ABSTRACT
The aim of this research was to evaluate the impact of chromium (III) nitrate on soil microbial activities and growth performance and phytoremediation potentials of two staple leguminous crops, namely cowpea (Vigna unguiculata) and groundnut (Arachis hypogea). Pristine sandy loam soil samples were polluted with nitrate salts of chromium (III) at four different levels (50, 100, 200 and 400 mg/kg) in triplicates. There was a significant (P < 0.05) retarding effect of this metal on the study parameters. A consistent decrease in the total bacterial count in response to increase in dosage of the metal salt was observed. Chromium was also observed to significantly (P<0.05) affect the microbial metabolism as indicated by the decline in microbial respiration shown by the lowering of CO2 evolution in the test samples. There was a reduction in the general growth performance of the two test plants treated with different levels of chromium when compared with the control. Phytoaccumulation experiment showed that only cowpea roots accumulated the pollutant from the 400 mg/kg treated soil, with no metal salt presence in aerial parts of the plants. This chromium-removal potential demonstrated by cowpea makes it a better candidate than groundnut for the phytoremediation of chromium-contaminated soils.
Key words: Chromium (III) nitrate, microbial activities, cowpea, groundnut, phytoremediation.
Chromium (Cr) is one of the naturally occurring elements found in rocks, soils, and in volcanic dust and gases. It occurs in two main forms in the environment, namely, chromium (III) and chromium (VI). Chromium is a toxic non-essential metal for microorganisms and plants. In plants, it reduces productivity by the imposition of chronic diseases, loss of chlorophyll and protein contents (Ma et al., 2016). Hexavalent chromium (Cr6+) is more toxic than trivalent chromium (Cr3+) (Saha et al., 2011). The trivalent form is a trace mineral important in human nutrition; in large doses however it can be harmful to health. Chromium (III) may react with carboxyl and sulfhydryl groups of enzymes causing alterations in their structure and activities. It also modifies DNA polymerase and other enzyme activities as a result of the displacement of magnesium ions by chromium ions (Snow, 1994).
Hexavalent chromium has been found to be carcinogenic. It causes mutation through the generation of free radicals as it is reduced to lower oxidation states in biological systems (Kadiiska et al., 1994). Chromium (VI) is a product of some industrial processes and can be found in such products as paints, dyes, tannery chemicals, wood preservatives, anti-corrosion agents, etc. Chromium occurs in neutral or alkaline soils in the trivalent form (Cr3+), which has low solubility and mobility. Shanker et al. (2005) observed that chromium impact on the physiological development of plants depends on the metal speciation, which is responsible for its mobilization, uptake and subsequent toxicity in the plant system. Heavy metal species commonly found in the soils as a result of human activities include, copper (Cu), zinc (Zn), nickel (Ni), lead (Pb), cadmium (Cd), cobalt (Co), mercury (Hg), chromium (Cr), arsenic (As). etc. Generally, metals are not degradable and so these heavy metals can persist in the environment indefinitely (Walker et al., 2003). Some of them act as micronutrients in small concentrations for the development of living organisms, but when bioaccumulated over time they become toxic to life.
The risk associated with polluted soils is contamination of the food chain. When plants grow on polluted soils, they become potential threats to human and animal health. Plants may also have their growth sharply reduced by high levels of toxic elements in their tissues, causing a decrease in crop yields and further economic loss. Uptake and accumulation of a number of metals by plants are affected by pH, clay content, organic matter content, cation exchange capacity, nutrient balance, mobility of the heavy metal species and soil moisture and temperature (Sauve et al., 1997).
Microorganisms, namely, bacteria, fungi, protozoa and algae coexist in the soil especially within the rhizosphere region. Some such as plant growth promoting rhizobacteria (PGPR), phosphorus solubilizing bacteria, mycorrhizal helping bacteria (MHB) and arbuscular mycorrhizal fungi (AMF) in the rhizosphere of plants growing on trace metal contaminated soils play an important role in phytoremediation (Khan, 2005; Ahemad, 2015; Stambulaka et al., 2018). These microorganisms can survive and serve as effective metal sequestering and growth-promoting bioinoculants for plants in metal stressed soils (Rajkumar and Freitas, 2008). They mitigate the toxic effects of these heavy metals on plants through secretion of acids, proteins, phytoantibiotics and other chemicals (Denton, 2007). In phytoremediation, a plant can be classified as an accumulator, excluder or an indicator according to the concentration of metals found in its tissue (Baker, 1981). Harnessing the phyto-remediation potentials of legumes is currently being advocated, though this move has been criticized in some quarters due to the belief that this might lead to health challenges and food scarcity, especially in the developing world such as Nigeria where these plants serve as staplefoods.
The growing increase in heavy metals pollution of the soil occasioned by industrialization, brings with it a concomitant concern for plants and microbial safety, considering their significant roles in the ecosystem. This research was therefore carried out to evaluate the impact of chromium (III) on soil microbial activities, growth performance and phytoremediation potentials of Arachis hypogea (groundnut) and Vigna unguiculata (cowpea).
Study area and sample collection
This study was carried out in Nsukka, Southeastern Nigeria. Soil samples were collected from the Plant Science and Biotechnology Garden, University of Nigeria, Nsukka at a depth of within 0 to 15 cm. Viable seeds of both cowpea and groundnut were purchased from Ogige market in Nsukka metropolis and stored at room temperature for 24 h. Seed viability testing was carried out using floatation technique. Analytical grade of chromium (III) nitrate salt (Cr (NO3)3.9H2O) was used.
Soil analysis
Physical and chemical properties of the soil samples were determined. Particle size was determined using the Boyoucos Hydrometer Method of Gee and Bauder (1987). Potential of hydrogen (pH) was analyzed according to Black (1965). Percentage organic matter, phosphorus, nitrogen, cation exchange capacity and soil moisture were also determined using the methods of Black (1965).
Determination of the effects of chromium on soil bacterial population
Pristine sandy loam soil was air-dried, sieved and dispensed in 100 g weights into twelve 250 ml conical flasks placed in four groups; each group comprising three flasks. Each triplicate group was polluted with one of four different levels (50, 100, 200, and 400 mg) of Cr(NO3)3. A control experiment made up of three unpolluted soil samples were also set up. The conical flasks were watered periodically to sustain the microorganisms. Bacterial analysis was done using 1.0 g of soil collected from each flask at one weekly interval over a period of four weeks. The population of viable bacterial cells in each soil sample was determined by a ten-fold serial dilution and spread plating technique as described by Wistreich (1997).
Determination of the effects of chromium on soil microbial respiration
Fifty grams of each polluted soil sample was weighed in duplicate into kliner jars and three unpolluted soil samples served as control. Sterile water was carefully sprinkled on the soil up to 60% water holding capacity to make it moist. In each jar containing the soil, a vial with 15 ml of already prepared 0.05 M NaOH was carefully placed at the centre. Three empty kliner jars, each containing a vial with 15 ml of 0.05 M NaOH, were used as blanks. The tops of the jars were greased properly to prevent the escape of CO2 and were tightly capped and incubated at room temperature. At weekly intervals, the vials were removed and 3 ml of 20% BaCl2 was added. Three drops of phenol red indicator were subsequently added and then titrated using 0.05 M HCl until a colourless end point was observed. This was repeated for four weeks.
Calculation of results
The rate of respiration was calculated by the following relationship:
CO2 (mg)/SW/T = (Vo - V) × 1.1 / DWT
Where, SW is the amount of soil dry weight in grams, T is the incubation time in hours, Vo is the total volume of HCl used for titration. V is the volume of HCl used for the soil sample, DWT is the dry weight of 1 g moist soil and 1.1 is the conversion factor (1 ml 0.05 NaOH equals 1.1 mg CO2).
Planting experiments
After the soil samples have been air dried and homogenized, they were sieved and dispensed in 3 kg weights into 24 (20 cm deep × 18 cm diameter) plastic pots, each perforated at the bottom. Each of the four different levels of Cr (NO3)3 9H2O (50, 100, 200 and 400 mg/kg) was used in triplicates to pollute the soil in the pots. The pots were kept in a green house and allowed to stabilize for seven days to compensate for the disturbance caused by sampling and sieving (Baath, 1998), and also to ease gradual distribution of the metal (Cr) in the soil. Soil samples which received no metal pollutants were prepared and kept as control. Seven days after soil pollution, four viable seeds each of groundnut or cowpea were planted in each plastic pot. The pots were watered with 200 ml of sterilized water every four days for eight weeks. Over-watering was avoided as much as possible to prevent water logging and leaching of the metal salt. The germination percentage and growth rates were monitored and recorded. Seeds were considered germinated when the radial reached a length of 1 mm. The germination percentage was calculated as:
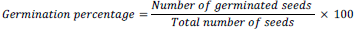
Length of shoot was measured with the help of a scale and reading was taken from both test plants and the control. This was done on a weekly interval. At the end of the planting experiment which lasted for eight weeks, the plants were gently uprooted and the following measurements carried out:
Root length
This was done by measuring the root length of the legume after harvesting to determine the length in relation to the control.
Nodulation
The number of nodules formed on each plant in each treatment was carefully counted and recorded.
Weight of plant
The uprooted plants were washed with distilled water. After air-drying, the wet weight was measured. This was followed by oven-drying at 105°C for 24 h to determine the dry weight.
Metal uptake potential
At the end of the weighing process, the plants were separated into root and shoot and pulverized using laboratory milling machine. The pulverized samples were digested using HCL/HNO3 (3:1 v/v) and the metal uptake determined using an Atomic Absorption Spectro-photometer (FS 240 AA Agilent Technology). The bioconcentration factor (BCF) and translocation factor (TF) were calculated. The BCF is the ratio of metal concentration in the roots to that in the soil or water and TF is the ratio of metal concentration in the shoots to that of the roots (Malik et al., 2010).
Plants are categorized as phytoextractor when TF > 1 (Fitz and Wenzel, 2002) and as phytostabilizer when BCF > 1 and TF < 1, respectively (Mendez and Maier, 2008).
Statistical analysis
All data were subjected to analysis of variance (ANOVA) using SPSS version 16 and reported as mean ± standard deviation (SD). Statistical value at p < 0.05 was considered to be significant.
Soil physicochemical analysis
Selected physico-chemical characteristics of the soil are shown in Table 1. From the analysis, the soil was classified as sandy loam with a moisture content of 6.2%, pH 6.3, organic matter of 1.98 and cation exchange capacity (CEC) of 8.0. The pH is considered an important parameter because it affects the availability of trace metals in the soil.
Effects of chromium on the germination, growth performance and phytoremediation potentials of V. unguiculata
Table 2 shows the effects of chromium on germination time, germination percentage, nodulation, weight and root length, metal uptake along with BCF and TF potentials of V. unguiculata.
The result presented in Table 2 shows that at a treatment level of 400 mg chromium/kg of soil, a prolonged germination time (9±1 days) was observed unlike the control (4±1 days). Germination time increased with increase in chromium dosage. In both control and 50 mg/kg treated soil, all the cowpea seeds sown in each pot germinated whereas in the other levels, there was a concomitant reduction in the number of germinated seeds as the dose increased. The uptake of chromium by cowpea was also found to be concentration dependent. The root was seen to be a better site for chromium accumulation than the shoot, though at 400 mg/kg treated soil only.
Effects of chromium on the germination, growth performance and phytoremediation potentials of A. hypogea
Table 3 shows the effects of chromium on germination time, germination percentage, nodulation, weight and root length, metal uptake along with BCF and TF potentials of A. hypogea.
A prolonged germination time of 8.75±1.7days was observed unlike the control (4±1 days). In both control and 50 mg/kg treated soil, all the groundnut seeds sown in each pot germinated as observed in cowpea. However, there was a reduction in the number of germinated seeds as the dose increased. Chromium accumulation was not observed in both the shoot and root parts of the plant. Nodulation as well as wet and dry weights were dose dependent.
Effects of chromium on microbial activities and shoot growth of the legumes
Figures 1 and 2 show the effects of different levels of chromium on the shoot lenghts of cowpea and groundnut, respectively. Chromium exhibited a dose-dependent retardative effect on the shoot growth of the legumes. This dose-dependent retardation was also observed on the bacterial growth as well as on microbial respiration (Figures 3 and 4).
The observed dose-dependent inhibitory effect of chromium on soil bacterial population is in conformity with the report of Wani et al. (2008) who stated in their work (though not specific to chromium) that metals have serious effects on both soil bacterial count and plant growth promoting rhizobacteria. The deleterious effect of chromium on soil bacterial population was found to increase with increase in levels of pollution. The higher the metal dose, the more significant (P < 0.05) the retardative effects on the total bacterial count. Ghorbani et al. (2002) in their study identified a reduction in microbial biomass as a result of heavy metal ecotoxicity in soil environment. Once a rise in soil metal build-up occurs, it becomes uninhabitable for microbial communities and unsuitable for crop production, thus inhibiting the growth and activities of various groups of microorganisms including symbiotic nitrogen fixers such as Rhizobium leguminosarum, Mesorhizobium ciceri, Bradyrhizobium species and Sinorhizobium.
Results of this study show that chromium contamination of soils has an inhibitory effect on carbon (iv) oxide evolution by microorganisms and this effect was observed to be concentration-dependent. Sethi and Gupta (2009) essayed the opinion that heavy metals are deleterious to microbial metabolism and that their effects might be more detrimental with rise in the level or dose of application. Soil respiration is a useful indicator for determining soil health. Microorganisms domiciled in the soil play a key role in the mineralization of nutrients, decomposition of organic matter and degradation/ transformation of toxic compounds.
Seed germination is the first visible evidence of plant growth. It is regulated by a number of physical and physiological processes. The investigation on the impact of chromium on the growth and development of the two legumes (A. hypogea and V. unguiculata) revealed an adverse effect on the plants general growth performance. Reports have shown that soil metal contents affect the growth and physiology of plants (Luilo and Othman, 2006; Trinh et al., 2014). The reduced germination (germination time and percentage) in both plants (cowpea and groundnut) as observed in the present finding could be attributed to metal toxicity. From the results it was observed that chromium had a more adverse effect on the germination and growth of groundnut than cowpea. This shows that cowpea probably has a higher intrinsic resistance to chromium than groundnut. Pandey et al. (2005) reported that growth inhibition of plants exposed to chromium might be due to the generation of free radicals and reactive oxygen species (ROS), which pose constant oxidative damage by degrading important cellular components. Shafiq et al. (2008) attributed it to accelerated breakdown of stored nutrients in seeds and alteration of selective permeability properties of cell membranes.
The observed decrease in the shoot lengths of the legumes could be the result of chromium toxicity which caused alteration in root cell functions leading to reduced nutrient uptake and water mobility. In a relevant work, Diwan et al. (2010) reported that reduction in shoot length caused by chromium stress may be due to its accumulation in the root region, or absence of its translocation from roots to other tissues, thus causing increase in chromium concentration in roots and inhibiting shoot development. Also Hu et al. (2015) suggested that damage of chloroplasts and possible reduction in photosynthesis could lead to significant reduction in shoot lengths of Crambe sativa and Eruca sativa when grown in chromium (VI) polluted soil.
The reduction in the number of nodules in the legumes planted in chromium (III)-polluted soil could also be the result of heavy metal toxicity. Ibekwe et al. (1996) similarly opined that the toxic effect of metals on the root hairs or rhizobia might be responsible for reduced nodulation when they examined alfalfa plant under zinc and cadmium stress. Manier et al. (2009) stated that the nodulation index of white clover could serve as a suitable bioindicator of increased heavy metal toxicity in soil. It is also possible that the chromium (III) inhibited nitrogenase activity and photosynthesis leading to retardation in nodulation and overall growth stagnation.
The observed decrease in the biomass of cowpea and groundnut is also an index of chromium phytotoxicity. Similar discovery on decreased biomass production in legumes was also reported as a direct toxic effect of chromium polluted tannery effluent used for irrigation (Santos et al., 2011). Klimek-Kopyra et al. (2015) in their recent work observed that increase in heavy metals pollution limit the longitudinal growth and biomass of roots with a corresponding reduction in nodule formation in field pea and spring vetch grown in contaminated soil.
Results from the present research show that the two plants used in the study were not able to facilitate the mobility of chromium (III) to the shoot region in all levels of treatment. However, a trace amount was observed to be domiciled within the root region in cowpea; in groundnut no metal presence was detected in the roots indicating a low mobility potential of chromium in the two plants. It could be that the two plants have barriers against chromium (III) transport or lack mechanisms for its transport from root to shoot. This is explained by the fact that the two forms of chromium, Cr (III) and Cr (VI) play no role in plants metabolism (Shanker et al., 2005) and nutrient uptake. The ability of plants to tolerate and take up heavy metals is useful for their classification for phytoremediation purposes (Yoon et al., 2006). The two legumes demonstrated low mobility for chromium. Kleiman and Cogliatti (1998) in their work with other plant species also reported a low mobility of chromium due to some mechanism that hindered its transportation.
This work has brought the following to limelight:
(1) The retardative effects of chromium (III) on soil microbial population and metabolism.
(2) The inhibitory effects of chromium (III) on the germination and general growth performance of groundnut and cowpea.
(3) The low translocation potentials for chromium exhibited by groundnut and cowpea. Cowpea however demonstrated a better phytoremediation capability than groundnut for soil heavily polluted with chromium (III) nitrate.
There is need for more research to delve into the mechanisms responsible for the higher resistance to chromium (III) toxicity exhibited by cowpea over groundnut. Understanding this could help enhance and engineer this trait for a more effective phytoremediation of environments contaminated with chromium (III) compounds.
The authors have not declared any conflict of interests.
The support given by the Microbiology Department, University of Nigeria, Nsukka through the provision of some of the hardware facilities used during the study was appreciated.
REFERENCES
|
Ahemad M (2015). Enhancing phytoremediation of chromium-stressed soils through plant-growth-promoting bacteria. Journal of Genetic Engineering and Biotechnology 13(1):51-58.
Crossref
|
|
|
|
Baath E (1998). Growth rate of bacterial communities in soil at varying pH: a comparison of the thymidine and leonine incorporation techniques. Microbial Ecology 36:316-327.
Crossref
|
|
|
|
|
Baker AJM (1981). Accumulators and excluders strategies in the response of plants to heavy metals. Journal of Plant Nutrition 3:1-4
Crossref
|
|
|
|
|
Black CA (1965). Methods of soil analysis. Agronomy No 9, part 2. Madison, Wisconsin: American Society of Agronomy.
|
|
|
|
|
Denton B (2007) Advances in phytoremediation of heavy metals using plant growth promoting bacteria and fungi. Basic Biotechnology 3:1-5.
|
|
|
|
|
Diwan H, Khan I, Ahmad A, Iqbal M (2010). Induction of phytochelatins and antioxidant defense system in Brassica juncea and Vigna radiate in response to chromium treatments. Plant Growth Regulation 61:97-107.
Crossref
|
|
|
|
|
Fitz WJ, Wenzel WW (2002). Arsenic transformations in the soil-rhizosphere-plant system: Fundamentals and potential application to phytoremediation. Journal of Biotechnology 99(3):259-278.
Crossref
|
|
|
|
|
Gee GW, Bauder JW (1987). Particle size analysis. In: Klute A (ed) Methods of soil analysis part 1: Physical and mineralogical method, Madison, Wisconsin pp. 383-411.
|
|
|
|
|
Ghorbani NR, Salehrastin N, Moeini A (2002). Heavy metals affect the microbial populations and their activities. Symposium 54:1-11.
|
|
|
|
|
Hu J, Deng Z, Wang B, Zhi Y, Pei B, Zhang G, Luo M, Huang B, Wu W, Huang B (2015). Influence of Heavy Metals on Seed Germination and Early Seedling Growth in Crambe abyssinica, a Potential Industrial Oil Crop for Phytoremediation. American Journal of Plant Science 6:150-156.
Crossref
|
|
|
|
|
Ibekwe AM, Angel JS, Chaney RL, Van Berkum P (1996). Zinc and cadmium toxicity to alfalfa and its microsymbiont. Journal of Environmental Quality 25:1032-10410.
Crossref
|
|
|
|
|
Kadiiska MB, Xiang QH, Mason RP (1994). In vitro free radical generation by chromium (VI): an electron resonance spin-trapping investigation. Chemical Research and Toxicology 7:800-805.
Crossref
|
|
|
|
|
Khan AG (2005). Role of soil microbes in the rhizospheres of plants growing on trace metal contaminated soils in phytoremediation. Journal of Trace Elements in Medicine and Biology 18(4):355-364.
Crossref
|
|
|
|
|
Kleiman ID, Cogliatti DH (1998). Chromium removal from aqueous solutions by different plant species. Environmental Technology 119:1127-1132.
Crossref
|
|
|
|
|
Klimek-Kopyra A, Baran A, ZajÄ…c T, Kulig B (2015). Effects of heavy metals from polluted soils on the roots and nodules formation. Bulgarian Journal of Agricultural Science 21:295-299.
|
|
|
|
|
Luilo GB, Othman OC (2006). Lead pollution in urban roadside environments of Dares-Salaam city. Tanzanian Journal of Science 32(2):61-67.
|
|
|
|
|
Ma J, Lv C, Xu M, Ghen G, Gao Z (2016). Photosynthesis performance, antioxidant enzymes and ultrastructural analyses of rice seedlings under chromium stress. Environmental Science and Pollution Research 23(2):1768-1778.
Crossref
|
|
|
|
|
Malik RN, Hussain SZ, Nazir I (2010). Heavy metals contamination and accumulation in soil and wild plant species from industrial area of Islamabad, Pakistan. Pakistan Journal of Botany 42:123-127.
|
|
|
|
|
Manier N, Deram A, Broos K, Denayer FO, Haluwyn CV (2009). White clover nodulation index in heavy metal contaminated soils - a potential bioindicator. Journal of Environmental Quality 38:685-692.
Crossref
|
|
|
|
|
Mendez MO, Maier RM (2008). Phytostabilization of mine tailings in arid and semi-arid environments - an emerging remediation technology. Environmental Health Perspectives 116(3):278-283
Crossref
|
|
|
|
|
Pandey V, Dixit V, Shyam R (2005) Antioxidative responses in relation to growth of mustard plants exposed to hexavalent chromium. Chemosphere 61:40-47.
Crossref
|
|
|
|
|
Rajkumar M, Freitas H (2008). Influence of metal-resistant-plant-growth-promoting bacteria on the growth of Ricinus communis in soil contaminated with heavy metals. Chemosphere 71:834-842
Crossref
|
|
|
|
|
Saha R, Nandi R, Saha B (2011) Sources and toxicity of hexavalent chromium. Journal of Coordination Chemistry 64(10):1782-1806.
Crossref
|
|
|
|
|
Santos JA, Nunes LA, Melo WJ, Araujo ASF (2011). Tannery sludge compost amendment rate on soil microbial biomass of two different soils. European Journal of Soil Biology 47:146-15.
Crossref
|
|
|
|
|
Sauve S, McBride MB, Norvell WA, Hendershot WH (1997). Copper solubility and speciation of in situ contaminated soil: effects of copper level, pH and organic matter. Water Air and Soil Pollution 164:1-7.
|
|
|
|
|
Sethi S, Gupta S (2009). Heavy metal Impact on Soil Microbial Biomass, Soil dehydrogenase activity and Soil Respiration rate. International Journal of Advanced Research in Biological Sciences 1(6):29-34.
|
|
|
|
|
Shafiq M, Zafar IM, Athar M (2008). Effect of lead and cadmium on germination and seedling growth of Leucaena leucocephala. Journal of Applied Science and Environmental Management 12(3):61-66
|
|
|
|
|
Shanker AK, Cervantes C, Loza-Tavera H (2005). Chromium toxicity in plants. Environment International 31:739-753.
Crossref
|
|
|
|
|
Snow ET (1994). Effects of chromium on DNA replication in vitro. Environmental Health Perspectives 3:41-44.
|
|
|
|
|
Stambulaka U, Bayliak MM, Lushchk VI (2018). Chromium (VI) toxicity in legume plants: Modulation effects of rhizobial symbiosis. Biomedical Research International.
Crossref
|
|
|
|
|
Trinh NN, Huang TL, Chi WC, Fu SF, Chen CC, Huang HJ (2014). Chromium stress response effect on signal transduction and expression of signaling genes in rice. Physiology of Plants 150(2):205-224.
Crossref
|
|
|
|
|
Walker AF, Marakis G, Christie S, Byng M (2003). Magnesium citrate found more bioavailable than other Magnesium preparations in a randomized double-blind.Magnesium. Research 16:183-191.
|
|
|
|
|
Wani PA, Khan MS, Zaidi A (2008). Effect of metal-tolerant plant growth-promoting Rhizobium on the performance of peas grown in metal-amended soil. Archives of Environmental Control and Toxicology 55:33-42.
Crossref
|
|
|
|
|
Wistreich GA (1997). Microbiology Laboratory: Fundamentals and applications. 6th edn. Prentice Hall, New Jersey pp. 37-76.
|
|
|
|
|
Yoon J, Cao X, Zhou Q, Ma LQ (2006). Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Science of the Total Environment 368:456-464.
Crossref
|
|