ABSTRACT
Diabetes is directly involved in oxidative stress production. Therefore, this study was conducted to investigate the morphological and functional alterations caused by oxidative stress and to evaluate the antioxidant effect of quercetin nanoparticles (QUNPs) in streptozotocin (STZ)-induced diabetic (type II) rats. Seventy two male albino adult rats were randomly distributed in 6 different experimental groups, with 12 animals per group: Normal Control (NC) group, Positive Control (PC) group received one dose of STZ (60 mg/kg body weight [bw]); QUNPs 10 mg/kg bw/day alone group; QUNPs 10 mg/kg bw/day + one dose of STZ (60 mg/kg bw) group; QUNPs 20 mg/kg bw/day alone group; and QUNPs 20 mg/kg bw/day + one dose of STZ (60 mg/kg bw) group. STZ-diabetic rats were treated with QUNPs (10 and 20 mg/kg bw/day) for 7 weeks to analyze their effects on markers of renal enzymes antioxidant [malondialdehyde (MDA), catalase (CAT), glutathione reductase (GR), and glutathione peroxidase (GPx)], total protein and albumin, and also on kidney tissues. The results showed that the particle size of QUNPs is 16.13 nm at flow rate 10 ml/min. QUNPs especially at the dosage of 20 mg/kg bw/day gave results close to normal values observed in NC compared to PC. Also, histopathology of kidney sections for QUNPs 20 mg/kg bw/day + STZ and QUNPs (10 and 20 mg/kg bw/day) alone, appeared similar to NC. It can be concluded that QUNPs could become a promising adjuvant in the treatment of diabetes mellitus and can act as an antioxidant agent.
Key words: Diabetic, streptozotocin, free radical, antioxidant.
Diabetes mellitus (DM) or hyperglycemia is a metabolic disorder that develops from cases of insufficient or absence of insulin release from β-cells (Vardi et al., 2003). Chronic hyperglycemia leads to many complications, such as cardiomyopathy, vascular damage, retinopathy, neuropathy, and nephropathy (Review of World Health Organ Tech Rep Ser.,1985). Streptozotocin (STZ), an antibiotic produced by Streptomyces achromogenes and used as an agent to induced diabetes, damages the insulin-producing β-cells membranes and results in the depletion of intracellular nicotinamide adenine dinucleotide in islet cells (Kanter et al., 2004; Coskun et al., 2004). Also, it is known to induce diabetic kidney injury with hyperlipidemia, inflammation, and hyperuricemia (Hovind et al., 2009; Tone et al., 2005). Kidney injury is the most common pathological disorder predisposing end-stage renal disease worldwide (Ayodele et al., 2004; Donath and Shoelson, 2011; Murea et al., 2010). Diabetic nephropathy is characterized with progression into glomerulosclerosis, interstitial fibrosis, and tubular atrophy by mesangial expansion and thickening of basement membranes, ultimately resulting in renal failure (Tsao et al., 1999; Voziyan et al., 2002; Feliers et al., 2001). Previous studies proposed a wide variety of mechanisms in the pathogenesis of diabetes, including oxidation of renal glycoproteins by reactive oxygen species (Reddy et al., 2002; Natarajan et al., 2002; Chen et al., 2001).
Oxidative stress is caused by highly toxic components, such as the overproduction of reactive nitrogen and oxygen radicals which interact with the lipid bilayer and produce lipid peroxides of cellular membranes and caused toxicity to all the components of the cells (Tatsuki et al., 1997). Higher oxidative stress is one of the factors for impaired antioxidant defense mechanisms or increased levels of free radicals because it is implicated in the progression and development of diabetic complications (Ceriello, 2000; Saxena et al., 1993). Therefore, in recent years, researchers have developed interest to prevent oxidative damage with high oxidative stress in DM by the role and usage of natural antioxidants. Flavonoids have the capacity to promote β-cell regeneration in islets, normalize blood glucose levels, and normal islets from STZ in rats (Un et al., 2006). Individually, the protective effects of polyphenols and flavonoids may exert in a variety of ways: they may scavenge chelating metal ions, reactive oxygen species, also, scavenging lipid peroxyl radicals to act as a chain-breaking antioxidant, or prevent lipid damage by partition into the lipid bilayer (Plumb et al., 1999; Laughton et al., 1991; Robak and Gryglewski, 1988). Moreover, some studies reported that the activity of flavonoids as antioxidant may be dependent on hydroxylation degree (Plumb et al., 1999; Rice-Evans et al., 1996).
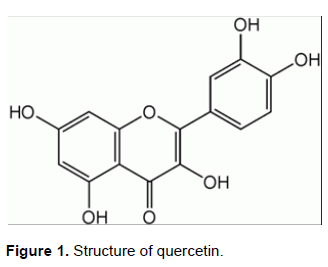
Quercetin (3,3,4,5,7-pentahydroxyflavone, QUE) is a lipid-soluble compound (Figure 1). It reduces lipid hydroperoxide production (Coldiron et al., 2002) and is capable of preventing lipid damage, inhibits biomolecule oxidation, radical scavenging, and alters antioxidant defense pathways in vitro (Candlish and Das, 1996; Morand et al., 1998). QUE is a well-documented bioflavonoid occurring in many foods and is known to be present in higher concentrations in green tea, red wine, broccoli, apples, and onions (Kiviranta et al., 1998; Weisburer, 2000). Previous studies have focused on the QUE beneficial properties, namely, anticarcinogenic properties, antioxidant, anti-inflammatory, antiproliferative, and antibacterial (Weisburer, 2000).
On the other hand, QUE is a challenging molecule to be delivered due to its poor water solubility. A water-soluble derivative of QUE has been synthesized but its bioavailability was only 20% (Mulholland et al., 2001) and it has such poor absorption in the gastro-intestinal tract. All these highlight the need for an improved formulation for QUE with enhanced dissolution so that its absorption can be greatly enhanced. Therefore, micro- and nanoparticle preparations are the most important approaches being investigated these days to improve bioavailability (Bilati et al., 2005). Nanoparticles are particularly useful in drug delivery for water-insoluble compounds such as ellagic acid (Bala et al., 2006) and coenzymeQ10 (Hsu et al., 2003), because their size (less than 1000 nm) can increase the absorption and the bioavailability of the delivered drug. Thus, an improved oral formulation of QUE is required with better bioavailability and higher efficacy. Therefore, this study aimed to prepare quercetin nanoparticles (QUNPs) and evaluate the antioxidant effect of QUNPs on renal histopathological and serum biochemical alterations in STZ- induced diabetic (type II) rats.
In this study, QUE (Sigma-Aldrich, Singapore) was used as received. All reagents used were of technical grade. Absolute ethanol (99.5 to 99.8%) was obtained from J.T. Baker (Avantor Performance Materials, Phillipsburg, NJ).
Preparation of QUNPs
To prepare QUNPs, magnetic stirring (1000 rpm) was used to mix water and ethanol (volume ratio 35:1, fixed flow rate of 10 ml/min) according to the nano participation technique (Kakran et al., 2012; Abd El-Rahman and Al Jameel, 2014). Then, commercial QUE was dissolved in predetermined concentration (5 mg/ml) of ethanol (the solvent). The syringe was filled with the prepared solution and secured onto a syringe pump. Quickly, drug solution was injected under magnetic stirring into the anti-solvent (deionized water) of definite volume at a fixed flow rate. The QUNPs were filtered and vacuum dried.
Morphology of the particles
Scanning electron microscopy (SEM; Quanta 3D FEG/FEI) with 20 kV, 300 V collector bias was used to observe the samples morphology. Before the SEM observations, the samples powder were spread on a SEM stub and sputtered with gold.
Biological methods
Male albino adult rats (72 animals weighing 170 ± 2 g) were obtained from Vaccination Center, Helwan, Giza, Egypt, then transported to Animal House of Ophthalmology Research Institute, Giza, Egypt. The rats fed on basal diet (casein 10%, salt mixture 4%, corn starch 70%, corn seed oil 10%, vitamins mixture 1% and cellulose 5%) for ten days after being housed in individual cages with screen bottoms. After equilibration and before administration of STZ, rats were divided into six groups (twelve animals per each) and weighted: G1, Negative Control (NC) group; G2, Positive Control (PC) group injected with single dose of STZ (60 g/kg bw); G3, treated group that received QUNPs (10 mg/kg bw/day) only; G4, treated group that received one dose of STZ (60 g/kg bw) + QUNPs (10 mg/kg bw/day); G5, treated group that received QUNPs (20 mg/kg bw/day) only; G6, treated group that received one dose of STZ (60 g/kg bw) + QUNPs (20 mg/kg bw/day) for 7 weeks. Fresh feed was provided every day; also at the beginning and during the experimental period, the animal total body and total feed consumption were weighed and recorded. The heparinized capillary glass tubes were used to collect the blood samples from the orbital plexus according to Schermer (1967). To obtain serum, samples were centrifuged (1500 ×g) at 4°C for 30 min. The study received institutional approval (2016-10-084).
Serum biochemical assays
Serum blood glucose, urea, creatinine, uric acid, albumin, total protein, and MDA were determined by kits obtained from bio diagnostic company (Dokki, Giza, Egypt).
Serum globulin was determined by the following formula:
Serum globulin = Total serum protein – Serum albumin Catalase (CAT), glutathione peroxidase (GPx) and glutathione reductase (GR) activity were assayed in serum. Catalase activity was determined in milliunits of enzymatic activity per mg of protein (mU/mg protein) contained in the samples by using Catalase Assay Kit (Cayman Chemical, Michigan, USA). The activity of GPx was determined according to Flohé and Günzler (1984). First, 20 µl of sample was mix with reaction mixture (180 µl) [pH 7.0, 50 mM potassium phosphate buffer, 1 mM glutathione (GSH, Roche, Mannheim, Germany), 0.5 mM EDTA, 1 mM sodium acid, 0.2-mM nicotinamide adenine dinucleotide phosphate (NADPH; Calbiochem) and 0.5 U GR (Roche)]. Then, adding 0.45 mM H2O2 (100 µl) to 0.15 mM (a final concentration) to initiated the reaction. The activity of GR was determined by recommended methods (Gutterer et al., 1999). Samples (30 µl) were mixed with reaction mixture (170 µl) (pH 7.0, 100 mM potassium phosphate buffer, 0.2 mM NADPH, 1 mM EDTA). Then, 100 µl of 3 mM GSSG (Roche) was added to 1 mM (a final concentration) to initiate the reaction. For both assays, the absorbance decreases because NADPH oxidation was recorded at 340 nm.
Statistical analysis
Mean (SEM) was used to express the results. One way analysis of variance (ANOVA) followed by Fischer’s least significant difference (LSD) test was used to measure the intergroup variation. Statistical significance was considered at (P≤0.05). Statistical analysis was done using the Jandel Sigma Stat Statistical Software version 2.0.
Histopathological assay
For microscopic evaluation, the kidneys were first fixed in neutral phosphate buffered formalin solution (10%). After dehydration in an ascending series of ethanol (70, 80, 96, and 100%), the samples’ tissue was cleared in xylene and embedded in paraffin. Tissue sections (5 μm) were stained with hematoxylin-eosin (H-E). Fields (10, a minimum) for each kidney slide were examined and assigned for severity of changes by pathologist blinded to the treatments of the animals.
Scanning electron microscope (SEM)
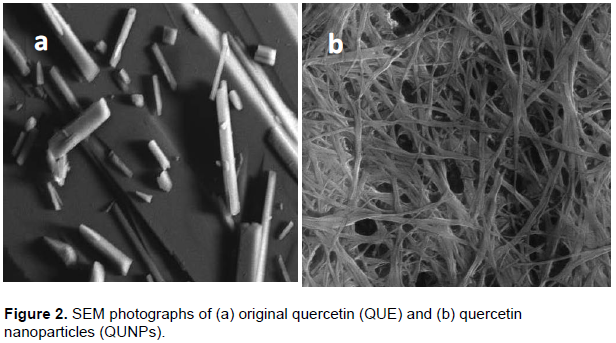
Morphology of original QUE and QUNPs were studied using SEM tool. As shown in Figure 2, QUNPs showed a particle size of 16.13 nm at flow rate 10 ml/min. The powder of original QUE (Figure 2a) exhibited particles lacking uniformity in size which was relatively much larger than the QUNPs. While, QUNPs prepared by syringe pump, exhibited less crystallinity, absence of larger particles, and particles uniformity in size (Figure 2b) (Abd El-Rahman and Al Jameel, 2014).
These results indicated that QUE made by syringe pump gave particle size more uniform and significantly smaller than the commercial QUE that was more evidenced in the case prepared sample at 5 mg/ml (lower drug concentration). This behavior can be explained by considering two factors: the concentration influence on the viscosity and the nuclei number formed in the interface of solvent/anti-solvent (Kakran et al., 2012).
Effect of QUNPs on blood sugars, urea, creatinine and uric acid
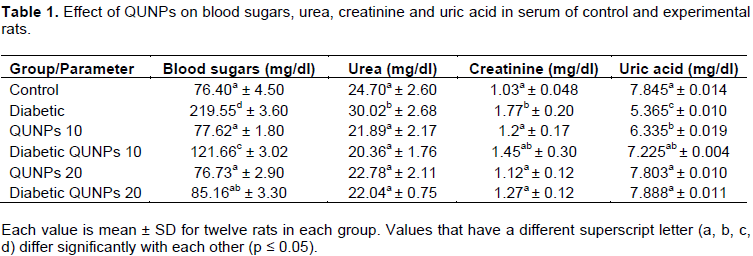
As shown in Table 1, STZ diabetic rats showed increases in blood sugars, urea and creatinine and decreased uric acid as compared to NC. Treatment of STZ diabetic rats with QUNPs (10 and 20 mg/kg bw/day) resulted in decreased serum blood sugars, urea and creatinine and increased uric acid levels in those treated rats as compared to PC. QUNPs (10 and 20 mg/kg bw/day) alone gave results close to normal values observed in NC. Also, STZ + QUNPs (20 mg/kg bw/day) gave similar results. The antihyperglycemic effect of quercetin might be due to its property of antioxidant, which inhibits the peroxidation of lipid by scavenging the free radicals produced by STZ and prevents oxidative stress induced by STZ, and also, helps the surviving β-cells to secrete more insulin and proliferation. Additionally, QUE enhances the sensitivity of insulin, leading to increased glucose utilization by the extrahepatic tissues and thereby decreasing the levels of blood glucose (Babujanarthanam et al., 2010). Vessal et al. (2003) reported that supplementation has proven to be beneficial in decreasing the concentration of blood glucose, promoting the pancreatic islets regeneration and increasing release of insulin in STZ treated diabetic rats; thus exerting its beneficial antidiabetic effects (Formica and Regelson 1995).
This is supported by the previous literature reports where these types of flavonoids enhance release of insulin up to 70% by its effect on function of islet at least in part, metabolism of cyclic nucleotide, and via alteration in Ca+2 fluxes (Yen et al., 2009; Hii and Howell, 1985a; Hii and Howell, 1985b). Blood glucose levels were significantly increased after 72 h following STZ injection compared to control group, while all QUE treatment groups had significantly decreased blood glucose concentrations compared to the diabetic group after 30 days (Elbe et al., 2015).
Previous studies reported that the administration of STZ decreased levels of insulin and increased levels of plasma glucose, while treatment with QUE resulted in decreased glucose in plasma and increased levels of insulin. Diabetic groups treated with QUE suspension showed significantly lower glucose levels as well as QUNPs as against diabetic control group (Babujanarthanam et al., 2011; Sinha, 1972). Serum urea and creatinine levels are the most important indicators of kidney functions. Lu et al. (2007) and Maciel et al. (2013) reported that urea and creatinine levels were increased in diabetic rats as compared to the control group. Also, urea level was increased in diabetic rats when administrated with 5, 25 and 50 mg/kg of QUE than healthy groups treated with the same QUE dosages (P < 0.05).
Effect of QUNPs on total protein, globulin and albumin
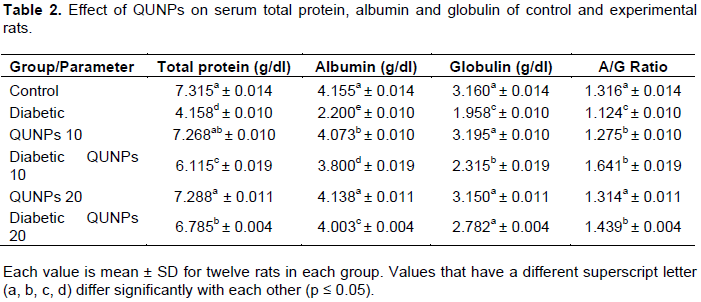
Data in Table 2 showed the total protein levels, albumin (A), globulin (G) and A/G ratio after treatment with STZ (60 mg/kg bw), QUNPs (10 and 20 mg/kg bw) and QUNPs (10 and 20 mg/kg bw) + STZ for 7 weeks. PC group showed decreased levels (P ≤ 0.05) of total protein, albumin, and globulin in relation to NC group. Diabetic rats treated with QUNPs (10 and 20 mg/kg bw) presented increased concentration of total protein, albumin and globulin to near NC levels (P ≤ 0.05) and these results agree with several studies (Maciel et al., 2013; Arya et al., 2014). The albumin level was significantly decreased in the diabetic group, which might be due to albumin leakage due to glomerular basement damage of membrane combined with an increase in the pressure of trans glomerular filtration or impaired reabsorption of tubular (Maciel et al., 2013). Also, the results should a significant reduction in concentration of serum albumin in diabetic rats, except the group of rats treated with QUE at 50 mg/kg (Naoum, 1999)(50). However, the QUE treatment significantly increased the serum albumin towards the negative levels, which was reflected in the reduction of kidney damage due to STZ-induced hyperglycaemia. Consistent with our results, Kandasamy and Ashokkumar (2012) reported that in diabetic nephrotoxic rats, flavonoids restore the reduced level of albumin. Thus, QUE has a beneficial pharmacological effect on diabetic, especially at a dose of 50 mg/kg on hepatic, protein levels, diabetic, and
functional markers.
Effect of QUNPs on MDA, CAT, GR and GPx
Oxidative stress is an imbalance between the production of free radicals and antioxidants defense capacity (Hamadi et al., 2012). It is well known that hyperglycaemia increases mitochondrial ROS production and impairs cellular antioxidant enzymes, which could represent a key event in the development and progression of the complications of diabetes (Hamadi et al., 2012; Chang et al., 2012).
Glutathione reductase, catalase and glutathione peroxidase are among those enzymes that metabolize endogenous free radicals and reactive oxygen species, often with the concomitant oxidation of reduced glutathione (GSH) to its oxidized form (GSSG) (Josephy, 1997). Reduced glutathione deficiency is also seen in tumorigenesis aminoaciduria, nephropathy (Meister, 1988) and cataract genesis (Nagasawa et al., 1996; Walsh and Aleo, 1997).
QUNPs significantly increased the serum activity of MDA, CAT, GR and GPx enzymes in normal rats after treatment for 7 weeks (Table 3). On the other hand, STZ had an opposite effect on the activity of serum malondialdehyde (MDA), catalase (CAT), GR and GPx enzymes, but treatment with QUNPs ameliorated its effect (Table 3). Similar results were noted by Elbe et al. (2015) where they found that QUE was beneficial in reducing diabetes-related alterations. QUE has a free radical scavenger, transfer electrons, chelate metals and superoxide radical inhibitor properties (Vessal et al., 2003; Ferrali et al., 1997). Beneficial effects of QUE are attributed to its antioxidant effects as well as protective effects on β-cell integrity. Also, Vessal et al. (2003) noted its increasing effect on the islets number of Langerhans in pancreas. Also, there is a significant decrease in the plasma level of glucose.
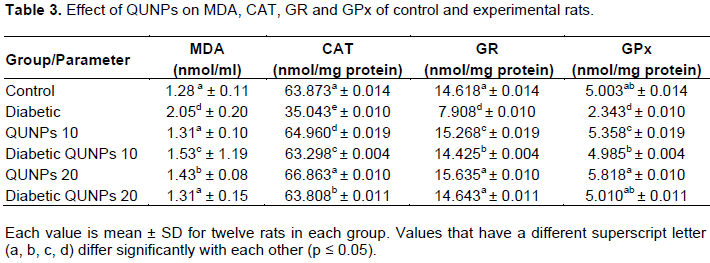
MDA is the most commonly used indicator of lipid peroxidation. Decreases in cellular antioxidant enzymes and increase in tissue level of MDA emphasize oxidative stress. Previous studies reported that QUE treatment significantly decreased diabetes-related oxidative damage in various organs by increasing the activities of antioxidant enzyme but decreasing the levels of MDA (Dias et al., 2005; Sirovina et al., 2013; Edremitlioglu et al., 2012).
Babujanarthanam et al. (2011) indicated that QUE decreases the levels of thiobarbituric acid reactive substances (TBARS) in plasma in STZ-induced diabetic rats. H2O2 may be an important mediator for tissue damage in STZ induced diabetes (Yanardag et al., 2005). CAT protects the cell from oxidative damage induced by H2O2, because it is localized in the microperoxisomes or the peroxisomes, which catalyzes the decomposition of H2O2 to water and oxygen (Abolfathi et al., 2012). If it is not decomposed by GSH peroxidase or CAT, it causes production of reactive hydroxyl radicals. Excess amounts of free radicals damage nucleic acids and cellular proteins by attaching to them and causing lipid peroxidation. STZ significantly increased the ROS and significantly decreased the activity of antioxidant enzyme. The activities of plasmatic GSH levels were increased significantly and the activities of antioxidant enzyme [CAT and superoxide dismutase (SOD)] were decreased significantly in STZ-diabetic rats. The diabetic rats treated with quercetin showed an increased in CAT, GSH and SOD activity. Quercetin directly scavenges free radicals and ROS; therefore, it is an important flavonoid which is known to be a potent antioxidant (Annapurna et al., 2009; Boots et al., 2008; Jeong et al., 2012). When treated with 50 mg/kg of QUE, the levels reverted close to normal values observed in control group (P < 0.05) (Maciel et al., 2013; Stanley and Menon, 2001). Also, Elbe et al. (2015) reported that in diabetic group, CAT activity was decreased significantly compared with the control group and were significantly increased in treated group compared with the diabetic group. QUE increases CAT activity and reduces lipid peroxidation, thus, it prevents oxidative stress (Elbe et al., 2015; Maritim et al., 1991).
GPx react with GSH, thus, it serves to detoxify peroxides (Sen, 1997). Low GPx activity in diabetic might be due to low GSH content, since GSH is a substrate and cofactor of this enzyme (Dominguez et al., 1998). In the process of converting H2O2 to water, GPx converts GSH to GSSG, which is reduced back to GSH by GRx (Maritim et al., 2003). Glutathione may contribute to antioxidant defense by networking with the other major antioxidants (Babujanarthanam et al., 2011).
STZ could decrease pancreatic GSH-Px and CAT, but QUE could enhances pancreatic GSH-Px and CAT activity and consequently antagonizes STZ effect on these antioxidant enzymes (Abdelmoaty et al., 2010). These results together suggest that QUNPs is effective to protect kidney against oxidative stress induced by STZ-induced diabetes.
Histopathological studies
As shown in Figure 3, the rat kidney sections were stained with hematoxylin and eosin. The NC rat kidney presents a normal glomerulus surrounded by Bowman’s capsule, distal convoluted and tubules proximal, normal podocytes (Pc) and normal capillaries (CP) (Figure 3A). Figure 3B shows the kidneys of the STZ-treated rats, which present a glomerular hypertrophy (Gh), thickening of the basement membrane and mesangial expansion. Also, degeneration of glomerular capillaries both tubular and proximal convoluted tubule exhibited edematous changes. Figure 3D shows the effect of QUNPs 10 mg + STZ treatment and the findings present less expansion of the glomerulus, features of healing, mildly dilated capillaries, and little damage of proximal and distal tubules. Moreover, the histopathological of kidney sections for QUNPs 20 mg/kg bw/day + STZ and QUNPs (10 and 20 mg/kg bw/day) alone, appeared similar to NC (Figure 3F, C and E).
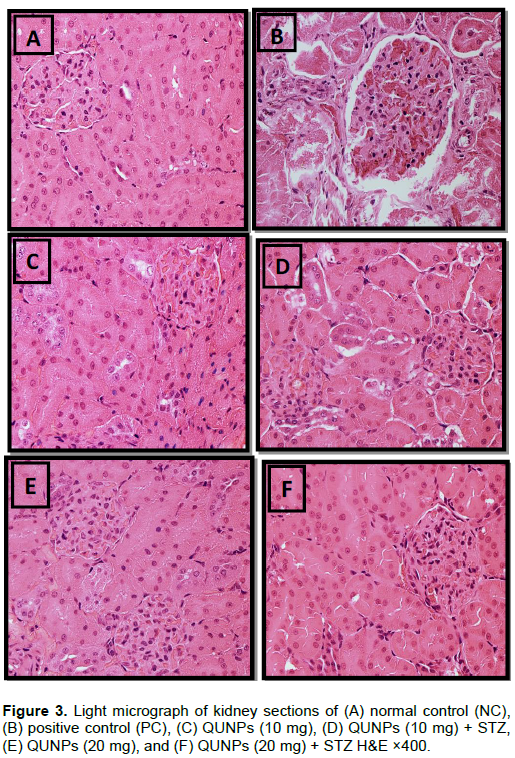
Our results agree with Elbe et al. (2015) who reported that diabetic group showed severe tubular and glomerular alterations. Cellular swelling, tubular changes including tubular basal membrane thickening, peritubular infiltration, epithelial desquamation, mesangial matrix expansion within glomerulus and capillary and intracytoplasmic vacuolization were obvious. Eddy (1996) and Babujanarthanam et al. (2011) suggested that tubular and glomerular changes were reduced in QUE administered groups. Also, Arya et al. (2014) and Bashir et al. (2014) reported that the diabetic rats treated with QUE demonstrated a recovery of the normal structure of kidney with intact tubules and glomerular epithelial cells.
The morphological and serum biochemical findings suggested that the administration of QUNPs to diabetic rats causes beneficial effect in terms of regeneration of cells in damaged kidney. Thus, it is concluded that QUNPs possess preventive and curative effect on STZ induced diabetes in rats and can be used as a natural herbal medicine to protect kidney and pancreatic cells.
The authors have not declared any conflict of interests.
REFERENCES
|
Abd El-Rahman SN, Al-Jameel SS (2014). Quercetin Nanoparticles: Preparation and Characterization. Indian J. Drugs 2(3):96-103.
|
|
|
|
Abdelmoaty MA, Ibrahim MA, Ahmed NS, Abdelaziz MA (2010). Confirmatory studies on the antioxidant and antidiabetic effect of quercetin in rats. Indian J. Clin. Biochem. 25:188-192.
Crossref
|
|
|
|
Abolfathi AA, Mohajeri D, Rezaie A, Nazeri M (2012). Protective effects of green tea extract against hepatic tissue injury in streptozotocin-induced diabetic rats. Evid. Based Complement. Alternat. Med. Feb 21; 2012.
Crossref
|
|
|
|
Annapurna A, Reddy CS, Akondi, R.B, Rao SR (2009). Cardioprotective actions of two bioflavonoids, quercetin and rutin, in experimental myocardial infarction in both normal and streptozotocin-induced type I diabetic rats. J. Pharm. Pharmacol. 61(10):1365-1374.
Crossref
|
|
|
|
Arya A, Al-Obaidi MM, Shahid N, Bin Noordin MI, Looi CY, Wong WF, Khaing SL, Mustafa MR (2014). Synergistic effect of quercetin and quinic acid by alleviating structural degeneration in the liver, kidney and pancreas tissues of STZ-induced diabetic rats: A mechanistic study. Food Chem. Toxicol. 71:183-196.
Crossref
|
|
|
|
Ayodele OE, Alebiosu CO, Salako BL (2004). Diabetic nephropathy, a review of the natural history, burden, risk factors and treatment. J. Natl. Med. Assoc. 96:1445-1454.
|
|
|
|
Babujanarthanam B, Kavitha P, MahadevaRao US, Pandian MR (2011). Quercitrin a bioflavonoid improves the antioxidant status in streptozotocin: induced diabetic rat tissues. Mol. Cell. Biochem. 358:121-129.
Crossref
|
|
|
|
Babujanarthanam R, Kavitha P, Rajasekara PM (2010). Quercitrin, a bioflavonoid improves glucose homeostasis in streptozotocin-induced diabetic tissues by altering glycolytic and gluconeogenic enzymes. Fundam. Clin. Pharmacol. 24:357-364.
Crossref
|
|
|
|
Bala I, Bhardwaj V, Hariharan S, Kharade SV, Roy N, Ravi Kumar MN (2006). Sustained release nanoparticulate formulation containing antioxidant ellagic acid as potential prophylaxis system for oral administration. J. Drug Target. 14:27-34.
Crossref
|
|
|
|
Bashir SO, Morsy MD, Sakr HF, El Refaey HM, Eid RA, Alkhateeb MA, Defallah MA (2014). Quercetin ameliorates diabetic nephropathy in rats via modulation of renal Na+, K+ -ATPase expression and oxidative stress. Am. J. Pharmacol. Toxicol. 9:84-95.
Crossref
|
|
|
|
Bilati U, Allemann E, Doelker E (2005). Development of a nano precipitation method intended for the entrapment of hydrophilic drugs into nanoparticles. Euro. J. Pharm. Sci. 24:67-75.
Crossref
|
|
|
|
Boots AW, Haenen GR, Bast A (2008). Health effects of quercetin: from antioxidant to nutraceutical. Euro. J. Pharmacol. 585:325-337.
Crossref
|
|
|
|
Candlish JK, Das NP (1996). Antioxidants in food and chronic degenerative diseases. Biomed. Environ. Sci. 9:117-123.
|
|
|
|
Ceriello A (2000). Oxidative stress and glycemic regulation. Metabolism 49:27s-29s.
Crossref
|
|
|
|
Chang CC, Chang CY, Huang JP, Hung LM (2012). Effect of resveratrol on oxidative and inflammatory stress in liver and spleen of streptozotocin-induced type 1 diabetic rats. Chin. J. Physiol. 55:192-201.
Crossref
|
|
|
|
Chen S, Cohen MP, Lautenslager GT, Shearman CW, Ziyadeh FN (2001). Glycated albumin stimulates TGF-beta 1 production and protein kinase C activity in glomerular endothelial cells. Kidney Int. 59:673-681.
Crossref
|
|
|
|
Coldiron AD, Sanders RA, Watkins JB (2002). Effects of combined quercetin and coenzyme Q10 treatment on oxidative stress in normal and diabetic rats. J. Biochem. Mol. Toxicol. 16(4):197-202.
Crossref
|
|
|
|
Coskun O, Ocakcı A, Bayraktaroglu T, Kanter M (2004). Exercise training prevents and protects streptozotocin-induced oxidative stress and -cell damage in rat pancreas. Tohoku J. Exp. Med. 203:145-154.
Crossref
|
|
|
|
Dias AS, Porawski M, Alonso M, Marroni N, Collado PS, González-gallego J (2005). Quercetin decreases oxidative stress, NF-B activation, and iNOS overexpression in liver of streptozotocin induced diabetic rats. J. Nutr. 135:2299-2304.
|
|
|
|
Dominguez C, Ruiz E, Gussinye M, Carrascosa A (1998). Oxidative stress at onset and in early stages of type I diabetes in children and adolescents. Diabetes Care 21:1736-1742.
Crossref
|
|
|
|
Donath MY, Shoelson SE (2011). Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol.11:98-107.
Crossref
|
|
|
|
Eddy AA (1996). Experimental insights into the tubule interstitial disease accompanying primary glomerular lesions. J. Am. Soc. Nephrol. 7:2494-2508.
|
|
|
|
Edremitlioglu M, Andic MF, Korkut O (2012). Quercetin, a powerful antioxidant bioflavonoid, prevents oxidative damage in different tissues of long-term diabetic rats. Balkan Med. J. 29:49-55.
Crossref
|
|
|
|
Elbe H, Esrefoglu M, Vardi N, Taslidere E, Ozero E, Tanbek K (2015). Melatonin, quercetin and resveratrol attenuate oxidative hepatocellular injury in streptozotocin-induced diabetic rats. Hum. Exp. Toxicol. 34:859-868.
Crossref
|
|
|
|
Feliers D, Duraisamy S, Faulkner JL, Duch J, Lee AV, Abboud HE (2001). Activation of renal signaling pathways in db/db mice with type 2 diabetes. Kidney Int. 60:495-504.
Crossref
|
|
|
|
Ferrali M, Signofrini C, Caciotti B, Sugherini L, Ciccoli D, Giachetti D, Comporti M (1997). Protection against oxidative damage of erythrocyte membranes by the flavonoid quercetin and its relation to iron chelating activity. FEBS Lett. 416:123-139.
Crossref
|
|
|
|
Flohé FL, Günzler WA (1984). Assays of glutathione peroxidase. Methods Enzymol.105:114-1121.
Crossref
|
|
|
|
Formica JV, Regelson W (1995). Review of the biology of quercetin and related bioflavonoids. Food. Chem. Toxicol. 33:1061-1080.
Crossref
|
|
|
|
Gutterer JM, Dringen R, Hirrlinger J, Hamprecht B (1999). Purification of glutathione reductase from bovine brain, generation of an antiserum, and immune cytochemical localization of the enzyme in neural cells. J. Neurochem. 73(4):1422-1430.
Crossref
|
|
|
|
Hamadi N, Mansour A, Hassan MH, Khalifi-Touhami F, Badary O (2012). Ameliorative effects of resveratrol on liver injury in streptozotocin-induced diabetic rats. J. Biochem. Mol. Toxicol. 26:384-392.
Crossref
|
|
|
|
Hii CS, Howell SL (1985a). Effects of epicatechin on rat islets of Langerhans. Diabetes 33:291-296.
Crossref
|
|
|
|
Hii CST, Howell SL (1985b). Effects of flavanoids on insulin secretion and 45Ca2+ handling in rat islets of langerhans. J. Endocrinol. 107:1-8.
Crossref
|
|
|
|
Hovind P, Rossing P, Tarnow L, Johnson RJ, Parving HH (2009). Serum uric acid as a predictor for development of diabetic nephropathy in type 1 diabetes: an inception cohort study. Diabetes 58:1668-1671.
Crossref
|
|
|
|
Hsu CH, Cui Z, Mumper RJ, Jay M (2003). Preparation and characterization of novel coenzyme Q10 nanoparticles engineered from microemulsion precursors. AAPS PharmSciTech. 4(3):24-35.
Crossref
|
|
|
|
Jeong SM, Kang MJ, Choi HN, Kim JH, Kim JI (2012). Quercetin ameliorates hyperglycemia and dyslipidemia and improves antioxidant status in type 2 diabetic db/db mice. Nutr. Res. Pract. 6(3):201-207.
Crossref
|
|
|
|
Josephy PD (1997). Molecular toxicology. New York: Oxford University Press.
|
|
|
|
Kakran M, Sahoo NG, Li L, Judeh Z (2012). Fabrication of quercetin nanoparticles by anti-solvent precipitation method for enhanced dissolution. Powder Technol. 223:59-64.
Crossref
|
|
|
|
Kandasamy N, Ashokkumar N (2012). Myricetin, a natural flavonoid, normalizes hyperglycemia in streptozotocin-cadmium-induced experimental diabetic nephrotoxic rats. Biomed. Prev. Nutr. 2:246-251.
Crossref
|
|
|
|
Kanter M, Coskun O, Korkmaz A, Oter S (2004). Effects of Nigella sativa on oxidative stress and -cell damage in streptozotocin-induced diabetic rats. Anat. Rec. 279:685-691.
Crossref
|
|
|
|
Kiviranta J, Huovinen K, Hiltunen R (1998). Variation of phenolic substances in onion. Acta Pharm. Fenn. 97:67-72.
|
|
|
|
Laughton MJ, Evans PJ, Moroney MA, Hoult JR, Halliwell B (1991). Inhibition of mammalian5-lipoxygenaseandcyclo-oxygenase by flavonoids and phenolic dietary additives. Relationship to antioxidant activity and to iron ion-reducing ability. Biochem. Pharmacol. 42:1673-1681.
Crossref
|
|
|
|
Lu Q, Yın XX, Wang JY, Gao YY, Pan YM (2007). Effects of Ginkgo biloba on prevention of development of experimental diabetic nephropathy in rats. Acta Pharmacol. Sin. 28:818-828.
Crossref
|
|
|
|
Maciel RM, Costa MM, Martins DB, França RT, Schmatz R, Graça DL, Duarte MM, Danesi CC, Mazzanti CM, Schetinger MRC, Paim FC, Palma HE, Abdala FH, Stefanello N, Zimpel CK, Felin DV, Lopes STA (2013). Antioxidant and anti-inflammatory effects of quercetin in functional and morphological alterations in streptozotocin-induced diabetic rats. Res. Vet. Sci. 95:389-397.
Crossref
|
|
|
|
Maritim AC, Moore BH, Sanders RA, Watkins JB (1991). Effects of melatonin on oxidative stress in streptozicin-induced diabetic rats. Int. J. Toxicol. 18:61-166.
|
|
|
|
Maritim AC, Sanders RA, Watkins JB (2003). Effects of a-lipoic acid on biomarkers of oxidative stress in streptozotocin induced diabetic rats. J. Nutr. Biochem. 14:288-294.
Crossref
|
|
|
|
Meister A (1988). Glutathione metabolism and its selective modiï¬cation. J. Biol. Chem. 263:17205-17208.
|
|
|
|
Morand C, Crespy V, Manach C, Besson C, Demigne C, Remesy C (1998). Plasma metabolites of quercetin and their antioxidant properties. Am. J. Physiol. 275:R212-R219.
|
|
|
|
Mulholland PJ, Ferry DR, Anderson D, Hussain SA, Young AM, Cook JE, Hodgkin E, Seymour LW, Kerr DJ (2001). Pre-clinical and clinical study of QC12, a water-soluble, pro-drug of quercetin. Ann. Oncol. 12:245-248.
Crossref
|
|
|
|
Murea M, Freedman BI, Parks JS, Antinozzi PA, Elbein SC, Ma L (2010). Lipotoxicity in diabetic nephropathy: the potential role of fatty acid oxidation. Clin. J. Am. Soc. Nephrol. 5:2373-2379.
Crossref
|
|
|
|
Nagasawa HT, Cohen JF, Holleschau AM, Rathbun WB (1996). Augmentation of human and rat lenticular glutathione in vitro by prodrugs of c-l-glutamyl-l-cysteine. J. Med Chem. 39:1676-1681.
Crossref
|
|
|
|
Naoum PC (1999). Eletroforese – Técnicas e diagnósticos. 2ed. Livraria. Santos. Editora.
|
|
|
|
Natarajan R, Gerrity RG, Gu JL, Lanting L, Thomas L, Nadler JL (2002). Role of 12lipoxygenase and oxidant stress in hyperglycaemia-induced acceleration of atherosclerosis in a diabetic pig model. Diabetologia 45:125-133.
Crossref
|
|
|
|
Plumb GW, Price KR, Williamson G (1999). Antioxidant properties of flavonol glycosides from green beans. Redox Rep. 4:123-127.
Crossref
|
|
|
|
Reddy MA, Thimmalapura PR, Lanting L, Nadler JL, Fatima S, Natarajan R (2002). The oxidized lipid and lipoxygenase product 12(S)-hydroxyeicosatetraenoic acid induces hypertrophy and fibronectin transcription in vascular smooth muscle cells via p38 MAPK and cAMP response element-binding protein activation. Mediation of angiotensin II effects. J. Biol. Chem. 277:9920-9928.
Crossref
|
|
|
|
Report of WHO study group on Diabetes Mellitus (1985). WHO Technol. Re. Ser. 727:1-113.
View
|
|
|
|
Rice-Evans CA, Miller NJ, Paganga G (1996). Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 20:933-956.
Crossref
|
|
|
|
Robak J, Gryglewski RJ (1988). Flavonoids are scavengers of superoxide anions. Biochem. Pharmacol. 37:837-841.
Crossref
|
|
|
|
Saxena AK, Srivastava P, Kale RK, Baquer NZ (1993). Impaired antioxidant status in rat liver – effect of vanadate. Biochem. Pharmacol. 45:539-542.
Crossref
|
|
|
|
Schermer S (1967). The Blood Morphology of Laboratory Animal Longmans, Green and Co. Ltd, P. 350.
|
|
|
|
Sen CK (1997). Nutritional biochemistry of cellular glutathione. J. Nutr. Biochem. 8:660-672.
Crossref
|
|
|
|
Sinha KA (1972). Colorimetric assay of catalase. Ann. Biochem. 47:389-394.
Crossref
|
|
|
|
Sirovina D, Orsolic N, Koncic MZ, Kovacevic G, Benkovic V, Gregorovic G (2013). Quercetin vs chrysin: effect on liver histopathology in diabetic mice. Hum. Exp. Toxicol. 32:1058-1066.
Crossref
|
|
|
|
Stanley MPP, Menon VP (2001). Antioxidant action of Tinosporacordifolia root extract in alloxan diabetic rats. Phytother. Res.15:213-218
Crossref
|
|
|
|
Tatsuki R, Satoh K, Yamamoto A, Hoshi K, Ichihara K (1997). Lipid peroxidation in the pancreas and other organs in streptozotocin diabetic rats. Jpn. J. Pharmacol. 75:267-273.
Crossref
|
|
|
|
Tone A, Shikata K, Sasaki M, Ohga S, Yozai K, Nishishita S, Usui H, Nagase R, Ogawa D, Okada S, Shikata Y, Wada J, Makino H (2005). Erythromycin ameliorates renal injury via anti-inflammatory effects in experimental diabetic rats. Diabetologia 48:2402-2411.
Crossref
|
|
|
|
Tsao TS, Stenbit AE, Factor SM, Chen W, Rossetti L, Charron MJ (1999). Prevention of insulin resistance and diabetes in mice heterozygous for GLUT4 ablation by transgenic complementation of GLUT4 in skeletal muscle. Diabetes 48:775-782.
Crossref
|
|
|
|
Un J, Mi-Kyung L, Yong B, Mi A, Myung-Sook C (2006). Effect of citrus flavonoids on lipid metabolism and glucose-regulating enzyme mRNA levels in type-2 diabetic mice. Int. J. Biochem. Cell. Biol. 38:1134-1145.
Crossref
|
|
|
|
Vardi N, Ucar M, Iraz M, Ozturk F (2003). Morphological changes of rat endocrine pancreas in experimental diabetes. T. Klin. J. Med. Sci. 23:27-32.
|
|
|
|
Vessal M, Hemmati M, Vasei M (2003). Antidiabetic effects of quercetin in streptozocin-induced diabetic rats. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 135:357-364.
|
|
|
|
Voziyan PA, Metz TO, Baynes JW, Hudson BG (2002). A post-Amadori inhibitor pyridoxamine also inhibits chemical modification of proteins by scavenging carbonyl intermediates of carbohydrate and lipid degradation. J. Biol. Chem. 277:3397-3403.
Crossref
|
|
|
|
Walsh CM, Aleo MD (1997). Mechanistic analysis of S(1,2-dichlorovinyl)-l-cysteine- induced cataractogenesis in vitro. Toxicol Appl. Pharmacol.146:144-155.
Crossref
|
|
|
|
Weisburer JH (2000). Approaches for chronic disease prevention based on current understanding of underlying mechanisms. Am. J. Clin. Nutr. 71:1710s-1719s.
|
|
|
|
Yanardag R, Ozsoy-Sacan O, Bolkent S, Orak H, Karabulut-Bulan O (2005). Protective effects of metformin treatment on the liver injury of streptozotocin-diabetic rats. Hum. Exp. Toxicol. 24:129-135.
Crossref
|
|
|
|
Yen FL, Wu TH, Lin LT, Cham TM, Lin CC (2009). Naringenin-loaded nanoparticles improve the physicochemical properties and the hepatoprotective effects of naringenin in orally-administered rats with CCl4-induced acute liver failure. Pharm. Res. 26:893-902.
Crossref
|