Full Length Research Paper
ABSTRACT
The current study assessed the anticoccidial efficacy of chitosan nanoencapsulated bromelain (CNB) against coccidiosis in goats. Bromelain was extracted from the pineapple peels using standard methods while oral solution of CNB was prepared using standard manufacturing methods. The in vivo study was done on fifteen healthy male goats naturally infected with coccidia. The goats were divided into three groups consisting of three treatment groups (90 and 270 mg/Kg CNB, Diclazuril, 5 mg/Kg). The drugs were administered orally for 3 days. Fecal Oocyst Counts (FOC) determined using the modified McMaster technique. The goats were observed for clinical signs on daily basis while body weight was recorded weekly. The level of packed cell volume (PCV), aspartate aminotransferases (AST), alanine aminotransferases (ALT), urea, and creatinine were assessed weekly. At the end of the study, goats were euthanized and gross pathology and histopathology conducted. The results showed that at day 28 post-treatment there was a significantly reduction of FOC of 98.42 and 82.30% for Diclazuril and 270 mg/Kg treatment groups, respectively. The reduction of FOC percentage was significantly higher (p ≤ 0.01) in Diclazuril group than that in 270 mg/Kg. During the monitoring period, there was no mortality or clinical signs observed in the goats. The PCV, AST, ALT, creatinine and urea were in normal ranges for goats. There were no pathological lesions on the goat organs. In conclusion, CNB had high anticoccidial efficacy and was safe for use in goats. Strategies to improve the efficacy of this potential drug should be further investigated.
Key words: Anticoccidial efficacy, bromelain, chitosan, goat, nanoencapsulation.
INTRODUCTION
Coccidiosis is an economically menacing disease affecting livestock and poultry (Mat Yusof and Md Isa, 2016). In small ruminants, coccidiosis is mainly caused by Eimeria species, which develop in both small and the large intestines and mainly affects young animals (Dakpogan et al., 2019). The disease is responsible for considerable losses in husbandry, due to reduced productivity, mortality, associated clinical and sub-clinical diseases and the cost of treatment and control measures (Etsay et al., 2020; Bawm and Htun, 2021). In Africa, coccidiosis is considered as a leading cause of mortality of young small ruminants and is compounded by the occurrence of other infectious and parasitic diseases such as helminthosis and pneumonia (Kagucia et al., 2020; Etsay et al., 2020). In Africa, prevalence of coccidiosis in goats was reported at 60% in Egypt (Mohamaden et al., 2018), 73% in Nigeria (Ikpeze et al., 2010), 85% in Ethiopia (Etsay et al., 2020) and 45% in Kenya (Maichomo et al., 2004).
Despite the aggressive use of anticoccidial drugs, coccidiosis continues to limit the productivity of livestock, especially that of small ruminants (Waller, 2006). This is due unfortunately to the rapid development of anticoccidials resistance (Hema et al., 2015), the higher cost of the latter and consumer concerns of drug residues in animal products. All these factors have led to a growing interest in alternative products to control coccidiosis. Phytochemicals from different types of botanical elements have been explored as sustainable alternatives to management of coccidiosis (Hady and Zaki, 2012). A wide variety of herbal extracts have been shown to have anti-parasitic activity, while others can enhance the immune system and growth performance, thereby helping the host to overcome coccidiosis infection (Zaman et al., 2015; Debbou-Iouknane et al., 2019). Among the plant extracts, bromelain obtained from pineapples (Ananas comosus) has been shown in a previous In vitro study, to have high activity against Eimeria spp. isolated from goats (Daiba et al., 2022). In furtherance of the latter study, we assessed the in vivo efficacy of chitosan nano-encapsulated bromelain (CNB) against coccidiosis in goats and investigated the possible toxic effects of the extract.
MATERIALS AND METHODS
Ethical approval
The approval for goats’ experiments was obtained from the Animal Ethics Committee of University of Nairobi (REF: FVM BAUEC/2020/339). The study followed the design, animal husbandry practices and protocols approved by the committee.
Experimental site
The study was carried out from January to March 2022, at the animal facility located in Jomo Kenyatta University of Agriculture and Technology (JKUAT), Juja, in Kiambu County, Kenya. The University is located at latitude 1°05 S and longitude 37°00 E, and it lies at an altitude of 1525 m above sea level with rainfall bimodal and ranges from 500 to 1,300 mm while average temperature is 19.5°C (Menge et al, 2014).
Extraction and encapsulation of bromelain in chitosan
Bromelain was extracted from the peels of pineapples (Ananas comosus) sold at local market in Juja Sub-county, Kenya. The enzyme was extracted using the procedure described previously (Daiba et al., 2022). Briefly, fresh ripe pineapples were ground in a blender in sodium acetate buffer (pH 7.4). The resultant crude extract was precipitated by adding 40% ammonium sulphate. Then, after 24 h of incubation at +4°C, extracted bromelain was purified using dialysis membrane (12 kDa). Encapsulation of bromelain in chitosan (Sigma Aldrich, USA) was done by ionic gelation method. The pellet obtained after encapsulation was frozen at -60°C and dried by placing in the freeze-dryer (MRC, Model FDL-10N-50-BA, Israel). The success of encapsulation of bromelain in chitosan nanoparticles was confirmed by Fourier transform infrared spectrophotometer analysis (FTIR).
Oral solution of chitosan nanoencapsulated bromelain (CNB) formulation
Oral solution of EB was made according the procedure described previously (Niazi, 2009a; Niazi, 2009b). The solvent contained in the excipients included: Tween 20 (surfactant), sucrose (to enhance solubility and stabilize against denaturation of enzymes), Potassium sorbate (antimicrobial preservative), xanthan gum (suspending and viscosity agent) and propylene glycol (humectant and stabilizing agent) (Rowe et al., 2009). Casein enzymatic assays were performed to evaluate the proteolytic activity and specific activity at diverse storage conditions (temperature, exposition to the light and pH) (Barnes, 2014).
Experimental animals
Fifteen (15) Small East African goats, which were naturally infected with coccidia were purchased from farmers in Makima Ward in Embu counties in Kenya. The average age of the goats was 15 months old and weighed between 22 and 31 Kg. Acclimatization was done for two weeks and animals were tagged with ear tags for easy identification before the start of the experiment. The goats were kept in JKUAT’ goat house, where they were fed with 1.5 kg of wheat hay thrice per day, 1 Kg of concentrate made up of beet liquid molasses, maize germ, and soybean meal (Aroma Feed Suppliers, Kenya), and supplemented with feed blocks minerals (Aroma Feed Suppliers, Kenya).
Study groups and sampling of animals
The fecal samples were collected using sterile gloves from the rectum of goats and analyzed to determine the number of coccidia oocysts per gram (OPG) of feces using the McMaster method (MAFF, 1986). All the animals used in the experiments had an OPG of more than 10,000. The goats were randomly allocated into three (3) treatment groups. Groups 1 and 2 received 90 and 270 mg/Kg of CNB, respectively, while group 3 acted as the positive control where goats were administered Diclazuril at 5 mg/Kg (Diclazuril®, Sigma-Aldrich). The above dosages were chosen based on th results of the bromelain toxicity and efficacy tests obtained in the previous studies (Wasso et al., 2020; Daiba et al., 2022). The treatment was done orally every morning for 3 days and the goats were monitored for 28 days after last day of drug administration.
In vivo assessment of anticoccidial efficacy
Fecal samples were collected from the rectum of goats, once per week, during the 4 weeks of monitoring. The fecal samples were analyzed using a modified McMaster technique to determine the coccidia oocyst counts (FOC) under light microscopy (Optical Element Corporation, Melville, USA) at 100× magnification (Joachim et al 2018). First, the burden of oocyst was evaluated before the treatment (pre-treatment samples at day 0), followed by evaluation of treatment efficacy, by assessing post-treatment fecal samples (days 7, 14, 21 and 28). Fecal Oocyst Count Reduction (FOCR) percentage was evaluated as previously described (Odden et al., 2018).
Assessment of acute toxicity effect
Following dosing, the animals were observed during the first 30 min and then periodically during the first 24 h. Special attention was given during the first 4 h, and daily thereafter, for a total of 28 days to observe any changes in general behavior and other physiological activities (OECD Guidelines, 2002; Parasuraman, 2011). Rectal temperature of goats was measured daily, each morning (8 h 30-9 h 30 am) using a digital thermometer (Kruuse Digital Thermometer; Jorgen Kruuse). The body weight of animals was recorded weekly. Two (2 mL) of blood was sampled weekly from each animal, from the jugular vein into blood collection tube 4 mL EDTA. The PCV was determined using the micro-hematocrit method (Shamaki et al., 2017). Afterward, blood samples were centrifuged for 14,000 rpm for 10 min to obtain the plasma. The plasma samples obtained were used to determine the levels of Aspartate aminotransferases (AST), alanine aminotransferases (ALT), urea, and creatinine using standard diagnostic test kits on Automated Clinical Biochemistry analyzer (Reflotron Plus System®, model: Cobas 4800 Detection Analyzer; India) (Emma et al., 2020; Wasso et al., 2020).
At 28th day, post last day of drug administration, the goats were euthanized and gross pathology conducted according the procedure described previously (King et al., 2013). Sections of liver, kidney, spleen and heart were collected and preserved in 10% buffered formalin for 24 h before being processed for histology as described previously (Rousselle et al., 2019).
Statistical analysis
The collected data were entered into and analyzed using Graph Pad Prism 8.4.3. for data analysis. Descriptive statistics (means and standard deviations) were determined before conducting other statistical tests. The FOCR, PCV, weight, temperature and biochemical parameters of different groups were compared using Students t-test, with p < 0.05 indicating statistical significance.
RESULTS
Bromelain oral solution pH and activity
The oral solution of CNB was preserved for 2 weeks at various temperatures. The protease activities of oral solution of nano-encapsulated bromelain were 0.1057±0.010 and 0.0839±0.009 U/mL, respectively for drug preserved at +4°C and room temperature (25°C). The activity of CNB stored at +4°C was significant higher (p < 0.002) compared to that kept under room temperature. The pH of the oral solution of CNB ranged from 4.68±0.36 to 4.61±0.4 and, there were no significant differences (p > 0.05) between pH of CNB oral solution kept at +4°C and that kept at room temperature (25°C).
In vivo assessment of efficacy
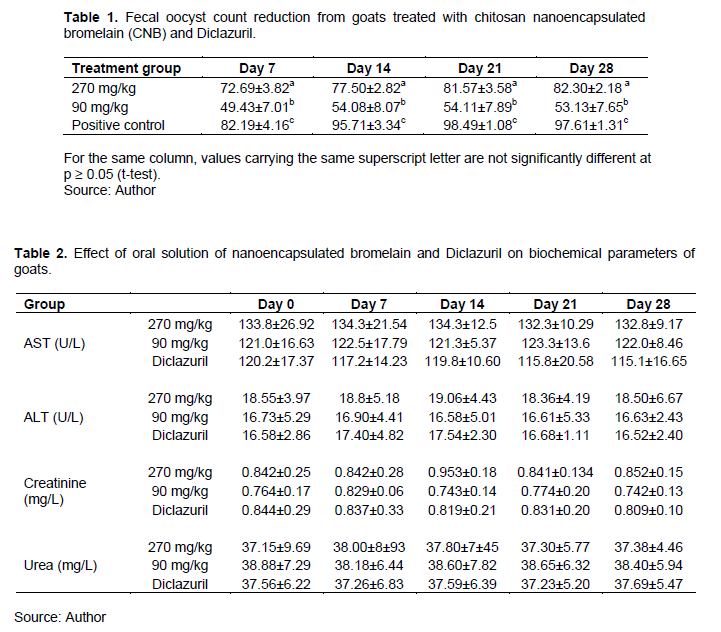
At day 0, the mean OPG in Groups 1, 2 and 3 goats were 11,600, 12,000 and 12,075, respectively. Following treatment, the FOCR% at day 7 post treatments were 82.12, 72.69 and 49.93% for goats treated with Diclazuril, 270 mg/Kg, 90 mg/Kg CNB, respectively. At the day 28 post treatment, FOCR% were 98.42, 82.30 and 53.16% for Diclazuril, 270 mg/Kg, 90 mg/Kg CNB treatment groups, respectively. The reduction of oocyst was significantly higher (p ≤ 0.018) for the goats treated with 270 mg/Kg than those treated with 90 mg/Kg. Further, there was a significant difference (p < 0.01) in FOCR% of the positive control group compared to that of 270 and 90 mg/Kg treatment groups (Table 1).
Toxicity assessment of oral solution of nanoencapsulated bromelain
Clinical observations
During the 28 days monitoring period, there were no mortalities or clinical signs reported in all treatment groups. The rectal temperature of animal body varied between 38.38 and 39.00°C and was thus within normal range for small East African goat. Following the treatment, there were no significant differences in body temperatures (p > 0.05) in goats from different groups. The body weights of the goats before treatment were 26.58±2.57, 27.00±1.41 and 26.75±3.40 Kg for Diclazuril, 270 mg/Kg and 90 mg/kg CNB groups, respectively. After three weeks since treatment, the weight had increased to 26.89±1.91, 28.00±1.63 and 28.00±3.56 Kg for Diclazuril, 270 mg/Kg and 90 mg/Kg CNB groups, respectively. The mean of body weights following treatments showed an increase ranging between 1 and 1.25 Kg at 28 days post treatment and the increase (compared to day 0) was statistically significant (p < 0.05).
Effect of treatments on PCV and biochemical parameters
The PCV levels, before treatment, were 31.23, 29.75 and 30.75% for Diclazuril, 270 mg/ Kg and 90 mg/ Kg CNB groups, respectively. During the treatment, the PCV levels ranged between 28 to 31% for 270 mg/Kg group and 27 to 32% for 90 mg/ Kg CNB group and for positive control, 28.79 to 30.24%. There were no significant differences (P > 0.05) between the treatment groups.
The mean of biochemical parameters of AST, ALT, creatinine and Urea for each of the dosed groups were in the normal ranges (Jackson and Cockcroft, 2002) (Table 2). During the treatment, AST and ALT ranged between 121 to 134 U/L and 16.58 to 19.06 U/L, respectively for CNB treatment groups and 115 to 120 U/L and 16.52 to 17.54 U/L for positive control group of animals. The creatinine level was 0.742 to 0.953 mg/L and urea was 37.15 to 38.88 mg/L for CNB treatment groups. There were no significant differences (p > 0.05) in levels of AST, ALT, creatinine and urea in different days and between treatment groups.
Necropsy findings
There were no gross changes at necropsy. All the organs were normal and similar in both treatment groups to control groups. There were no histological lesions observed in the organs in both the treated and control groups (Figure 1).

DISCUSSION
The current study is a follow-up of the previous study which had assessed the in vitro activity of nano-encapsulated bromelain (CNB) on oocysts of Eimeria spp. isolated from goats in Kenya (Daiba et al., 2022). The previous study reported high anticoccidial activity of CNB comparable to that of commercial drug (Diclazuril). In the current study, CNB was been administered as an oral solution made by adding other excipients in order to increase its activity in the animal’s body (Rowe et al., 2009). The current study showed that, the proteolytic activity of oral solution of CNB was higher than crude encapsulated bromelain reported in previous studies (Hunduza et al., 2020; Wasso et al., 2020; Daiba et al., 2022). This high activity of the oral solution of CNB could be due to the lower pH (pH = 4.7 to 5) which would contribute to accelerate the release of bromelain from chitosan nanocarriers during casein protease assay. As described by Hunduza (2018), acidic conditions chitosan nanocarriers released more bromelain into solution than at neutral pH, and thus had a higher proteolytic activity. Further, the oral solution prepared using Tween 20, sucrose, potassium sorbate, xanthan gum and propylene glycol could have enhanced the stability of CNB in the gut of the goat making it more effective against the coccidial parasites. Previous studies have used water as the solvent (Wasso et al., 2020).
The results of the present study showed that the administration of oral solution of CNB for three days, significantly reduced the excretion of coccidia oocysts during the monitoring period. Our findings are in concordance with experiments using cysteine proteinase from others plants, which show that the enzyme having an efficacy against coccidian infections (Abdel-Tawab et al., 2020; Dakpogan et al., 2019; Juasook et al., 2017; Molan et al., 2009). Juasook et al. (2017) treated Eimeria tenella infected broilers with pineapple (Ananas comosus) crude extracts, and observed that the oocyst output decreased significantly and also inhibited sporulation. In their experiment, Dakpogan et al. (2019) reported a reduction of 59% of oocyst per gram (OPG) in chicks treated with Carica papaya crude extracts. On the other hand, Abdel-Tawab et al. (2020) showed that Astragalus membranaceus extracts reduced oocyst output and sporulation of Eimeria papillata infection in mice by 57%. As reported in a recent study in vitro efficacy (Daiba et al., 2022), the activity of CNB was due to the damage it causes to coccidia oocysts. The drug causes degradation of the coccidia shell wall, softening and destruction of the central cytoplasmic mass (Daiba et al., 2022, Juasook et al., 2017).
The present study also evaluated the toxicity effect of CNB on goats. As reported in a recent study evaluating the acute toxicity (Wasso et al., 2020), during the present study, there were no mortalities and clinical signs in the experimental goats. On the other hand, a slight weight gain of the treated animals and this could show that the drug enhanced the growth performance of the goats.
During the current study, the levels of the assessed biochemical parameters were normal and were similar to the results reported by Wasso et al. (2020) where the goats were treated with EB at a dose of 30 mg/kg for 14 days, and in a single dose of 90 and 270 mg/kg. Likewise, this was confirmed by the absence of macroscopic and microscopic changes in the examined organs (Moss et al., 1963; Pavan et al., 2012). Moss et al. (1963) in rats have shown that bromelain when administered at 500 and 1500 mg/Kg/day was not toxic. Further, Pavan et al. (2012) did not observe toxic effects after 6 months administrating bromelain at 750 mg/Kg/day to dogs. All these results show that the drug is quite safe for use in different animals.
CONCLUSION
The current study reported high efficacy of CNB against coccidial infections in goats. Further the oral solution of CNB had no toxic effects at 270 mg/Kg on goats. Accordingly, this formulation of bromelain can contribute to management of coccidiosis in small ruminants. Further studies should be carried out to determine dosing regimens which can increase the efficacy of CNB against coccidiosis to 100%.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
REFERENCES
|
Abdel-Tawab H, Abdel-Baki AS, El-Mallah AM, Al-Quraishy S, Abdel-Haleem HM (2020). In vivo and in vitro anticoccidial efficacy of Astragalus membranaceus against Eimeria papillata infection. Journal of King Saud University-Science 32(3):2269-2275. |
|
|
Barnes A (2014). A Standard Protocol for Deriving and Assessment of Stability Part 3-Oral Liquid Medicines (Solutions, Emulsions, Suspensions and Syrups). August, 16. |
|
|
Bawm S, Htun LL (2021). Management and Control of Eimeria Infection in Goats. In (Ed.), Goat Science - Environment, Health and Economy. Intech Open. |
|
|
Daiba AR, Kagira JM, Ngotho M, Kimotho J, Maina N (2022). In vitro anticoccidial activity of nanoencapsulated bromelain against Eimeria spp. oocysts isolated from goats in Kenya. Veterinary World 15:397-402. |
|
|
Dakpogan HB, Mensah S, Attindehou S, Chysostome C, Aboh A, Naciri M, Salifou S, Mensah GA (2019) Anticoccidial activity of Carica papaya and Vernonia amygdalina extract. International Journal of Biological and Chemical Sciences 12(5):2101-2108. |
|
|
Debbou-Iouknane N, Neri?n C, Amrane M, Ghemghar M, Madani K, Ayad A (2019). In Vitro Anticoccidial Activity of Olive Pulp (Olea europaea L. var. Chemlal) Extract Against Eimeria Oocysts in Broiler Chickens. Acta Parasitologica 64(4):887-897. |
|
|
Emma N, Judith S, Peter M, Naomi M (2020). Sub-acute and chronic toxicity of silver nanoparticles synthesized by Azadirachta indica extract. African Journal of Biotechnology 19(6):320-331. |
|
|
Etsay K, Megbey S, Yohannes H (2020). Prevalence of sheep and goat coccidiosis in different districts of Tigray region, Ethiopia. Nigerian Journal of Animal Science 22(3):61-69. |
|
|
Hady MM, Zaki MM (2012). Efficacy of some herbal feed additives on performance and control of caecal coccidiosis in broilers. APCBEE Procedia 4:163-168. |
|
|
Hema S, Arun T, Senthilkumar B, Senbagam D, Sureshkumar M (2015). In vivo anticoccidial effects of Azadirachta indica and Carica papaya L. with salinomycin drug as a dietary feed supplement in broiler chicks. Pakistan Journal of Pharmaceutical Sciences 28(4):1409-1415. |
|
|
Hunduza A (2018). Anthelmintic efficacy of bromelain encapsulated chitosan nanocarrires against Haemonchus contortus, Pan African University thesis. Available at: |
|
|
Hunduza A, Kagira J, Maina N, Andala D, Cheruiyot K, Kahiro S (2020). In vitro anthelmintic activity of chitosan encapsulated bromelain against eggs, larval and adult stages of Haemonchus contortus. Journal of Applied Life Sciences International 23(3):28-38. |
|
|
Ikpeze O, Eneanya C, Ikerionwu P (2010). Prevalence of Coccidiosis in West African Dwarf (WAD) goats at Mgbakwu, Anambra state, south-eastern Nigeria. Zoologist (1):162-167. |
|
|
Jackson PG, Cockcroft PD. (2002) Clinical Examination of Farm Animals. Wiley-Blackwell Science Limited, 312 pages. |
|
|
Joachim A, Altreuther G, Bangoura B, Charles S, Daugschies A, Hinney B, Lindsay DS, Mundt HC, Ocak M, Sotiraki S (2018). WAAVP Guideline for Evaluating the Efficacy of Anticoccidials in Mammals (Pigs, Dogs, Cattle, Sheep), Veterinary Parasitology 253:102-119. |
|
|
Juasook A, Aengwanich W, Chalalai T, Watwiengkam N, Asawapattanakul T, Promsud W (2017). Changes in sporulation, packed cell volume, malondialdehyde level, fecal oocyst count and histopathology of eimeria tenella-infected broilers treated with pineapple (Ananas comosus) crude extracts. International Journal of Poultry Science 16(5):189-195. |
|
|
Kagucia AW, Kagira JM, Maina N, Karanja SM, Njonge FK (2020). Characterisation of productivity and diseases affecting dairy goats in smallholder systems of greater thika region, Kenya. Journal of Agriculture and Rural Development in the Tropics and Subtropics 121(2):243-249. |
|
|
King JM, Roth-Johnson L, Dodd DC, Newsom ME (2013). The necropsy book: A Guide for Veterinary Students, Residents, Clinicians, Pathologists, and Biological Researchers. The Internet-First University Press pp. 33-35. |
|
|
MAFF (Ministry of Agriculture, Fisheries and Food) (1986). Fisheries and Food, Reference Book, 383 Manual of Veterinary Parasitological Laboratory Techniques, Vol. 418, Ministry of Agriculture, 384 HMSO, London, 5 pp. |
|
|
Maichomo MW, Kagira JM, Walker T (2004). The Point Prevalence of Gastro- Intestinal Parasites in Calves, Sheep and Goats in Magadi Division, South-Western Kenya. Onderstepoort Journal of Veterinary Research 71(4):257-261. |
|
|
Mat Yusof A, Md Isa ML (2016). Prevalence of gastrointestinal nematodiasis and coccidiosis in goats from three selected farms in Terengganu, Malaysia. Asian Pacific Journal of Tropical Biomedicine 6(9):735-739. |
|
|
Menge DMS, Makobe M, Monda EO, Okemo PO (2014) Effects of crude extracts on some selected physio-logical parameters of French beans (Phaseolus vulgaris) infected with rust (Uromyces appendiculatus). African Journal of Plant Science 8(7):356-363. |
|
|
Mohamaden WI, Sallam NH, Abouelhassan EM (2018). Prevalence of Eimeria species among sheep and goats in Suez Governorate, Egypt. International Journal of Veterinary Science and Medicine 6(1):65-72. |
|
|
Molan AL, Liu Z, De S (2009). Effect of pine bark (Pinus radiata) extracts on sporulation of coccidian oocysts. Folia Parasitologica 56(1):1-5. |
|
|
Moss JN, Frazier CV, Martin GJ (1963). Bromelains. The pharmacology of the enzymes. Archives Internationales de Pharmacodynamie et de The?rapie 145:166-189. |
|
|
Niazi S (2009a). Pharmaceutical Manufacturing Formulations. In Handbook of Pharmaceutical Manufacturing Formulations, Second Edition. |
|
|
Niazi S (2009b). Pharmaceutical Manufacturing Formulations. Handbook of Pharmaceutical Manufacturing Formulations, Second Edition, 5:146-405. |
|
|
Odden A, Denwood MJ, Stuen S, Robertson LJ, Ruiz A, Hamnes IS, Hektoen L, Enemark HL (2018). Field evaluation of anticoccidial efficacy: A novel approach demonstrates reduced efficacy of toltrazuril against ovine Eimeria spp. in Norway. International Journal for Parasitology: Drugs and Drug Resistance 8(2):304-311. |
|
|
Organization for Economic Cooperation and Development (OECD Guidelines) (2002). Test No. 423: Acute Oral toxicity-Acute Toxic Class Method. OECD Guideline for Testing of Chemicals pp. 1-14. Available at: |
|
|
Parasuraman S (2011). Toxicological screening. Journal of Pharmacology and Pharmacotherapeutics 2(2):74-79. |
|
|
Pavan R, Jain S, Shraddha K (2012). Properties and Therapeutic Application of Bromelain: A Review. Biotechnology Research International 2012(1):1-6. |
|
|
Rousselle SD, Wicks JR, Tabb BC, Tellez A, O'Brien M (2019). Histology Strategies for Medical Implants and Interventional Device Studies. Toxicologic Pathology 47(3):235-249. |
|
|
Rowe RC, Sheskey PJ, Quinn ME (2009). Handbook of Pharmaceutical Excipients. Pharmaceutical Press and American Pharmacists Association. 6th Edition P 917. |
|
|
Shamaki BU, Sandabe UK, Abdulrahaman FI, Ogbe AO, Hassan ZI, Yusuf IL, Bitrus W, Zongoma Y, Isikhuemhen OS (2017). Toxicity Studies and Body Weights Changes in Wistar Rats Following Oral Administration of Methanol Extract From Indigenous Ganoderma Sp. In Nigeria. MOJ Biology and Medicine 1(5):138-141. |
|
|
Waller PJ (2006). From discovery to development: Current industry perspectives for the development of novel methods of helminth control in livestock. Veterinary Parasitology 139(1-3):1-14. |
|
|
Wasso S, Maina N, Kagira J (2020). Toxicity and Anthelmintic Efficacy of Chitosan Encapsulated Bromelain against Gastrointestinal Strongyles in Small East African Goats in Kenya. Veterinary World 13(1):177-83. |
|
|
Zaman MA, Iqbal Z, Abbas RZ, Ehtisham-Ul- Haque S (2015). In vitro efficacy of herbal extracts against Eimeria tenella. International Journal of Agriculture and Biology 17(4):848-850. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0