ABSTRACT
Data from the snail farm of the University of Calabar, Nigeria were collected and utilized to investigate the distribution and gene frequencies of egg shell colour of two ectotypes of Giant African Land Snails (Archachatina marginata var. saturalis), black skinned (BS) × black skinned mating group, and white skinned (WS) × white skinned mating group. The eggs collected were scored for the presence of yellow (Yp), light yellow (Lp) and milky white (Mp) egg shell colour. The egg shell colour distributions between the two ectotypes (BS × BS and WS × WS) were 46.50 vs. 3.77%, 42.68 vs. 48.11% and 10.83 vs. 48.11% for yellow, light yellow and milky white, respectively. In both ectotypes, the dominant gene for yellow, light yellow and milky white egg shell colour segregated at low frequencies (0.26 vs. 0.02, 0.24 vs. 0.31 and 0.06 vs. 0.31). The lowest value being yellow shell of the white skinned × white skinned mating group with frequency of 0.02, followed by milky white shell of the black skinned × black skinned mating group with frequency of 0.06. These values were much lower than the Mendelian value of 0.75. This is an indication that snails have not been purified through artificial breeding. Estimate of genetic distance between the two ectotypes were 0.060, 0.005 and 0.063 at yellow, light yellow and milky white loci, respectively. This shows that the ectotypes are closely related at the egg shell colour loci.
Key words: Genotype, phenotype, snail egg, shell colour, variations.
Snail farming in Nigeria has received numerous attentions in the past decades (Akinnusi, 2002). Snail meat is widely consumed all over the world by both the rich and the poor (Murphy, 2001; Ebenso, 2003). The flesh of the Giant African Land Snail is of remarkable nutritive value, with high iron content (Ogbeide, 1974), and a protein content of 37.00 to 51.30 g/100 g dry matter (Udedibie, 1989). It is possible that snail eggs might also be of high nutritive value, though it is not consumed by humans in Nigeria at the present. Okon et al. (2013) reported that snail eggs are good sources of protein and basic minerals (K+,Na+ ,Ca++, Fe++, Mg++ and Zn++) that compare favourably with the flesh and chicken eggs. With growing awareness of the role of cholesterol in various heart and arterial diseases, the demand for low cholesterol meat like snails and by extension snail eggs will become more acute. Characterising snail eggs will help consumers’ choice and/or preference, just as does chicken eggs colour.
Among the most common land snails in West Africa are Achatina achatina, Achatina fulica, Archachatina marginata and Limicolaria aurora (Ejidike, 2002; Smith and Fowler, 2003). According to Smith and Fowler (2003), among the most common snails, A. marginata and A. fulica are truly Giant African Land Snails.
A. marginata is the second largest snail and most popular breed of snail kept and reared in Nigeria (Venette and Larson, 2004; Okon et al., 2012b). A. marginata produces a peristome with a reflected lip and reaches maturity 2 to 4 months later than A. fulica (Raut and Barker, 2002). The columella and parietal callus of A. marginata are either white or red in colour (Venette and Larson, 2004).
The physiological adaptability to the environment and genetic variation among and within breeds has a marked effect on the performance and productivity of snails than other factors (Okon et al., 2012a). The diversity of gene pool, natural selection and free mating among individuals has given rise to different population of snails. In genetic analysis, knowledge of relatedness or variation is used to estimate the genetic parameters such as heritability and genetic correlations (Falconer and Mackay, 1996). In artificial selection, estimation of breeding values relies on the knowledge of relatedness of individuals (Lynch and Walsh, 1998).
Characterisation of breed is the first approach to sustainable use of animal genetic resources. Studies on diversity and variability between and within breeds of Giant African Land Snails (GALS) on the basis of quantitative and qualitative variables have become inevitable (Okon et al., 2011; Ugwu et al., 2011; Okon et al., 2012b; Ibom et al., 2012). Information on the egg shell colour of this animal species is not available in the literature. The present study is aimed at providing information on the variation of egg shell colour among eggs produced from the mating between black skinned × black skinned and white skinned × white skinned ectotypes of A. marginata var. saturalis snails.
The study was carried out at the Botanical Garden, University of Calabar, Calabar, Nigeria. Calabar is located within the geographical area of longitude 8°17’ and 10°43’ E of the Greenwich meridian, and latitude 4°58’ and 15°39’ N of the equator. The annual rainfall and temperature ranges between 1260 and 1280 mm and 25 to 30°C, respectively. The botanical garden is described in Okon et al. (2009a,b).
Eighty (80) adult snails comprising of 40 black skinned and 40 white skinned ectotypes of A. marginata snails were used for the study. The snails were purchased from a local market in Calabar. They were allowed to acclimatize for 35 days. This was to allow them shed the eggs they came with from the wild. They were allotted into two mating groups (black skinned × black skinned) and (white skinned × white skinned) on the basis of skin (foot) colour. There were two snails per cell and replicated 20 times to ensure that any egg emerging from the cell is a product of mating(s) between the two. The snails were housed in wooden cells measuring 40 × 40 cm by 30 cm within a hutch compartments.
The cells were filled with treated soil up to 15 cm depth from the bottom. Eggs were collected within 24 h of lay as the soil is turned and moistened. Moistening the soil helped maintain the humidity and moisture content.
The snails were fed a combination of concentrate and pawpaw leaves. The diet was formulated to contain 24% crude protein and 2580.36 Kcal/kg.
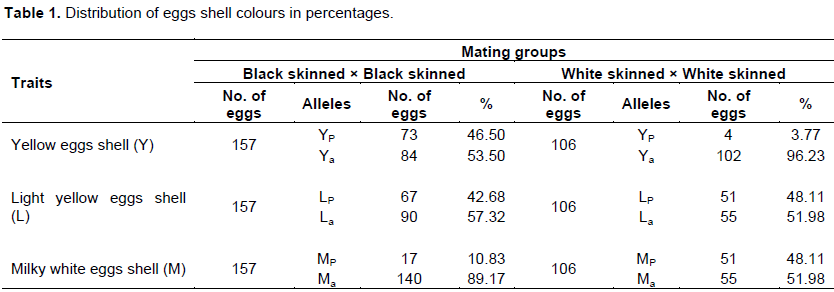
Eggs collected from the mating groups were classified according to their colours. The white skinned × white skinned mating group had a total of 106 eggs, comprising of 4 yellow shelled eggs, 51 light yellow shelled eggs and 51 milky white shelled eggs. The black skinned × black skinned mating group recorded a total of 157 eggs in the order of 73: 67:17 (yellow shelled eggs: light yellow shelled eggs: milky white shelled eggs), respectively.
Statistical analysis
The distribution of the various colours, yellow shelled eggs (Y), light yellow shelled eggs (L) and milky white shelled eggs (M) were expressed in percentages (Table 2). The frequencies of the recessive alleles (Ya for recessive yellow colour, La for recessive light yellow colour and Ma for recessive milky white colour) were estimated using Hardy-Weinberg equilibrium (Falconer and Mackay, 1996) as follows:
q =√N/M
That is q = √M – d/M, where N = M – d
The frequency of the dominant allele (Yp for dominant yellow colour, Lp for dominant light yellow colour and Mp for dominant milky white colour) were calculated as follows:
p = q – 1
Where q is the frequency of the recessive gene, N is the observed number of eggs exhibiting the particular recessive trait, d is the dominancy or dominant gene observed, M is the total number of eggs collected, and p is the frequency of a particular dominant allele.
The observed frequencies were then tested against the Mendelian ratio of 3:1 corresponding to the value of 0.75 for dominant allele and 0.25 for the recessive allele using Pearson’s Chi- square test. Pearson’s Chi-square test for goodness of fit is as follows:
X2 = ∑ (Observed – Expected)2 / Expected
The level of significance of the test was examined at P<0.05.
Genetic distance between the black skinned and black skinned ectotypes were estimated at the different egg colour locus using their respective gene frequencies. The method of Bodmer and Cavalli-Sforza (1976) was adopted as follows:
d2 = (p1– p2)2
Where d2 is the genetic distance estimate between the two populations, p1 and p2 are genetic frequencies of population one and population two, respectively.
Table 1 shows the distribution of the shell colour in percentages. The black skinned × black skinned mating group recorded 46.50% over white skinned × white skinned mating group with 3.77% for yellow egg shell colour. The same percentages were recorded by black skinned × black skinned mating group for both light yellow egg shell colour (48.11%) and milky white egg shell colour (48.11%). The black skinned × black skinned mating group recorded lower percentages in light yellow egg shell colour (42.68%) and milky white egg shell colour (10.83%). This apparent wide variation in egg shell colour is an indication that the population has not been purified through impeccable selective breeding (Yakubu et al., 2010).
The gene frequencies of the two ectotypes are presented in Table 2. The frequencies of the dominant alleles for both black skinned and white skinned ectotypes were 0.26 (YP,) vs. 0.02 (YP,), 0.24 (LP) vs. 0.31 (LP) and 0.06 (MP) vs. 0.31 (MP) (Table 2). These values were quite lower than the expected Mendelian value of 0.75. At the recessive allele, higher frequencies were observed for both black skinned and white skinned ectotypes; 0.74 (Ya) vs. 0.98 (Ya), 0.76 (La) vs. 0.69 (La) and 0.94 (Ma) vs. 0.69 (Ma), respectively.
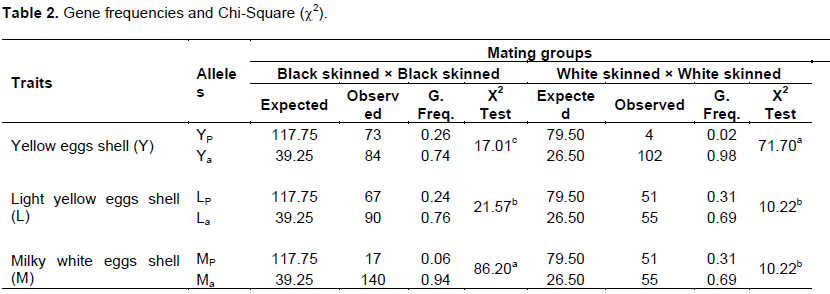
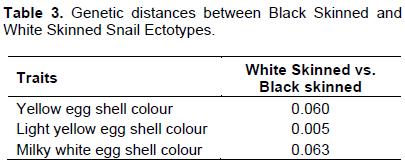
Table 3 shows the genetic distance between the black skinned and the white skinned ectotypes. The genetic distance between the black skinned and the white skinned ectotypes of A. marginata var. saturalis studied were 0.06, 0.005 and 0.063 at the yellow, light yellow and milky white egg shell colour loci, respectively. Genetic distance makes it possible to evaluate the degree of genetic similarity between two populations by measuring the probability of one or more characters appearing in one population but not in the other (Sournia, 1991). The smaller value (0.005) obtained at the light yellow locus is an indication of phylogenetic relationship between the two ectotypes, while the higher values at the yellow and milky white loci are indicative of genetic differentiation which could be used to classify the two ectotypes into distinct population (Yakubu et al., 2010).
This study has shown that the ectotypes of snail influenced the gene frequencies of yellow, light yellow and milky white egg shell colours. The dominant alleles in both ectotypes were found to segregate at lower frequencies. Estimate of genetic distance showed the relatedness of white skinned and black skinned ectotypes. Efforts should be geared towards constructing genetic study that will find the genes associated with these morphological differences.
The authors have not declared any conflict of interests.
REFERENCES
|
Akinnusi O (2002). Introduction to Snail Farming. Triolas Publishing Company, Abeokuta. P 70.
|
|
|
|
Bodmer WF, Cavalla-Sforza LL (1976). Genetic Evaluation and Man. Am. Anthropol. 79(2):485-486.
|
|
|
|
Ebenso IE (2003). A Dietary Calcium Supplement for Edible tropical land snail, Archachatina marginata in Niger Delta, Nigeria. Livest. Res. Rural Dev. 15(5):82-85.
|
|
|
|
Ejidike BN (2002). Snail Rearing Practices in southern Nigeria. Proc., 27th Annual Conference of Nigerian Society for Animal Production (NSAP) March 17 – 21, Federal University of Technology, Akure, Nigeria. pp. 307-308.
|
|
|
|
Falconer DS, Markay TFC (1996). Introduction to quantitative genetics. 4th Edn. Longman, London. P 464.
|
|
|
|
Ibom LA, Okon B, Bassey BE (2012). Egg traits, hatchability and Snailet Survivability of black skinned, white skinned and crossbred Archachatina marginata snails. Int. J. Agric. Sci. Bioresour. Eng. Res. 1(1):10-18.
|
|
|
|
Lynch M, Walsh JB (1998). Genetics and Analysis of Quantitative Traits. Sinaver Associates, Sunderland, M.A. 980.
|
|
|
|
Murphy B (2001). Breeding and growing snails commercially in Australia. A report for rural industries research and development cooperation publication No 00/188 project No ARH-IA 29.
|
|
|
|
Ogbeide O (1974). Nutritional hazards of food taboos and preferences in Mid-Western Nigeria. Am. J. Nutr. 27:213-216.
|
|
|
|
Okon B, Ibom LA, Ekpo IA, Ewa EC (2009a). Evaluation of reproductive performance and some egg quality parameters of Albino snails (Archachatina marginata var. saturalis). J. Appl. Sci. 12(1):77-80.
|
|
|
|
Okon B, Ibom LA, Ettah HE, Udoh UH (2012b). Comparative Differentiation of Morphometric Traits and Body Weight prediction of Giant African Land Snails with four whorls in Niger Delta Region of Nigeria. J. Agric. Sci. 4(10):205-211.
Crossref
|
|
|
|
Okon B, Ibom LA, Ettah HE, Ukpuho IE (2012a). Effects of Genotype, Dietary Protein and energy on the Reproductive and Growth Traits of Parents and F1 Hatchlings of Achatina achatina (L) Snails in Nigeria. Int. J. Appl. Sci. Technol. 2(1):179-185.
|
|
|
|
Okon B, Ibom LA, Ifut OJ, Bassey AE (2013). Intraspecies variation in nutritive potentials of eggs from two ectotypes of giant African land snail (Archachatina marginata var. saturalis) in Calabar, Nigeria. Afr. J. Agric. Res. 8(15):1310-1314.
Crossref
|
|
|
|
Okon B, Ibom LA, Odidio EE (2011). Reproductive performance and egg traits of crossbreeding between two strains of snails. Arch. Zootec. 60(229):153-156.
Crossref
|
|
|
|
Okon B, Ibom LA, Williams ME, Akpankpan IE (2009b). Comparative Evaluation of Reproductive Performance and some egg quality parameters of Black and White skinned snails. Glob. J. Agric. Sci. 8(1):77-80.
|
|
|
|
Raut SK, Barker GM (2002). Achatina fulica (Bowdich) and other Achatinidae as pests in Tropical Agriculture. In. G. M. Barker [ed], Molluscs as crop pests. Hamilton, New Zealand. CABI publishing. pp. 55-114.
|
|
|
|
Smith JW, Fowler G (2003). Pathway risk assessment for Achatinidae with emphasis on the Giant African Land Snails, Achatina fulica (Bowdich) and Limicolaria aurora (JAY) from the Caribbean and Brazil, with comments on related taxa Achatina achatina and Archachatina marginata (Swainson) intercepted by PPQ, USDA- APHIS, Center for Plant Health Science and Technology (internal concept), Raleigh, NC. pp. 1-7.
|
|
|
|
Sournia JC (1991). Dictionary of Genetics. The International Council of French Language. ET. Intercultural Foundation Postgraduate, Paris. P 351.
|
|
|
|
Udedibie ABI (1989). The Giant Land Snails and Prospects of Snails Farming in Nigeria. In. 25th Annual Conference of Agricultural Society of Nigeria, Owerri. pp. 33-36.
|
|
|
|
Ugwu SOC, Ogbu CC, Ikechiuno IK (2011). Reproductive Characterisation of three species of Giants African Land Snails (GALS) in Captivity. Afr. J. Biotechnol. 10(50):10315-10319.
https://doi.org/10.5897/AJB11.875
|
|
|
|
Venette RC, Larson M (2004). Mini risk Assessment Giant African Land Snail, Achatina fulica (BOWDICH) [Gastropoda: Achatinidae]. Department of Entomology, University of Minnesita, ST. PAUL MN 55108. pp. 1-30.
|
|
|
|
Yakubu A, Raji AO, Omeje JN (2010). Genetic and Phenotypic Differentiation of Qualitative Traits in Nigerian indigenous Goats and Sheep Populations. ARPN J. Agric. Biol. Sci. 5(2):58-65.
|