ABSTRACT
Launaea taraxacifolia is used by the traditional health practitioners in Sokoto to ease labour pains and augment labour thereby facilitating child birth. This study examined the effect of the aqueous extract of the whole plant on isolated uterus from non-pregnant rats pre-treated with stilbestrol and from pregnant rats in late gestation. This was compared to the effects of uterine contraction agonists, namely; oxytocin, histamine and acetylcholine. The possible mechanism of uterotonic activity was investigated using antagonists such as piroxicam, mepyramine and atropine. The aqueous extract of L. taraxacifolia produced a dose-dependent uterotonic activity. In the stilbestrol treated non-pregnant uterus, the force generated at 400, 800 and 1600 mg/ml was 1.469, 1.624 and 1.793 times greater than the control, respectively. In the pregnant rat uterus, a dose of 400, 800 and 1600 mg/ml generated a force of contraction that was 1.36, 1.51 and 1.66 times greater than the control, respectively. When the relative potency was compared to oxytocin, the gold standard uterotonin, L. taraxacifolia at 1600 mg/ml was found to be 0.08 times more potent than 0.4 µg/ml oxytocin in the stilbestrol treated non-pregnant rat uterus. In the pregnant rat uterine strip however, oxytocin was 0.17 times more potent than 1600 mg/ml L. taraxacifolia. Pre-treating the tissue with either atropine or mepyramine before administering the extract showed an inhibitory effect while piroxicam completely abolished its uterotonic effect, showing a probable moderate stimulation of muscarinic and histamine receptors but majorly the oxytocin receptors by L. taraxacifolia. A preliminary phytochemical screening of the extract shows the presence of saponins, tannins, flavonoids and steroids. A dose of 2000 mg/kg/oral of L. taraxacifolia was found to be well tolerated in rats with no sign of toxicity. Thus, L. taraxacifolia contains phytochemicals with uterotonic properties thereby justifying its ethnobotanical use in easing labour.
Key words: Launaea taraxacifolia, uterotonic, labour, isolated uterus.
Since time immemorial, plants have been used for their effects upon sex hormones particularly for suppressing fertility, regularizing menstrual cycle, relieving dysmenorrheal, treating enlarged prostrate, menopausal symptoms, breast pain and during and after childhood (Williamson et al., 1996). This is of importance especially in developing countries where modern medicine and health care is both inaccessible and unaffordable hence more than 80% of the populace continues to rely on traditional form of medicine (WHO, 2003). This form of medicine is also used in solving female reproductive health issues.
Uterotonic plants are plants that stimulate uterine contraction and are therefore used to assist labour, remove retained placenta, control postpartum bleeding and as an abortificient (Watcho et al., 2010). They are also of importance in facilitating uterine contraction following a miscarriage to reduce hemorrhage (Roqaiya et al., 2015). Various plants have been used to regulate a number of issues relating to pregnancy and delivery as well as post-partum complications. Examples of such plants are Monechma ciliatum (Uguru et al., 1998), Musanga cecropioides (Ayinde et al., 2006), Harpagophylum procumbens (Mahomed and Oyewole, 2006), Ficus asperifolia (Watcho et al., 2011), Ananas comosus (Monji et al., 2016), unripe fruits of Carica papaya (Praveena et al., 2017) and Steganotaenia Araliacea (Goma et al., 2017); all of which are used to ease the birthing process due to their uterotonic and oxytocic properties; plants such as Ricinus communis (Raji et al., 2006), Strychnos potatorum (Shah et al., 2009) and Macrotyloma axillare (Odhiambo et al., 2017) have been reported to have contraceptive property, while Lawsonia inermis (Mudi et al., 2011), Bamusa vulgaris (Yakubu et al., 2009) and Millettia aboensis (Onyegeme-Okerenta et al., 2016) possess abortificient properties. In a review of herbs with uterotonic property, Roqaiya et al., (2015) reported 16 plants species whose uterotonic property have been validated in both in vivo and in vitro models.
Launaea taraxacifolia common names wild lettuce or African lettuce and locally known as Noomen barewa in Hausa or Yanrin in Yoruba is one of the plants that are claimed by the locals to ease birthing process and alleviate labour pains and it is therefore used for that purpose. It grows as a weed along road sides and in bushes in many African countries. It is a perennial herb up to 150 cm tall, with creeping root system. Its stem is erect, often woody at the base (Adebisi, 2004). In Nigeria, the plant is found in the far north (Sokoto, Kebbi and Zamfara States) as well as among the Yoruba speaking states in the south. In Nigeria, the plant is fed to nursing cattle to increase milk production and also given to livestock to induce multiple births. In Benin, it is used as a febrifuge while in Ghana; the leaves are rubbed on the limbs of backward children to induce them to walk (Burkill, 1997). It is sometime burnt for its ash which is used as vegetable salt (Adebisi, 2004). It has also been reported as antimicrobial as it is established to have activity against Escherichia coli and Pseudomonas aeruginosa (Gbadamosi et al., 2012). L. taraxacifolia provides protection against cisplatin-induced hepato-renal damage through its antioxidant activities (Adejuwon et al., 2014). A review of ethnopharmacological and nutraceutical relevance of L. taraxacifolia documented effects such as antidiabetic, antihypertensive, anticancer, antimalarial, antibacterial and antiarthritis properties (Adinortey et al., 2018). Aboderin et al. (2017) however reported some degree of toxic effects on the liver and kidney of albino rats treated with aqueous of L. taraxacifolia and emphasized the need for caution in its use for medicinal purposes. The aim of this study was to investigate the uterotonic property of this plant and the possible mechanism of action in order to validate its ethnobotanical use. This study could provide a useful guide to the discovery of lead compounds with oxytocic properties which can be of great benefit in the management of aforementioned gynecological conditions.
Plant material
The whole plant of L. taraxacifolia was collected fresh from the wild in Talata Mafara area of Zamfara State. The plant was identified and authenticated at the herbarium of Department of Biological Sciences, Usmanu Danfodiyo University, Sokoto where an herbarium specimen with voucher number UDUH/ANS/0010 was deposited.
Experimental animals
Healthy female rats weighing between 130-150 g were obtained from the animal house of the Department of Pharmacology and Toxicology, Usmanu Danfodiyo University, Sokoto. The animals were kept under standard environmental conditions with access to feed and water ad libitum. The animals were divided into two categories.
Drugs and chemicals
The following drugs were purchased from Sigma Chemical (St Louis, MO, USA): oxytocin, histamine, mepyramine, acetylcholine hydrochloride, stilbestrol; while piroxicam and atropine were purchased from a local pharmacy. All other chemicals used were of analytical grades.
Preparation of extract
The whole plant was dried under shade to constant weight and ground manually using mortar and pestle. Forty gram (40 g) of the powdered plant material was extracted by maceration using distilled water. It was evaporated to dryness over water bath at 50°C and the percentage yield was calculated.
Oestrogen- dominated non-pregnant rats
All the animals in this category were pretreated with stilbestrol (0.1 mg/kg subcutaneous) for 24 h prior to use in order to induce oestrus phase. Vaginal smears were taken immediately before the animals were sacrificed in order to ascertain that the animals were in oestrus phase. Female rats in oestrus were used for the study.
Pregnant rats
Female animals were mated with male overnight. The morning after mating occurred, each female was examined for the presence of a vaginal plug or a vaginal swab was taken to detect any sperm under light microscopy. The presence of a vaginal plug or sperm positivity was designated as day 0 of gestation. Pregnant animals were housed two per cage with access to water and feed ad libitum.
They were kept till late pregnancy (Day 16 to 20) before use.
Preliminary phytochemical analyses
The quantitative phytochemical analysis was done using standard protocols. These include Van Buren and Robinson (1981) method for tannins; Obadoni and Chuko (2001) method for saponins; Borham and Kocipai-Abyzan (1994) method for flavonoids;, Molischi’s test for carbohydrate (Trease and Evans, 1999); and; Okeke and Elekwa, (2003) method for steroids.
Acute oral toxicity
The acute oral toxicity study was done using the “Up and Down method” in healthy adult female albino rats according to OECD guidelines No. 425 (OECD, 2008). A limit dose of 2000 mg/kg was used for the study. Five female rats were labeled for identification. An animal was picked at a time, weighed and dosed with equivalent volume of extract containing 2000 mg/kg body weight dissolved in distilled water as a vehicle after overnight fasting. Oral administration of drug was done using gastric feeding tube. Each animal was observed after dosing for the first 5 min for signs of regurgitation and then kept in a metallic cage. Each was then observed every 15 min in the first 4 h after dosing, every 30 min for 6 h and daily for 48 h for the short-term outcome according to the specifications of the OECD. The animals were monitored for a total of 14 days for the long term possible lethal outcome.
Experimental design
Each of the pregnant and stilbestrol- pretreated non-pregnant female rats were anaesthetized using chloroform. The uteri were promptly removed, cleaned of the connective tissues and cut into strips of about 1 cm in length. Each uterine strip was mounted in an organ bath of 25 25-ml capacity containing De Jalon solution of the following composition (mM) NaCl 153.85, KCl 5.64, CaCl2 0.55, MgSO4 0.08, NaOH 12.5 and glucose 2.78. The physiological salt solution was maintained at 37°C and continuously aerated with carbogen (that is, 5% carbon dioxide + 95% oxygen gas mixture). Each preparation was subjected to a resting tension of 1.0 g and allowed to equilibrate for 30 min before it was challenged with L. taraxacifolia / other drugs used.
Drug challenges
After an equilibration period of 30 min, normal myometrial contractions were recorded at baseline. Uterine contractile responses were elicited by adding oxytocin (0.4 µg/ml), acetylcholine (0.4 µg/ml), histamine (0.4 µg/ml) and aqueous extract of L. taraxacifolia (400, 800 and 1800 mg/ml) to the De Jalon’s solution. Each dose of the drug was allowed to act for 10 min and the amplitude of the contraction recorded by means of an isotonic transducer connected to a single channel recorder which was calibrated to record change in the tension generated on g versus displacement basis. Piroxicam (0.4 µg/ml), atropine (0.4 µg/ml) and mepyramine (0.4 µg/ml) were then used to antagonize the responses of the isolated uterus to oxytocin, acetylcholine or histamine and to the aqueous extract of L. taraxacifolia.
Statistical analysis
Results were expressed as mean ± standard error of mean (SEM).
Data was analysed using student t-test. P<0.05 was considered to be statistically significant (Figure 1).
Percentage yield
The percentage yield obtained for the aqueous extract of the whole plant of L. taraxacifolia was 17.38%.
Preliminary phytochemical analysis
The result of the preliminary qualitative phytochemistry showed the presence of carbohydrate, saponins, tannins, flavonoids and steroids.
Acute oral toxicity
At a limit dose of 2000 mg/ml, all the rats in the short and long term observation survived and no mortality or obvious signs of toxicity was recorded after the 14 day observation period. The LD50 is therefore more than 2000 mg/ml.
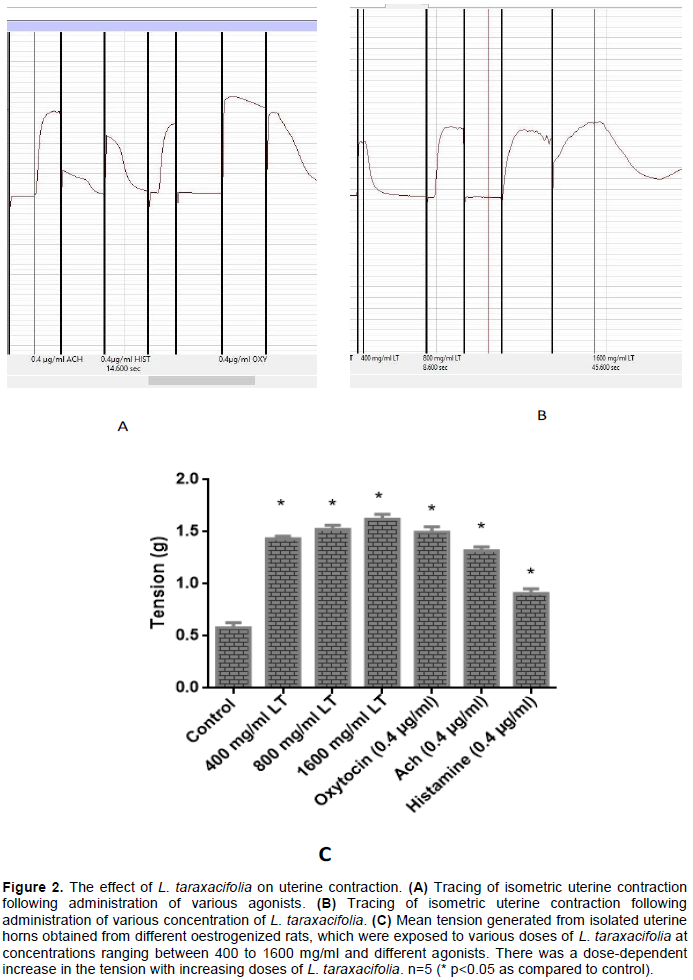
Dose dependent effect of L. taraxacifolia on uterine contraction on non-pregnant rats
Aqueous extract of L. taraxacifolia evoked a dose dependent contraction of the uterine smooth muscle. In the control, the force of contraction recorded was 0.58 g, which was the baseline contraction in oestrogenised rat’s uteri. At 400 mg/ml, the force generated was 1.469 times greater than the control. Meanwhile, the force increased by 1.624 and by 1.793 times, following administration of 800 and 1600 mg/ml L. taraxacifolia, respectively, with 1600 mg/ml L. taraxacifolia producing the maximum tension (Emax). The agonists used also elicited contractions of varying degree with oxytocin having the highest amplitude, with a force of contraction 1.577 times greater than the control while the force increased by 1.272 and 0.567 for acetylcholine and histamine, respectively. The dose of L. taraxacifolia that produced the Emax (1600 mg/ml) showed a force of contraction greater than that of 0.4 µg/ml oxytocin. This is shown in Figure 2.
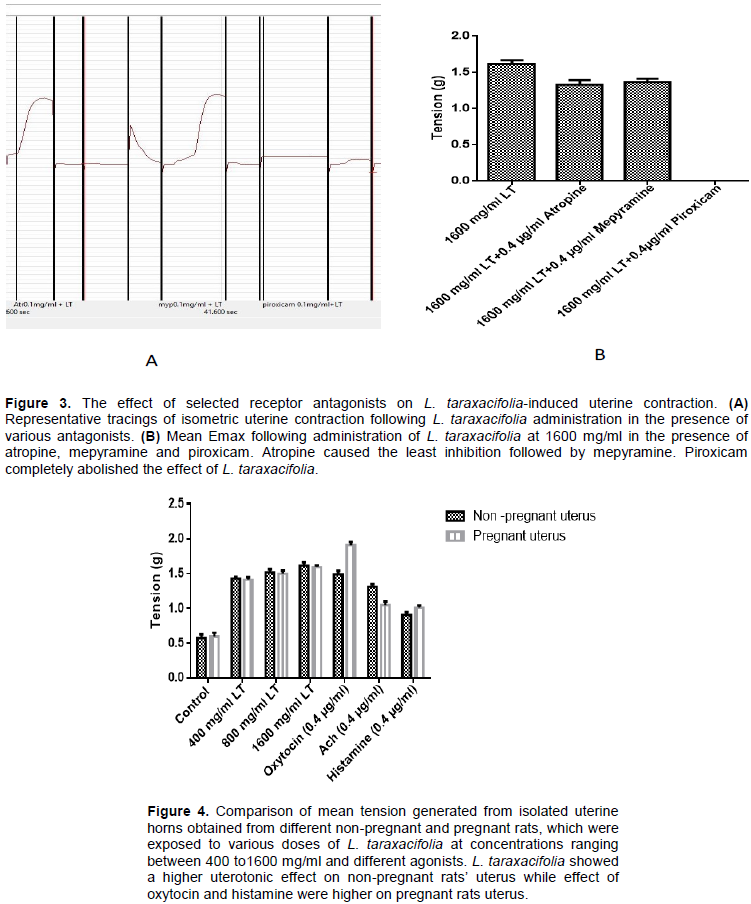
Effect of atropine, mepyraine and piroxicam on the Emax induced by 1600 mg/ml L. taraxacifolia in non- pregnant uterine strip
In Figure 3, administration of muscarinic receptor antagonist, atropine into the bathing solution containing isolated uterine tissue pre-exposed to 1600 mg/ml L.
taraxacifolia resulted in the Emax decreasing by 0.17 times. Meanwhile, administration of a histamine H1 antagonist mepyramine resulted in the Emax decreasing by 0.16 while a prostaglandin (PG) inhibitor, piroxicam an antagonist of oxytocin completely abolished its effect.
Dose dependent effect of L. taraxacifolia on uterine contraction on pregnant rats
The aqueous extract of L. taraxacifolia also evoked a dose dependent contraction of the uterine smooth muscle obtained from pregnant uterus in the third trimester (day 18 gestation). In the control, the force of contraction recorded was 0.60 g, which was the baseline contraction in pregnant rat’s uteri. At 400 mg/ml, force generated was 1.36 times greater than the control. Meanwhile, the force increased by 1.51 and by 1.66 times, following administration of 800 and 1600 mg/ml L. taraxacifolia, respectively. The agonists used also elicited contractions of varying degree with oxytocin having the highest amplitude, with a force of contraction 2.19 times greater than the control while the force increased by 0.755 and 0.69 for acetylcholine and histamine, respectively. The amplitude of contraction obtained with L. taraxacifolia and acetycholine in the pregnant rats uterine strips were lower than that obtained in the non- pregnant rats. However, for oxytocin and histamine the forces of contraction in the pregnant rat uterine strip were 0.28 times and 0.11 higher than that obtained in the non-pregnant rats, respectively. This is presented in Figure 4.
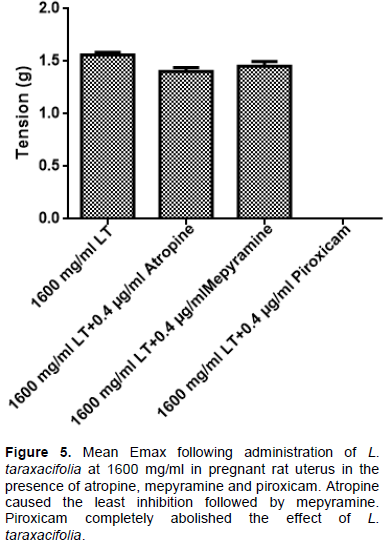
Effect of atropine, mepyramine and piroxicam on the Emax induced by 1600 mg/ml L. taraxacifolia in pregnant rat uterine strip
In Figure 5, administration of muscarinic receptor antagonist, atropine into the bathing solution containing isolated uterine tissue pre-exposed to 1600 mg/ml L. taraxacifolia resulted in the Emax decrease by 0.12 times. Meanwhile, administration of a histamine H1 antagonist mepyramine resulted in the Emax decreasing by 0.09 while a prostaglandin (PG) inhibitor, piroxicam completely abolished its effect as obtained in the non- pregnant rats uterine strips.
Relative potency of L. taraxacifolia as uterotonin
In Table 1, the relative potency of L. taraxacifolia was compared to other uterotonin. L. taraxacifolia at 1600 mg/ml was 0.08 times more potent than 0.4 µg/ml oxytocin in the non- pregnant rat uterine strips. The Emax produced following administration of 1600 mg/ml L. taraxacifolia on non-pregnant rats uterine strip was 1.620 ±0.048 g. Meanwhile, the Emax produced following administration of 0.4 µg/ml oxytocin, 0.4 µg/ml acetylcholine and 0.4 µg/ml histamine were 1.495±0.053, 1.318±0.032 and 0.909±0.046 g, respectively. In the pregnant rat uterine strip, however, oxytocin was 0.17 times more potent than 1600 mg/ml L. taraxacifolia.
This study has shown the uterotonic effect of aqueous extract of L. taraxacifolia and the possible mechanism of action. To the best of our knowledge, this study is the first to display this effect, which justifies the claim that this plant eases birthing process and alleviate labour pain. L. taraxacifolia at 1600 mg/kg was 0.08 times more potent than oxytocin, which is a gold standard uterotonin (Sheldon et al., 2012) in non-pregnant rats. This was however not the case with the pregnant rat uterus. The reason for this is not fully understood but may be due to the presence of different component in the crude extract used, some of which may even have antagonistic effect. Chan et al. (1988) reported that in gravid uterus, just prior to term labour, the parturient uterus usually becomes highly active and responsive to oxytocin. The agonist (oxytocin), been a pure compound will therefore likely result in greater uterine contraction.
The results obtained showed that L. taraxacifolia effect is mediated via muscarinic, oxytocin and histamine receptors. The presence of these receptors in the uterus has been previously established (Hay et al., 2010; Abdalla et al., 2004; Kobayashi et al., 1999). These mechanism were confirmed from inhibition of Emax produced by 1600 mg/ml L. taraxacifolia following administration of the antagonists to these receptors. Our findings suggest that LH-induced uterine contraction was mediated mainly via the oxytocin receptor. This is because piroxicam, an antagonist of oxytocin completely abolished the effect of the aqueous extract. Moderate inhibition of the Emax by atropine and mepyramine suggest that LH binding to muscarinic and histamine receptor produced a moderate degree of contraction. There is a possibility that the greatest effect of L. taraxacifolia produced following it binding to the oxytocin receptors (as evidenced by the complete inhibition of the Emax by piroxicam) was due to a high number of this receptor expression in the uterus. Previous studies have shown that of all the receptors reported to be present in the uterus, the oxytocin receptor expression in the uterus is the highest (Sanborn, 2001; Grigsby et al., 2006). This is however, not the case with F. asperifolia which elicited uterotonic activity via the histamine receptor (Watcho et al., 2011), M. cecropiodes, M. ciliatum and Agapanthus africanus which elicited their uterotonic activity significantly via the muscarinic and oxytocin receptors (Uguru et al., 1998; Ayinde et al., 2006; Veale et al., 1999), and Ananas comosus which elicited its uterotonic activity through serotonergic pathway (Monji et al., 2016; Monji et al., 2018).
Pharmacological activities observed in plant extracts are due to the presence of various secondary metabolites they contain (Ayinde et al., 2006). The observed uterine contractility effect in this extract is invariably due to these secondary metabolites. The phytochemical analysis of L. taraxacifolia revealed the presence of saponins and steroids both of which have been shown to possess uterine stimulating effect (Watcho et al., 2011; Guo et al., 2008). Similar investigation of different plants with uterotonic properties including F. asperifolia (Watcho et al., 2011; M. ciliatum (Uguru et al., 1998), M. cecropiodes (Ayinde et al., 2006) and Nymphaea alba (Bose et al., 2014) all revealed the presence of saponins, tannins, flavonoids and steroids.Tannis, flavoniods and saponins have been shown to be present in uterotonic plants such as Ficus deltoidea (Amiera et al., 2014) and Calotropis procera (Shamaki et al., 2015). Tannins are thought to elicit their uterotonic effect through affecting calcium availability for uterine tissue and cardiac muscle contraction (Polya et al., 1995; Calixto et al., 1986) while flavonoids, on the other hand, act directly on oestrogen receptors to cause uterine contraction (Revuelta et al., 1997).
The oral acute toxicity studies show that it is safe and well tolerated in rats as no sign of toxicity was observed in all the treated animals. A study which assessed the safety of the ethanol extract of L. taraxacifolia in rodents revealed no negative effect on physical, hematological and serum biochemical parameters when doses ranging from 10-5000 mg/kg were administered (Kuatsienu et al., 2012). The findings in this study suggest that the use of L. taraxacifolia should be contra-indicated in pregnancy. It however authenticates the folkloric obstetric use of the plant for the induction or acceleration of labour as well as expelling retained placenta among pregnant women in Sokoto, North-west Nigeria.
The authors have not declared any conflict of interests.
The authors wish to appreciate the technical support of Mr Osaro Ibisuyi and Mallam Abdulahi Sulaiman of the Pharmacology Laboratory, UDUS for their technical support.
REFERENCES
|
Abdalla FM, Maróstica E, Picarelli ZP, Abreu LC, Avellar MC, Porto CS (2004). Effect of estrogen on muscarinic acetylcholine receptor expression in rat myometrium. Molecular and Cellular Endocrinology 213(2):139-148.
Crossref
|
|
|
|
Adebisi AA (2004). Launaea taraxacifolia (wild). Amin ex Jeffrey. In: Grubben GJH and Denton OA (editors). PROTA 2: Vegetables/legumes. PROTA Wageningen, Netherlands
View
|
|
|
|
|
Adejuwon AS, Femi-Akinlosotu O, Omirinde JO, Owolabi OR, Afodun AM (2014). Launaea taraxacifolia ameliorates cisplatin-induced hepato-renal injury. European Journal of Medicinal Plants 4(5):528-541.
Crossref
|
|
|
|
|
Nutraceutical Relevance of Launaea taraxacifolia (Willd.) Amin ex C. Jeffrey. Evidence-Based Complementary and Alternative Medicine Article ID 7259146
View
Crossref
|
|
|
|
|
Amiera ZUR, Nihayah M, Wahida IF Rajab NF (2014). Phytochemical characteristic and uterotonic effect of aqueous extract of Ficus
Crossref
|
|
|
|
|
deltoidea. Pakistan Journal of Biological Sciences 17(9):1046-1051.
|
|
|
|
|
Ayinde BA, Onwukaeme DN, Nworgu ZAM (2006). Oxytocic effects of the water extract of Musanga cecropioides R. Brown (Moraceae) stem bark. African Journal of Biotechnology 5(14):1350-1354.
|
|
|
|
|
Bose AN, Moumita SR, Sudhanshu SC (2014). Uterotonic properties of Nymphaea alba on isolated myometrium model. International Journal of Pharmacy and Pharmaceutical Science 6(6):490-493.
|
|
|
|
|
Calixto BJ, Nicolau N, Rae GA (1986). Pharmacological actions of tannic acid. I. Effects on isolated smooth and cardiac muscles and on blood pressure. Planta Medica 52(1):32-35.
Crossref
|
|
|
|
|
Chan WY, Berezin I, Daniel EE (1988). Effects of inhibition of prostaglandin synthesis on uterine oxytocin receptor concentration and myometrial gap junction density in parturient rats. Biology of Reproduction 39(5):1117-1128.
Crossref
|
|
|
|
|
Gbadamosi AE, Alia IT, Okolosi O (2012). In-vitro antimicrobial activities and nutritional assessment of roots of ten Nigerian vegetables. New York Science Journal 5(12):234-240.
|
|
|
|
|
Goma FM, Ezeala C, Nyirenda J, Chuba D, Prashar L, Simfukwe N, Lengwe C (2017). Extraction and demonstration of uterotonic activity from the root of Steganotaenia araliacea Hochst. Medical Journal of Zambia 44(3):125-132.
|
|
|
|
|
Grigsby PL, Sooranna SR, Adu-Amankwa B, Pitzer B, Brockman DE, Johnson MR, Myatt L (2006). Regional expression of prostaglandin E2 and F2 alpha receptors in human myometrium, amnion, and choriodecidua with advancing gestation and labor. Biology of Reproduction 75(2):297-305.
Crossref
|
|
|
|
|
Guo L, Su J, Deng BW, Yu ZY, Kang LP, Zhao ZH, Shan YJ, Chen JP, Ma BP, Cong YW (2008). Active pharmaceutical ingredients and mechanisms underlying phasic myometrial contractions stimulated with the saponin extract from Paris polyphylla Sm. var. yunnanensis used for abnormal uterine bleeding. Human Reproduction 23(4): 964-971.
Crossref
|
|
|
|
|
Hay A, Wood S, Olson D, Slater DM (2010). Labour is associated with decreased expression of the PGF2α receptor (PTGFR) and a novel PTGFR splice variant in human myometrium but not decidua. Molecular Human Reproduction 16(10):752-760.
Crossref
|
|
|
|
|
Kobayashi M, Akahane M, Minami K, Moro M, Ajisawa Y, Inoue Y, Kawarabayashi T (1999). Role of oxytocin in the initiation of term and preterm labor in rats: Changes in oxytocin receptor density and plasma oxytocin concentration and the effect of an oxytocin antagonist, L-366,509. American Journal of Obstetrics and Gynecology 180(3):621-627.
Crossref
|
|
|
|
|
Kuatsienu LE, Ansah C, Woode E (2012). Safety Assessment of the Ethanolic Leaf Extract of Launaea taraxacifolia (Willd) of the Family Asteraceae in Rodents. Master's Thesis, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana.
View
|
|
|
|
|
Mahomed IM, Ojewole JA (2006). Oxytocin-like effect of Harpagophytum procumbens DC [Pedaliaceae] secondary root aqueous extract on rat isolated uterus. African Journal of Traditional, Complementary and Alternative Medicines 3(1):82-89.
Crossref
|
|
|
|
|
Monji F, Adaikan PG, Lau LC, Said BB, Gong Y, Tan HM, Choolani M (2016). Investigation of uterotonic properties of Ananas comosus extracts. Journal of Ethnopharmacology 193:21-29.
Crossref
|
|
|
|
|
Monji F, Adaikan PG, Lau LC, Siddiquee AAM, Said BB, Yang LK, Yoganathan K, Choolani MA (2018). Role of the serotonergic pathway in uterotonic activity of Ananas comosus (L.) Merr.-an in vitro and in vivo study. Phytomedicine 48:32-42.
Crossref
|
|
|
|
|
Mudi SY, Ibrahim H, Bala MS (2011). Acute toxicity studies of aqueous root extract of Lawsonia inermis Linn in rats. Journal of Medicinal Plants Research 5(20):5123-5126.
|
|
|
|
|
Obadoni BO, Ochuko PO (2001). Phytochemical studies and comparative efficacy of the crude extracts of some homostatic plants in Edo and Delta States of Nigeria. Global Journal of Pure and Applied Sciences 8(2):203-208.
Crossref
|
|
|
|
|
Odhiambo RS, Kareru PG, Mwangi EK, Onyango DW (2017)
|
|
|
|
|
Contraceptive and Phytochemical Profile of Lime-Yellow Pea (Macrotyloma axillare, E. Mey) Verdc: A Tropical Climber. European Journal of Medicinal 19(2):1-13
Crossref
|
|
|
|
|
OECD (2008). OECD guideline for testing of chemicals. Acute oral toxicity studies. Up and down procedure. View
|
|
|
|
|
Okeke CU, Elekwa I (2003). Phytochemical study of the extract of Gongronema latifolium Benth. Journal of Health and Visual Science 5(1):47-55.
|
|
|
|
|
Onyegeme-Okerenta BM, Anacletus FC, Offor K (2016). Aborticient potential effect of aqueous extract of Millettia aboensis on reproductive health of matured wistar rats. IOSR Journal of Pharmacy and Biological Sciences 11(6):13-19.
|
|
|
|
|
Polya GM, Wang BH, Foo YL (1995). Inhibition of signal-regulated protein kinases by plant-derived hydrolysable tannins. Phytochemistry 38(2):307-314.
Crossref
|
|
|
|
|
Praveena P, Jethinlalkhosh JP, Doss VA (2017). Evaluation of uterotonic activity of hydroethanolic extract of unripe fruit of Carica papaya Linn using Wistar albino rats. Indian Journal of Pharmaceutical Education and Research. 51(4S):S615-S22
Crossref
|
|
|
|
|
Raji Y, Oloyo AK, Morakinyo AO (2006). Effect of methanol extract of Ricinus communis seed on reproduction of male rats. Asian Journal of Andrology 8(1):115-121.
Crossref
|
|
|
|
|
Revuelta MP, Cantabrana B, Hidalgo A (1997). Depolarization-dependent effect of flavonoids in rat uterine smooth muscle contraction elicited by CaCl2. General Pharmacology 29(5):847-857.
Crossref
|
|
|
|
|
Roqaiya M, Begum N, Majeedi SF, Saiyeed A (2015). A review on herbs with uterotonic property. The Journal of Phytopharmacology 4(3):190-196.
|
|
|
|
|
Sanborn BM (2001). Hormones and calcium: Mechanisms controlling uterine smooth muscle contractile activity. Experimental Physiology 86(2):223-237.
Crossref
|
|
|
|
|
Shah GM, Khan MA, Ahmad M, Zafar M, Khan AA (2009). Observations on antifertility and abortifacient herbal drugs. African Journal of Biotechnology 8(9):1959-1964.
|
|
|
|
|
Shamaki BU, William A, Abdullah A, Sadiq AA (2015). Phytochemical constituents and uterotonic effects of aqueous extract of Calotropis procera leaves on excised uterine segments of albino rats. European Journal of Pharmaceutical and Medical Research 2(2):20-29.
|
|
|
|
|
Sheldon WR, Blum J, Durocher J, Winikoff B (2012). Misoprosol for the prevention and treatment of postpartum hemorrhage. Expert Opinion on Investigational Drugs 21(2):235-250.
Crossref
|
|
|
|
|
Trease and Evans (1999). Pharmacognosy, edited by Evan W.L. 14th ed.: W.B. Saunder Company Ltd. London pp. 109-334.
|
|
|
|
|
Uguru MO, Okwusaba FK, Ekwenchi EE, Uguru VE (1998). Uterotonic properties of Monechma ciliatum. Journal of Ethnopharmacology 62(3):203-208.
Crossref
|
|
|
|
|
Van Buren JP, Robinson WB (1969). Formation of complexes between protein and tannin acid. Journal of Agricultural and Food Chemistry 17(4):772-777.
Crossref
|
|
|
|
|
Veale DJ, Havlik I, Oliver DW, Dekker TG (1999). Pharmacological effects of Agapanthus africanus on the isolated rat uterus. Journal of Ethnopharmacology 66(3):257-262.
Crossref
|
|
|
|
|
Watcho P, Ngadjui E, Efouet PAN, Ngelafack TB, Kamanyi A. (2010). Evaluation of in-vitro uterotonic activities of fruits extracts of Ficus asperifolia in rats. African Health Science 9(1):49-53.
|
|
|
|
|
Watcho P, Ngadjui E, Alango Nkeng-Efouet P, Benoît Nguelefack T, Kamanyi A (2011). Evaluation of in vitro uterotonic activities of fruit extracts of Ficus asperifolia in rats. Evidence-Based Complementary and Alternative Medicine 783413.
Crossref
|
|
|
|
|
World Health Organization (WHO) (2003). WHO calls on African governments to formally recognize traditional medicine. 31 August, 2003. Johannesburg, South Africa.
View
|
|
|
|
|
Williamson EM, Okpako DT, Evans FJ (1996). Pharmacological methods in phytotherapy research, volume I: selection preparation and pharmacological evaluation of plant material. John Wiley and Sons Ltd., London pp. 191-212.
|
|
|
|
|
Yakubu MT, Bukoye BB (2009). Abortifacient potentials of the aqueous extract of Bambusa vulgaris leaves in pregnant Dutch rabbits. Contraception 80(3):308-313.
Crossref
|
|