investigation (axillary bud method) does not usually produce some clones. The result of acclimatization showed that 100% of plantlets survived and grew under greenhouse conditions and were morphologically similar to mother plants. The leaves also started thickening in shade house. The tissue culture plants were hardened and these plants could face ambient environmental conditions (Bhojwani and Razdan, 1992).
Rooted plantlets were transferred from culture bottles to plastic pots containing 1:1 ratio of soil: rice husk. Natali et al. (1990) suggested the mixture of soil, sand and perlite. While Aggarwal and Barna (2004) suggested mixture of soil and farmyard manure, and Hashemabadi and Kaviani (2008) suggested the mixture of cocopeat and perlite. There is decrease in the glycoprotein, verectin (Yagi et al., 2000) and barbaloin content in the clonally regenerated plants of A. vera. According to Yagi et al. (2006), the clonally induced mutations are associated with the phenotypic variation observed in A. vera. The present results show the occurrence of variation in morphological and biochemical characteristics when compared with the mother plants and their tissue culture generated plants. The extent of variation, however, differs from accession to accession. This could be related to differences in the genotypes of various accessions.
Hardening of plantlets
After 30 days of culture on rooting media, the plantlets were successfully acclimatized. The pots (8×6 cm) were kept and readily filled with garden soil, compost and sand in the proportion of 2:1:1, respectively. The plants were then transplanted into the pots, then thoroughly watered and kept under plastic house having 80% humidity and 31°C temperature for ten days. Then the plants were shifted to shade house with less humidity and indirect sunlight. After 15 days, the hardened plantlets were transferred to the soil. The result of acclimatization showed that 100% of plantlets survived to grow under greenhouse conditions and were morphologically similar to mother plants (Figure 5a to c). The plants were watered periodically and upper layer of the soil mulched occasionally whenever necessary. The overall procedure of micro propagation flow diagram is as shown in Figure 6. The tissue culture showed the rapid production of large number of true type plants. Somaclonal variations are therefore undesirable. However, in the present case, the somaclonal variants can be a valuable source of genetic variation in the germplasm. The somaclonal variants thus obtained need detailed genetic characterization before being put to use. Genetic variation is an essential component of any conventional breeding programme. Aloe germplasm lacks natural genetic recombination mechanism due to the absence of sexual reproduction. The tissue culture technology has great potential for induction of genetic variability.
