ABSTRACT
In vitro storage of prolific potato cultivars increases labour, space and material costs due to frequent sub-culturing. A method that reduces frequent sub-culturing can lower maintenance costs of potato storage. A study was undertaken to assess in vitro growth of potato under either (1) complete dark exposure, (2) dark exposure for three days only and then to light or (3) light exposure throughout. Also, micro shoots were placed either (1) horizontally, (2) upright or (3) inverted on culture media. There were significant differences between treatments with respect to number of shoots (P=0.0099) and roots (P=0.0279), but not with respect to shoot length when placed horizontally. Micro shoots placed upright and under light condition were significantly (P=0.0092) longer than those in the dark. Similarly, those under light exposure had significantly (P=0.0168) higher number of roots than those under dark condition. The number of leaves under light condition was significantly (P=0.0318) higher than those under dark condition. For inverted plantlets, significant differences between treatments with respect to shoot length (P=0.0114), number of roots (P=0.0045) and number of leaves (P=0.0004) were obtained. There were significant differences (P=0.003) with respect to explant position. Placing micro shoots under dark condition and at an upright position severely reduced explant growth. Exposing micro shoots to dark condition for three days and then to light also reduced explant growth. This study concludes that exposing potato micro shoots to dark and then to light condition in an upright position can reduce sub-culturing frequency and enables explants to recover from chlorosis. This procedure is necessary for in vitro potato storage as it reduces maintenance costs due to low sub-culturing frequency.
Key words: Chlorosis, conservation, explants, in vitro, regeneration, Thandizo.
Potato (Solanun tuberosum L.) is an important food crop and a source of income. It is being produced in more than 125 countries in the world and consumed almost daily by more than a billion people (FAO, 2008). In Malawi, potential potato yield is 15 to 20 tons per hectare (Government of Malawi, 2003), but farmers achieve only 10 tons or lower than this per hectare of fresh weight. This low yield is attributed to poor quality seed and accumulation of degenerative viral diseases during clonal propagation. Recycling of potato seed has resulted in losses in seed vigor and increase in disease (viral, fungal and bacterial) infestation. Traditional potato storage practices such as underground pits and use of a dark room result in seed weight loss, excessive sprouting, and pest and disease attack (Gachango et al., 2008). In vitro potato storage is necessary as field conservation makes potato vulnerable to losses.
In vitro potato plantlets are commonly used for seed potato production as they restore plant vigor, enable mass multiplication and are free from pests and diseases (Kumar et al., 2007; Sharma and Pandey, 2013; Lommen, 2015). High in vitro potato propagation rates (ten times per month) on Murashige and Skoog basal media (Murashige and Skoog, 1962) without plant growth regulators (hormones) were obtained with plantlets spontaneously producing roots (Hussey and Stancey, 1981). Such high shoot multiplication rates and root development increase in vitro storage costs due to frequent sub-culturing to maintain plants. This increases labour and material costs and also takes a lot of space. Reducing frequent sub-culturing of plants can minimize in vitro storage costs.
There are several factors influencing in vitro plant growth. These include light quality and intensity, culture media, temperature, humidity, hormones, ventilation, explant orientation among other factors (Gachango et al., 2008; Mohamed and Alsadon, 2010; Saez et al., 2012; Rani et al., 2013). Light promoted in vitro Castanea sativa shoot regeneration (Saez et al., 2012) and tomato (Lycopersicon esculentum) shoot regeneration depended on orientation on the culture media (Rani et al., 2013). Light (both quality and quantity) is required for photo-synthesis and photo-morphogenesis hence influences plant growth and development (George et al., 2008; Bello-Bello et al., 2016). Cybularz-Urban et al. (2007) reported that Blue light increased micro-propagation efficiency of Cattleya hybrid plant. Explant orientation also affects in vitro plant growth. According to Chow et al. (1993), inversion of Lillium leaf explants improved both shoot and root regeneration.
In vitro potato regeneration rate depends on cultivar (Saker et al., 2012; Kumar et al., 2007). Cultivar Desiree has a high regeneration frequency on several culture media (Yee et al., 2001) and in vitro potato plantlets (4 cm tall) were produced after three weeks (Lommen, 2015). Shahriyar et al. (2015) reported higher potato (cultivar: cardinal) shoot regeneration on Murashige and Skoog (MS) basal media without plant growth regulators than on media supplemented with 6-benzyl aminopurine and gibberellic acid. The rapid regeneration capacity increases sub-culturing frequency during in vitro storage.
A lot of labour (for culture media preparation, culture inoculation and cleaning of used culture tubes), materials (culture media, test tubes or glass jars, disinfectants and others) and laboratory space are needed to manage frequent sub-culturing. The costs can be high, especially for a longer period of in vitro storage. Reducing the costs can make clean potato seed available and affordable and increased crop productivity. A higher rate of photo-synthetic potential is obtained from in vitro plantlet leaves than those from ex vitro (Cassana et al., 2010) and this translates into a high crop yield.
In this study, plant growth regulators were excluded from the culture media as they could induce high shoot and root regeneration rates. The objective of this study was to assess in vitro potato regeneration capacity under different explant orientation positions and light or dark exposure conditions.
Experimental site and plant material
An experiment was carried out in tissue culture laboratory at the Lilongwe University of Agriculture and Natural Resources, Bunda Campus in June 2015. A newly released potato variety, Thandizo was used as a stock plant. According to Demo et al. (2012), this variety was introduced during 2006/2007 season from International Potato Center (CIP) to improve productivity, food security and income to farmers. Potato micro-cuttings (micro shoots) were obtained from plantlets already cultured on MS basal media for a month. Micro shoots were excised with at least two nodes and then sub-cultured.
Culture media
Hormone free MS basal media (10 ml) were dispensed into 25 × 125 mm test-tubes and 60 ml into glass jars. All culture MS media contained 20 g/l of sucrose and 7 g/l of agar to solidify the culture media. All culture tubes were placed in an incubation chamber. For those under light condition (fluorescent bulbs), they were at an intensity of 40 µmol m-2s-1 PAR for 16 h and with temperature of 18°C. Observations were done for 25 days.
Horizontal position
Potato micro shoots were horizontally placed on MS basal media. Treatments included five culture tubes of micro shoots placed either in (1) dark condition (covered with aluminum foil to exclude light) throughout culturing period of 25 days, (2) dark condition for three days and then exposing them to light until the end of culturing period or (3) light condition throughout the culturing period. This experiment was laid out in a completely randomized design (CRD) with three replications.
Upright position
A second set of experiment involved placing five micro shoots in test tubes in an upright position and then exposing them either to (1) dark condition throughout the culturing period of 25 days, (2) dark condition for three days and then placing them under light condition until the end of culturing period or (3) light condition throughout the culturing period. This experiment was laid out in a CRD with three replications.
Inverted position
In the third experiment, all micro-cuttings were inverted (upside down position). Treatments included placing five culture tubes of micro shoots either in (1) dark condition throughout culturing period of 25 days, (2) dark condition for three days and then exposing them to light until the end of the culturing period or (3) light condition throughout the culturing period. This experiment was laid out in a CRD with three replications.
Micro shoots on one culture media
This experiment involved placing micro shoots in one large glass jar containing hormone free MS basal media. Each glass jar contained six (6) micro shoots placed either (1) on an upright position, (2) horizontal position or (3) inverted position. The glass jars were then placed either under (1) dark condition throughout the culturing period of 25 days, (2) dark condition for three days and then exposing them to light condition until the end of culturing period or (3) light condition throughout the culturing period. This experiment was a two-way CRD arranged in a factorial fashion (3 × 3) with three levels of micro shoot orientation positions and three levels of dark/light exposure. It was replicated three times.
Data collection and analysis
Data collected included number of shoots, roots and leaves regenerated. Also, shoot length and culture contamination was recorded for a period of 25 days. Observations were also made in the presence of plant necrosis and growth. Data were subjected to analysis of variance using PROC MIXED of the SAS procedure. Means were separated by Fischer’s Least Significant Difference (LSD) at 5% level of significance.
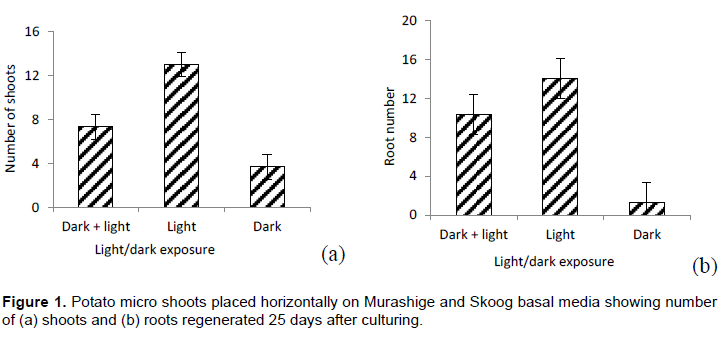
Horizontal position
There were significant variations between treatments with respect to number of shoots (P=0.0099) (Figure 1a) and roots (P=0.0279) regenerated (Figure 1b), but there were no significant differences between treatments with respect to shoot length. Exposure of micro shoots to light condition throughout the culturing period significantly increased the number of shoots and roots regenerated.
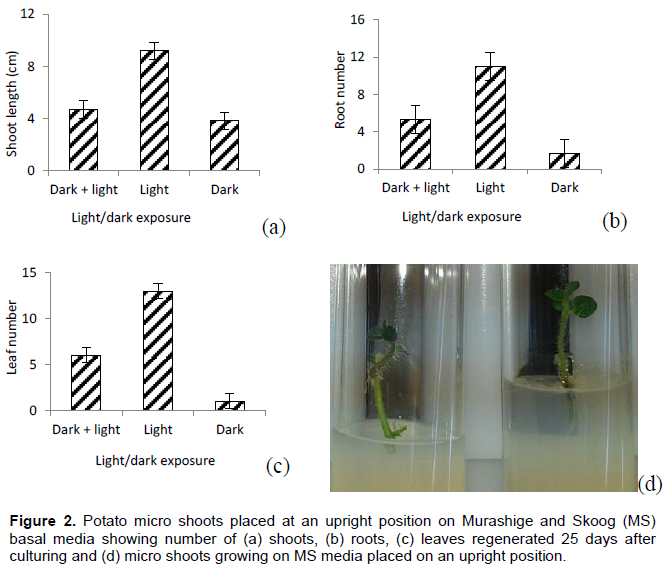
Upright position
Micro shoots placed under light condition throughout the experiment regenerated significantly (P=0.0092) longer shoots than those under dark exposure condition (Figure 2a). Similarly, micro-cuttings under light condition throughout the experiment had significantly (P=0.0168) more roots than those under dark exposure condition (Figure 2b). The number of leaves was also significantly (P=0.0318) higher than those under dark condition (Figure 2c). No significant variations were obtained with respect to number of shoots regenerated.
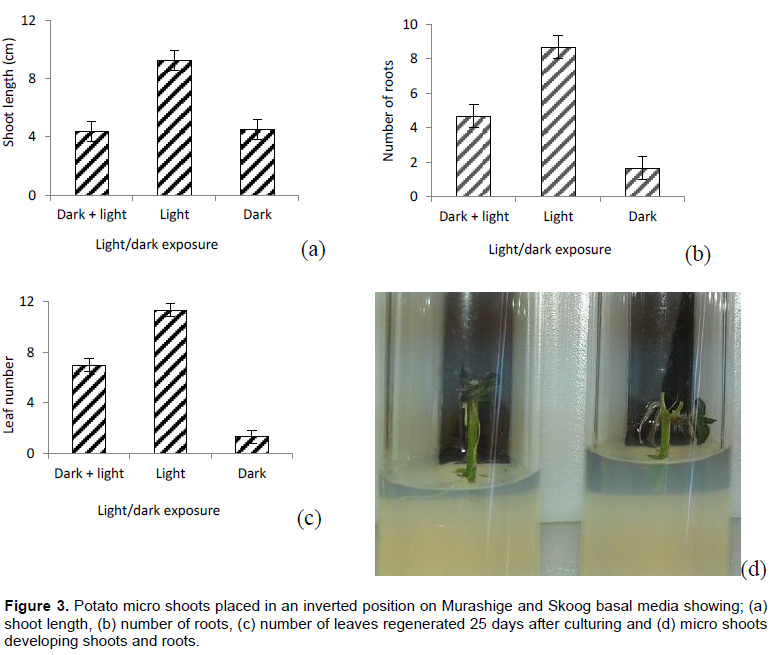
Inverted position
There were significant variations between treatments with respect to shoot length (P=0.0114) (Figure 3a), number of roots (P=0.0045) (Figure 3b) and number of leaves (P=0.0004) (Figure 3c). Exposing micro shoots to light condition throughout the culturing period significantly increased shoot length, number of roots and leaves.
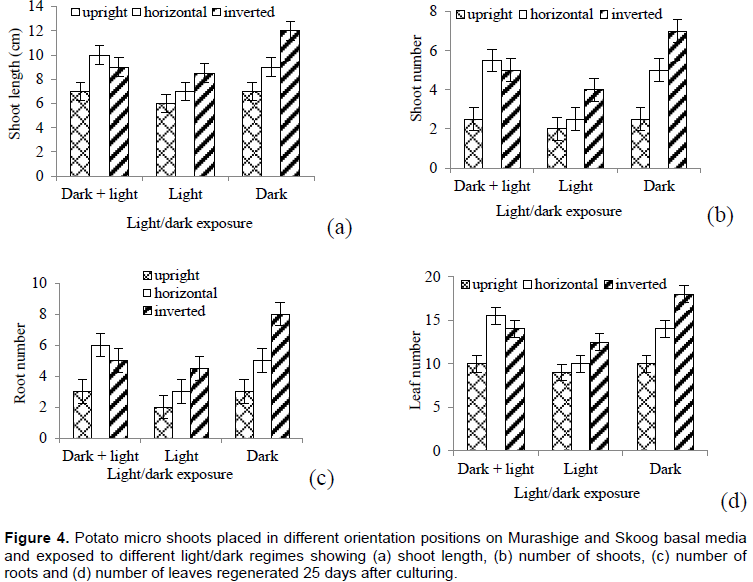
Micro shoots on one culture media
There were significant differences between explant orientations (P=0.0021), light/dark exposure condition (P=0.0192), but not their interactions with respect to shoot length (Figure 4a). Significant variations were obtained between explant orientations (P=0.004) and light/dark condition exposure (P=0.0056), but not their interactions with respect to shoot number (Figure 4b). There were also significant variations between explant orientations (P=0.0021) and light/dark condition exposure (P=0.0192), but not their interactions with respect to number of roots (Figure 4c) regenerated. Similarly, significant differences were obtained between explant orientations (P=0.003) and light/dark condition exposure (P=0.0043), but not their interactions with respect to number of leaves (Figure 4d).
Horizontal position
In this study, exposing micro shoots on a horizontal position to light condition increased number of shoots and roots. Dark conditions had an inhibitory effect on regeneration of shoots and roots. Weak and etiolated shoots were regenerated under dark condition. According to Seabrook (2005), light promotes potato morphogenesis and growth as it promotes photosynthesis and accumulation of photo-assimilates. According to George et al. (2008), light promotes plant growth and development as it is required for photosynthesis and photo-morphogenesis processes. In this current study, placing micro-cuttings on a horizontal position had no effect on explant shoot length despite an increase in shoot number, especially under light condition.
Upright position
Exposing micro shoots to light condition increased the number of roots and leaves, and shoot length. Exposing explants to dark conditions inhibited growth with respect to the number of roots and leaves and also shoot length. However, there was no effect on the number of shoots regenerated. More roots were produced when explants were on a horizontal position than when on an upright position (Figures 1 and 2). This could be due to the fact that a large portion of micro shoots/explants on a horizontal position was in contact with culture media and this increased the area from which shoots and roots were regenerated. Consequently, each node or internode produced shoots and/or adventitious roots from several parts of the explants. Micro shoots were etiolated under dark condition (three days exposure) and then regained their vigour when exposed to light.
Inverted position
Exposing micro shoots in an inverted (upside down) position to light condition increased number of roots and leaves and also shoot length. According to Chow et al. (1993), inversion of Lillium leaf explants improved both shoot and root regeneration. In the current study, the opposite was true when micro shoots were exposed to a dark condition. Exposing micro shoots to dark condition for three days before exposing them to light condition for the rest of the culturing period in an inverted position showed a remarkable reduction in shoot length just like exposing them to dark condition throughout the experiment. The leaves were tiny, weak and chlorotic when exposed to dark conditions throughout the experiment. This indicates that these leaves were less functional due to chlorosis. Micro shoots exposed to the dark and then light conditions were able to recover (be functional) with time as they gained chlorophyll when exposed to light condition. Also, new leaves were regenerated under light conditions. The loss of growth vigor under dark condition contributed to reduced shoot length.
Micro shoots on one culture media
Micro shoots on an upright position had the lowest growth with respect to shoot length, number of shoots, roots and leaves regenerated under light and/or dark conditions. Exposure to light reduced micro shoot growth as plantlets had a few shoots, roots and leaves. Plantle tgrowth in this experiment (micro shoots on one media) could be affected by several factors as there were three different explant orientation positions in a single glass jar. Plant exudates (phytochemicals) in the media could have affected growth of other plants. Explants exude phytochemicals such as amino acids, organic acids, sugars, phenolics, polysaccharides, proteins and others (Cai et al., 2012). These can affect growth of other plants in the same culture media.
In this study, micro shoots in an upright position could have released phytochemicals that promoted growth of micro shoots on either horizontal or inverted position. Also, phytochemicals from those on an inverted position could have negatively affected growth of micro shoots on an upright position. Exposure to light condition could increase phytochemical production, especially phenolics that negatively affect explant growth. Hussein et al. (2010) reported increased production of phenolics under light condition unlike under dark condition from Brassica nigra L. culli although there are exceptions. However, potato phytochemical production, especially phenolics warrants further investigation.
In vitro plantlet growth
In all the experiments in this study, plantlet leaves produced under dark conditions were tiny and chlorotic. Although frequent sub-culturing (short passages) can reduce culture necrosis (Mng’omba et al., 2007), this increases in vitro storage costs. An ideal condition is to reduce in vitro growth in order to reduce frequent sub-culturing.
Improved growth with respect to number of shoots, roots and leaves was obtained when micro shoots were exposed to dark and then light conditions compared to those under dark condition throughout the culturing period. This could be attributed to light exposure duration as light promotes growth of plant cultures (Shin et al., 2008). Exposing micro shoots to dark condition (three days) reduced plantlet growth, but growth improved once exposed to light condition. This study indicates that the longer the culture period under light condition (light exposure throughout), the higher the frequency of shoot, root and leaf formation. This indicates that it is important to expose potato micro shoots to dark condition before to light condition in order to slow down shoot, root and leaf proliferation. Exposing micro shoots to dark condition throughout the culture duration has shown a drastic reduction in plantlets growth in this study. It is possible that some plantlets may not recover (gain functional leaves) due to leaf chlorosis and weak plantlets hence this procedure is not suitable for in vitro potato (Thandizo). However, further studies are warranted to ascertain suitable duration of dark exposure for potato micro shoots before exposing them to light condition.
This study indicates that dark condition reduced in vitro multiplication of cultivar Thandizo potato micro shoots when grown in separate test tubes. Furthermore, growth of micro shoots was severely reduced under dark condition throughout the culturing period. Although this reduces sub-culturing frequency, it may lead to a low or no plant recovery due to prolonged leaf chlorosis and weak plantlets. Exposing micro shoots to dark condition for three days and then to light slightly reduced plant growth hence reduced sub-culturing frequency. This short exposure to dark condition enables plants recover from chlorosis and expand leaves once exposed to light condition. This procedure has a potential to reduce frequent sub-culturing for in vitro potato storage. However, micro shoots exposure duration to dark condition before exposing them to light condition requires further investigations to optimize the procedure.
The authors have not declared any conflict of interests.
REFERENCES
|
Bello-Bello JJ, Martínez-Estrada E, Caamal-Velázquez JH, Morales-Ramos V (2016). Effect of LED light quality on in vitro shoot proliferation and growth of vanilla (Vanilla planifolia Andrews). Afr. J. Biotechnol. 15(8):272-277.
Crossref
|
|
|
|
Cai Z, Kastell A, Knorr D, Smetanska I (2012). Exudation: an expanding technique for continuous production and release of secondary metabolites from plant cell suspension and hairy root cultures. Plant Cell Rep. 31(3):461-477.
Crossref
|
|
|
|
|
Cassana FF, Falqueto AR, Braga EJB, Peters JA, Bacarin MA (2010). Chlorophyll a fluorescence of sweet potato plants cultivated in vitro and during ex vitro acclimatization. Braz. J. Plant Physiol. 22(3):167-170.
Crossref
|
|
|
|
|
Chow YN, Selby C, Fraser TW, Harvey BMR (1993). Basal plate tissue in narcissus bulbs and in shoot clump cultures: Its structure and role in organogenic potential of single leaf cultures. Ann. Bot. 71(5):437-443.
Crossref
|
|
|
|
|
Cybularz-Urban T, Hanus-Fajerska E, Åšwiderski A (2007). Effect of light wavelength on in vitro organogenesis of a Cattleya hybrid. Acta Biol. Crac. Ser. Bot. 49(1):113-118.
|
|
|
|
|
Demo P, Mwenye OJ, Pankomera P, Chimwala L, Benesi I, Chipungu F, Torrence L, Kumar N (2012). New improved high yielding potato varieties selected and released in Malawi.
View. Accessed on 22 June 2017.
|
|
|
|
|
FAO (2008). The International Year of Potato: the Global Crop Diversity Trust and FAO's plant production and protection division. Rome, Italy.
|
|
|
|
|
Gachango E, Shibairo SI, Kabira JN, Chemining'wa GN, Demo P (2008). Effects of light intensity on quality of potato seed tubers. Afr. J. Agric. Res. 3(10):732-739.
|
|
|
|
|
George EF, Hall MA, De Klerk G (2008). Plant Propagation by Tissue Culture. 3rd edition. Springer, Dordrecht, The Netherlands.
|
|
|
|
|
Government of Malawi (2003). Guide to Agricultural Production, Lilongwe, Malawi.
|
|
|
|
|
Hussein EA, Taj-Eldeen AM, Al-Zubairi AS, Elhakimi AS, Al-Dubaie AR (2010). Phytochemical screening, total phenolics and antioxidant and antibacterial activities of callus from Brassica nigra L. hypocotyl explants. Int. J. Pharmacol. 6:464-471.
Crossref
|
|
|
|
|
Hussey G, Stacey NJ (1981). In vitro propagation of potato (Solanum tuberosum L). Ann. Bot. 48(6):787-796.
Crossref
|
|
|
|
|
Kumar D, Singh V, Singh RP, Singh BP, Naik PS (2007). Performance of in vitro plantlets for production of minitubers in vector free environment. Potato J. 34(1-2):131-132.
Crossref
|
|
|
|
|
Lommen WJM (2015). How age of transplants from in vitro derived potato plantlets affects crop growth and seed tuber yield after field transplanting. Potato Res. 58(4):343-360.
Crossref
|
|
|
|
|
Mng'omba SA, du Toit ES, Akinnifesi FK, Venter HM (2007). Repeated exposure of jacket plum (Pappea capensis) micro-cuttings to indole-3-butyric acid (IBA) improved in vitro rooting capacity. S. Afr. J. Bot. 73:230-235.
Crossref
|
|
|
|
|
Mohamed MAH, Alsadon AA (2010). Influence of ventilation and sucrose on growth and leaf anatomy of micropropagated potato plantlets. Sci. Hortic. 123:295-300.
Crossref
|
|
|
|
|
Murashige T, Skoog F (1962). A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 15:473-97.
Crossref
|
|
|
|
|
Rani T, Yadav RC, Yadav NR, Kumar M (2013). Effect of explant orientation on shoot regeneration in tomato (Lycopersicon esculentum). Indian J. Agric. Sci. 83(5):514-517.
|
|
|
|
|
Saez PL, Bravo LA, Latsague MI, Sanchez ME, Rios DG (2012). Increased light intensity during in vitro culture improves water loss control and photosynthetic performance of Castanea sativa grown in ventilated vessels. Sci. Hortic. 138:7-16.
Crossref
|
|
|
|
|
Saker MM, Moussa TAA, Heikal NZ, Aboellil AH, Abdel-Rahman RMH (2012). Selection of an efficient in vitro micropropagation and regeneration system for potato (Solanum tuberosum L.) cultivar Desirée. Afr. J. Biotechnol. 11(98):16388-16404.
|
|
|
|
|
Seabrook JEA (2005). Light effects on the growth and morphogenesis of potato (Solanum tuberosum) in vitro: A review. Am. J. Potato Res. 82(5):353-367.
Crossref
|
|
|
|
|
Shahriyar S, Akram S, Khan K, Miya Md. F, Sarkar AR (2015). In vitro plant regeneration of potato (Solanum tuberosum L.) at the rate of different hormonal concentration. Asian J. Med. Biol. Res. 1(2):297-303.
Crossref
|
|
|
|
|
Sharma AK, Pandey KK (2013). Potato mini-tuber production through direct transplanting of in vitro plantlets in green or screen houses. Potato J. 40(2):95-103.
|
|
|
|
|
Shin KS, Murthy HN, Heo JW, Hahn EJ, Paek KY (2008). The effect of light quality on the growth and developmentof in vitro cultured Doritaenopsis plants. Acta Physiol. Plant. 30:339-343.
Crossref
|
|
|
|
|
Yee S, Stevens B, Coleman S, Seabrook JEA, Li X (2001). High- efficiency regeneration in vitro from potato petioles with intact leaflets. Am. J. Potato Res. 78:151-157.
Crossref
|
|