ABSTRACT
Insect chitinases are hydrolytic enzymes that cleave chitin of the cuticle and peritrophic membrane during molting. Multiple genes encode insect chitinases, which are characterized as having diverse chemical and enzymatic properties depending on the time and the site of expression. This work was done to isolate and characterize chitinase genes from the red palm weevil (RPW), Rhynchophorus ferrugineus (Oliver), a cryptic pest of many palm trees. The isolated five genes were phylogenetically clustered into five different groups (I, II, III, VI, and VII) of the glycoside hydrolase family 18 (GH18). Domain structure analysis revealed that RfCht1 (group I), RfCht3 (group VI), and RfCht5 (group VII) each retained a single catalytic domain of the GH18, whereas RfCht2 (group III)) and RfCht4 (group II) possessed two and five GH18 catalytic domains, respectively. RfCht1, RfCht2, and RfCht3 each retained a single chitin-binding domain (CBD) and RfCht4 retained five CBDs, but RfCht5 lacked CBD. Developmental and tissue expression profiles showed high levels of transcripts of the five genes in the newly hatched first instar larvae. RfCht1 and RfCht2 transcripts were expressed constitutively almost with high levels in young and mature eggs, in all tested larval instars, pre-pupae, pharate pupae, and adults; whereas RfCht3 and RfCht5 transcripts were expressed as low levels in the early instars larvae few hours before molting. In late developmental stages and mature eggs, RfCht3, RfCht4, and RfCht5 were expressed as low levels mainly in the cuticle. This study presents the first report on chitinase genes in the RPW and suggests that these genes have additional roles in the weevil development, which require further elucidation.
Key words: Chitinase, conserved motifs, cuticle, domain structure, expression profile, Rhynchophorus ferrugineus
Insect body contains rigid and insoluble chitin, as a component of the exoskeleton, trachea, and the peritrophic membrane (PM) that surrounds the food in the midgut. Chitin in these organs provides protection to insects against environmental and mechanical injuries but it limits the growth and development of insects. Therefore, the cuticles and PM are degraded periodically and reshuffled to allow growth and development (Merzendorfer and Zimoch, 2003; Muthukrishnan et al., 2016). Insect chitinases play crucial roles to degrade chitin in the old cuticles and PM during the larval molting and pupation and act defensively to prevent bacteria and fungi from penetrating the PM. They belong to family 18 glycosyl hydrolases (GH18). The GH18 genes of chitinases have potential use for pest management as biopesticides (Kramer and Muthukrishnan, 1997). Insect chitinase genes have been suggested as targets for gene silencing via RNA interference (RNAi) (Al-Ayedh et al., 2016; Cao et al., 2017; Su et al., 2016; Zhu et al., 2008a) and have also been proposed as appropriate candidates in host-mediated silencing of pest genes (HMSPG) for control of diseases and insect pests of date palm (Niblett and Bailey, 2012).
Chitinases have been isolated and characterized in many insects including Anopheles gambiae, Bombyx mori, Chilo suppressalis, Drosophila melanogaster, Manduca sexta, Mythimna separata, Nilaparvata lugens, Ostrinia nubilalis, Tribolium castaneum, and economically important other species (Shen and Jacobs-Lorena, 1997; Zhu et al., 2008b; Khajuria et al., 2010; Zhang et al., 2011a; Huang et al., 2012; Pan et al., 2012; Tetreau et al., 2015; Xi et al., 2015; Su et al., 2016; Cao et al., 2017). Functional analyses of particular chitinases revealed that insect chitinases belong to a large family of enzymes with diverse domain architecture, expression patterns, tissue specificity, and function. They have been grouped into, at least, eleven groups based on domain arrangement and/or tissue specificity of expression and phylogenetic analyses as well as functional analyses (Nakabachi et al., 2010; Tetreau et al., 2015). Groups I and II chitinases are found in molting fluid (Koga et al., 1992; Qu et al., 2014). Groups III and VIII members have a membrane-spanning domain and are involved in regulating abdominal contraction and wing expansion. Group V members are mainly imaginal disc growth factor genes that are necessary for adult eclosion (Zhu et al., 2008a). It has been reported that in N. lugens at least four groups of chitinases (groups I, II, III, and V) are involved in nymph-nymph molt (Xi et al., 2015). The presence of several functional chitinases with distinct domain configuration suggests that they have other functions besides the digestion of chitin in the old cuticle.
These could include providing primers for elongation of chitin and processing of mature chitin chains for a higher level of organization (Muthukrishnan et al., 2016). The red palm weevil (RPW), Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae), is a noxious internal feeder attacking many palm species including coconut, Cocos nucifera, date palm, Phoenix dactylifera, Canary Islands palm, Phoenix canariensis, and African oil palm, Elaeis guineensis (Dembilio et al., 2012; Fiaboe et al., 2012; Hoddle et al., 2013). The entire larval life cycle of the weevil is concealed within the palm trunk, which makes its early infestations difficult to detect (Faleiro et al., 2012; Hoddle et al., 2013; Llácer et al., 2010; Murphy and Briscoe, 1999). Food and Agriculture Organization of the United Nations (FAO) has classified RPW as category-1 pest on date palm in the Middle East (Al-Dosary et al., 2016). To date, the large-scale dataset on the RPW transcriptome analyses though provided substantial information on the weevil’s development that could have important practical applications (Wang et al., 2013); however, no information exists about particular genes, especially those functions relating to processes of ecdysis and metamorphosis of the developmental stages of the RPW. Thus, we isolated, amplified, cloned, and sequenced five genes for chitinase were isolated from the RPW to gain insight into their structural and functional domains architecture and also to study the mRNA expression patterns of these genes in different developmental stages of the RPW.
RPW rearing and tissue collection
RPW was reared in the laboratory and the insectary facilities of the Date Palm Research Center of Excellence, King Faisal University. For egg laying, male and female adults were fed on sugarcane kept in TATAY storage boxes (51 cm ´ 38 cm ´ 26 cm) made of polypropylene and bisphenol A (BPA) free (www.tatay.com) with perforated lids as described elsewhere (El-Shafie et al., 2013). Eggs were removed with a brush and placed into Petri dishes that contained cotton and moist filter paper and incubated at 28°C until the eggs hatch. First instar larvae were collected daily and reared on pineapples and date palm trunk. Samples of different developmental stages were collected periodically for integument and tissue collection. Larvae were dissected by cutting off their heads using a standard stainless steel entomology dissection set. The integument was cut longitudinally to separate the adipose tissues and the guts. The dissected tissues were immediately frozen in liquid nitrogen. Eggs, elytra, forewings, and the adult’s body were directly frozen in liquid nitrogen. All samples were stored at -80°C for the subsequent experiments.
BLAST® search and sequence alignment
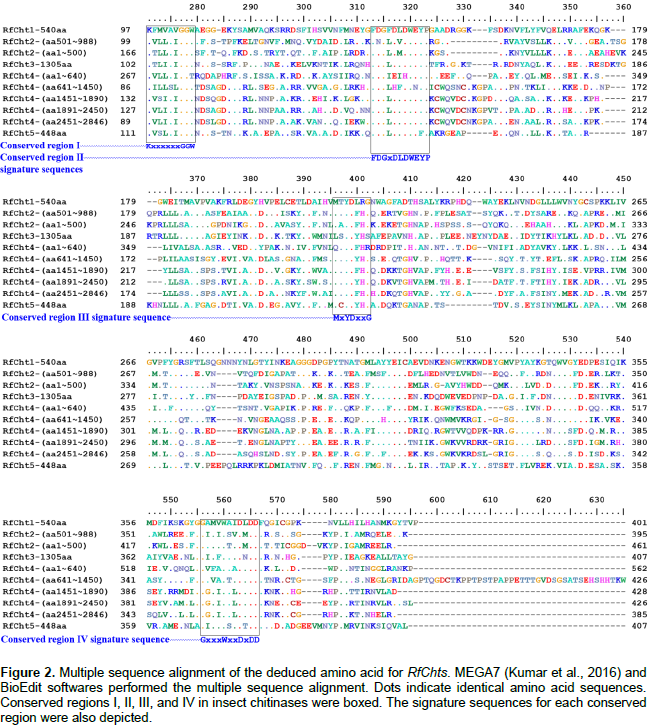
The online Basic Local Alignment Search Tool (BLAST) was used to search for potential chitinase genes sequences in the RPW Transcriptome Shotgun Assembly (TSA) (Wang et al., 2013). T. castaneum and B. mori chitinase gene sequences available in the GenBank (https://www.ncbi.nlm.nih.gov/genbank/) were used to search for similar sequences in the RPW TSA dataset. The identified RPW TSA sequences were pools of unannotated sequences with gaps in sequenced contigs. Multiple sequence alignment was done using MEGA7 (Kumar et al., 2016) and BioEdit (http://www.mbio.ncsu.edu/BioEdit/BioEdit.html) softwares in order to locate the four highly conserved signatures in the amino acids of all known insect chitinases (Zhang et al., 2011b). Only RPW TSA contigs with the conserved four regions were used to synthesize the primers (Table 1) used for partial amplification of chitinase genes.
RNA isolation and first strand cDNA synthesis
Frozen RPW tissues were ground into fine powder in liquid nitrogen using mortar and pestle. Total RNA was isolated using RNeasy Plus Universal Mini Kit (QIAGEN) according to the manufacturer’s protocol. Elongase™ enzyme mix was obtained from Invitrogen® and the recombinant Taq DNA polymerase was purchased from Fermentas®. Reverse transcription of RNA to synthesize first strand cDNAs for RfChts was done using a random hexamer primer and RevertAid RT Kit obtained from Thermo Fisher Scientific according to the manufacturer’s protocol. Double-stranded cDNA was amplified using the first strand cDNA as template and a gene-specific primer (Table 1). Primers used to amplify the full-length cDNAs and to study the expression patterns of the RPW chitinase genes, and for sequencing are shown in Table 1. The thermocycler used for cDNA amplification was Veriti® Thermal Cycler (96 well) supplied by Applied Biosystems™. Amplified PCR products were electrophoresed on 0.7% agarose D1 (Pronadisa) gel, stained with ethidium bromide, visualized using INGENIUS Syngene Bio Imaging System, and documented using GeneSnap software from Syngene. Then, the cDNAs were purified either from the excised gel using QIAquick® Gel extraction kit (Qiagen) or directly from the PCR products using the DNA Pure Kit (Geneaid®) following the manufacturers’ protocols. The recovered cDNAs were used for the subsequent PCR amplification, cloning, or direct sequencing.
Gene cloning and sequencing
The PCR-amplified cDNAs were cloned into pGEM®-T Easy vector (Promega, Madison, WI, USA) according to the manufacturer’s protocol. Ligation, cloning, and transformation processes were carried out according to the standard protocols (Sambrook et al., 1989). The manipulated plasmids were transformed into Escherichia coli strain DH5α. Plasmids maintained by the bacterium were isolated using Wizard® Plus SV Minipreps DNA Purification System (Promega, Madison, WI, USA) according to the supplier’s instructions. Due to the big sizes of some cDNA clones, multiple sequencing rounds were carried out to clarify dubious and long uncovered reads. Sequencing was done at Macrogen service facilities (Seoul, South Korea).
Domain structure and phylogenetic analyses
Multiple sequence alignment of the deduced amino acids of RfChts and the molecular phylogenetic analyses were performed using the software MEGA7 (Kumar et al., 2016). Chitinase domain structure analysis was done using InterPro: protein sequence analysis and classification database (https://www.ebi.ac.uk/interpro/).
Expression profiles of RfChts genes in different developmental stages and tissues
The expression profiles of the five RfCht genes at different stages of development including eggs, larvae, pre-pupae, pupae, adults and appendages were tested. Eggs were collected 12- and 24-h after laying and tissues of the middle-aged larvae were collected at 0- to 96-h pre-molting. Reverse transcription PCR was done in a 25-ml reaction mixture containing 1 ml template first strand cDNA, 10 pmol/ml each primer, 12.5 ml Master Mix (Biomatik Corporation, Canada), and nuclease-free water. The thermocycler program for RT-PCR was as follows: initial denaturation cycle at 94°C for 3 min followed by 30 cycles at 94°C for 25 s, 60°C for 25 s, and 72°C for 2 min, and a final extension at 72°C for 10 min. PCR products were analyzed on 1% agarose gel. Experiments were replicated at least three times using independent total RNA preparations. RPW’s ribosomal protein S3 (RfRpS3) was used as an internal reference gene for RT-PCR analysis.
Sequence analysis of cDNAs for the genes of chitinase
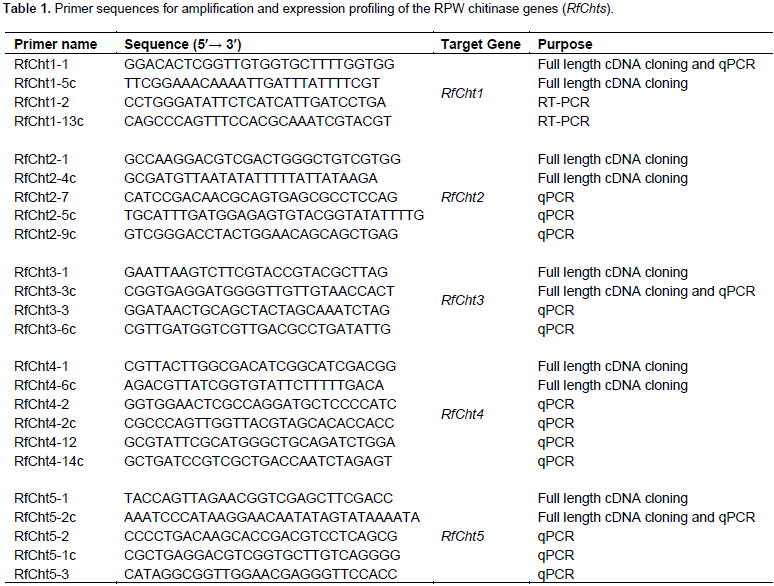
Five chitinase cDNA amplicons from R. ferrugineus were sequenced (RfCht1, RhCht2, RfCht3, RfCht4, and RfCht5) and the sequences were deposited in the GenBank database and the accession numbers are shown in Table 2. The RfCht1 full sequence is 1,828 bp long. It consists of 1620 bp open reading frame (ORF), 102 bp 3¢-untranslated region, and 106 bp 5¢-untranslated region (Table 2). The translated region consists of 540 amino acids (Figure 1). The first 20 amino acids constitute a putative signal peptide, as predicted by the online SignalP 4.1 Server (Petersen et al., 2011) that targets the protein to the extracellular space or sorts it into plasma membrane to face in both cases carbohydrates of the extracellular matrix (Kawamura et al., 1999; Royer et al., 2002; Arakane et al., 2003).
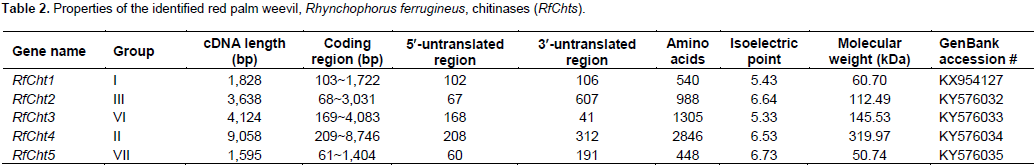
Analysis of the putative consensus signature domains of the isolated cDNA revealed that the protein consists of a single catalytic domain of the glycoside hydrolase family 18 (GH18). The catalytic domain covers the deduced amino acids span from 18 to 383. The amino acids from 270 to 346 form a chitinase insertion domain (CID). The C-terminus amino acids from 484 to 539 constitute a chitin-binding domain (CBD), which belongs to family 14 of carbohydrate-binding modules (CBM14). RfCht2 is 3,638 bp long covering an ORF of 2,964 bp that encodes a putative protein of 988 amino acids including a putative signal peptide, two GH18 catalytic domains in which two CIDs are embedded, and has a single C-terminal CBD. RfCht3 is 4,124 bp long. It consists of 3,915 bp ORF that encodes a putative protein of 1,305 amino acids including a putative signal peptide, a GH18 catalytic domain in which a CID is embedded, and has a single C-terminal CBD. On the other hand, RfCht4 is the largest gene for chitinase from the RPW found in this study. The gene is 9,058 bp long with an ORF of 8,538 bp that encodes a putative protein of 2,846 amino acids. The identified sequence of RfCht4 lacks a signal peptide but it contains five GH18 catalytic domains in which five CIDs are embedded, and has five CBDs. One CBD located between the first and the second catalytic domains, three CBDs located between the second and the third catalytic domains, and one CBD located between the fourth and the fifth catalytic domains (Figure 1 and Table 2).
In comparison, RfCht5 is the smallest gene among the RfChts identified in this study. RfCht5 consists of 1,595 bp with an ORF of 1,344 bp that encodes a putative protein of 448 amino acids (Table 2). It contains a predictable signal peptide, a single GH18 catalytic domain but lacks CBD (Figure 1). The five RfChts have many serine/ threonine (S/T) residues in the sequences between the catalytic domains and the CBDs. Similar to other insect chitinases, all CBDs of RfChts retain six conserved cysteines (C) that probably form three disulfide bridges that increase the affinity of chitinase for the insoluble substrate during chitin hydrolysis. GenBank database homology searches and the sequence alignments revealed that the deduced amino acids of the five RfChts share common consensus signature sequences that identify them as GH18 chitinases (Figure 2) and these are well-characterized from many insects belonging to different orders and species (Zhu et al., 2008b; Su et al., 2016). All the five RfChts retain the signature sequences in the conserved region I (KxxxxxGGW) and in the conserved region IV (GxxxWxxDxDD) of GH18 chitinases. It is notable that the conserved region II (FDGxDLDWEYP) is common in all identified RfChts except RfCht5 where the residue tyrosine (Y) was replaced by phenylalanine (F). Moreover, in the RfCht4 the conserved glutamate residue (E) in the second catalytic domain was replaced by asparagine (N) (Figure 2).
Phylogenetic analysis of deduced protein sequences of five RfCht genes
A phylogenetic tree was constructed by using deduced amino acids of RfChts and sequences of chitinase and/or chitinase-like proteins from 21 insect species belonging to seven orders (Figure 3). The tree was generated using maximum likelihood method. Phylogenetically, RfCht1 belongs to group I chitinases and is closely related to a hypothetical protein from the mountain pine beetle, Dendroctonus ponderosae (Keeling et al., 2013) and to chitinase 5 (TcCht5) of the red flour beetle, T. castaneum (Zhang et al., 2011a). RfCht2, RfCht3, RfCht4, and RfCht5 phylogenetically belong to the groups III, VI, II, and VII, respectively (Figure 3).
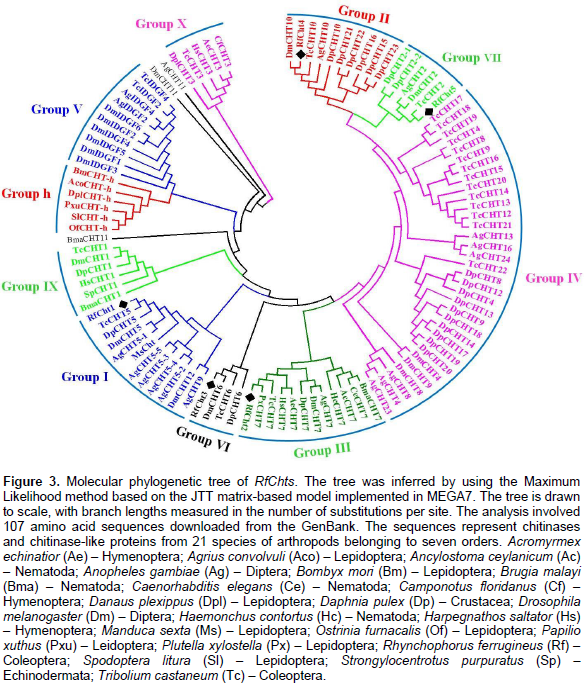
Expression profiling of RfChts in the tissues and developmental stages
Expression profiles of RfChts primary transcripts in eggs, larvae, adults, and the appendages were determined by RT-PCR. Transcripts of RfCht1 and RfCht2 were expressed in the larval cuticles 96-h pre-molting and the expression continues to the onset of molting (Figure 4). RfCht2 seems more steadily expressed 14-h pre-molting; however, 96- to 24-h pre-molting, fewer transcripts were expressed relative to the transcripts found 14- to 0-h pre-molting. The transcripts of both chitinases were found in the adipose tissues and the gut of larvae, as well as in the guts of both males and females, the elytra, and the hindwings (Figure 4). The transcripts of RfCht3 were uniformly detectable 14- to 0-h pre-molting but in less quantity relative to RfCht1 and RfCht2 transcripts. RfCht3 was not detected in cuticular tissues 96- to 24-h pre-molting and it was not found in adult weevils or their appendages. RfCht4 transcripts were detected only in cuticles that were collected 96-, 8- and 4-h pre-molting. It seems there is no steady expression titer for RfCht4 in the cuticle. Transcripts of RfCht4 were not detected in adults or their appendages. Faint transcripts of RfCht5 were detected in larval cuticular tissues 48- to 0-h pre-molting but were not found in other tissues and developmental stages.
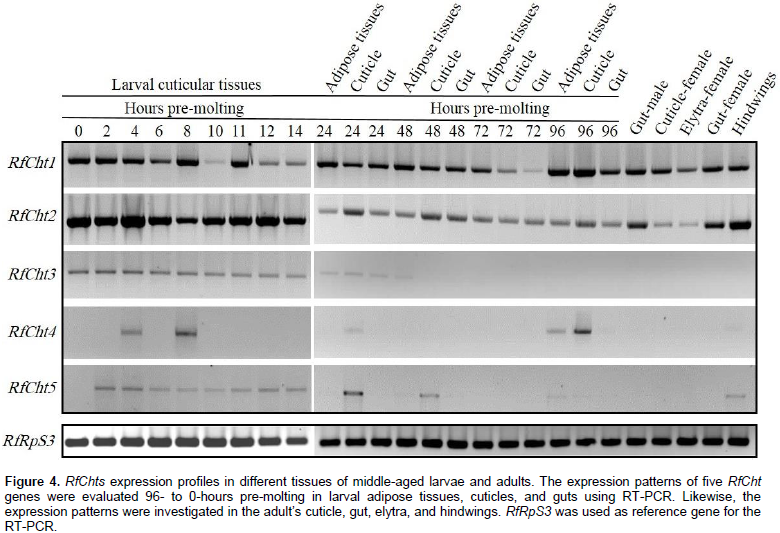
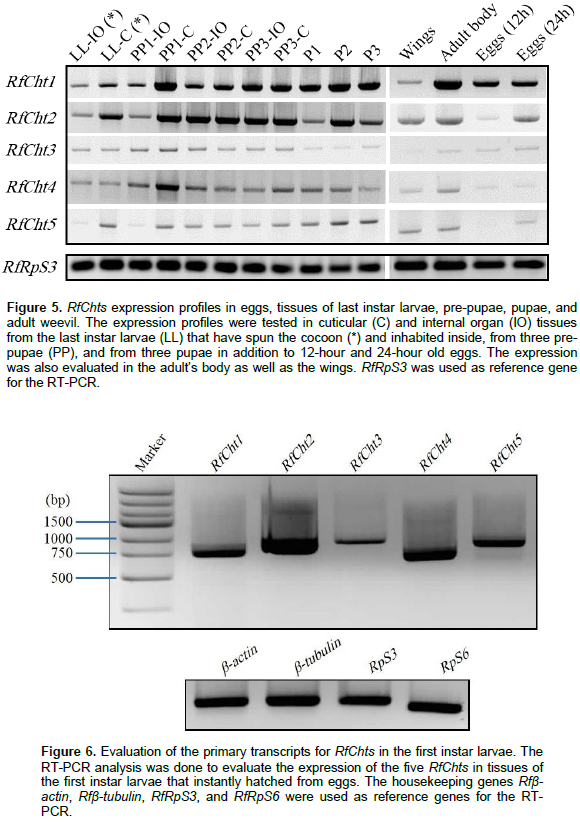
Transcripts of the five chitinases were further investigated in the cuticles and internal organs of the larvae inhabiting the cocoon, pre-pupae, and pupae (Figure 5). RfCht1 and RfCht2 were found in all tested stages and tissues including adult wings, 12- and 24-h old eggs. RfCht3, RCht4, and RfCht5 transcripts were very low relative to those for RfCht1 and RfCht2. It is clear that the transcripts of RfCht3, RCht4, and RfCht5 were steadily expressed but in low quantities in the last instar larvae within the cocoon, pre-pupae, and pupae. Transcripts of all the five RfCht genes were expressed in 24-h old eggs though more transcripts of RfCht1 were expressed followed by RfCht2, RfCht3, RfCht5, and RfCht4. RfCht2 transcripts were prominent in 12-h old eggs and hardly detectable for the other chitinases (Figure 5). Apparently, the transcripts of RfCht2 were higher followed by the transcripts of RfCht4, RfCht5, RfCht1, and RfCht3 in first instar larvae just emerged from the eggs (Figure 6).
Chitinases are indispensable enzymes involved in chitin metabolism leading to molting and eclosion of insects and other arthropods. The matrix polymer chitin has been designed to provide the cuticle with flexibility in response to the need to adapt to extensively diverse environmental stresses (Muthukrishnan et al., 2016). Insect chitinases have been characterized as potential targets for pest management either via directly targeting them (Su et al., 2016; Cao et al., 2017) or via manipulating their inhibitors as biopesticides (Arakane and Muthukrishnan, 2010; Hirose et al., 2010). R. ferrugineus lives and develops inside the palm trunk, where there are various biomass-degrading microbes that do not harm the weevil. In this context, it has been reported that polar surface cuticular extracts from adults and larvae inhibited the growth of Gram-positive bacteria and the entomopathogenic fungi Beauveria bassiana (Mazza et al., 2011). Here, cDNAs have been isolated and synthesized for the chitinase genes from R. ferrugineus larvae that had not yet undergone apolysis. During this time the molting fluid fills the space between the digested old cuticle and the newly synthesized cuticle (Kramer et al., 1985). The identified chitinase genes shared the common consensus signature sequences found in all investigated insect chitinases, namely the conserved regions I, II, III, and IV that classify them into GH18. The largest gene for chitinase isolated from the RPW is RfCht4 and the smallest one is RfCht5. The five RfChts identified in this study have divergent numbers of catalytic domains, CBDs, and CIDs that are believed to interact with oligosaccharides during catalysis (Li and Greene, 2010).
The CID facilitates orienting and binding to longer chitin substrates when inserted into the triose phosphate isomerase (TIM) barrel, a conserved protein fold consisting of eight a-helices and eight parallel β-strands that alternate along the peptide backbone (Li and Greene, 2010). Moreover, with the exception of RfCht5 that has no CBD, the CBDs of RfChts have linker sequences with six conserved cysteine residues. It has been reported that the common spacing between the conserved cysteines in the CBDs of GH18 chitinases appears to be as follows: 1Cx11-24-2Cx5-6-3Cx9-19-4Cx10-17-5Cx4-14-6C, where x is any other amino acid (Arakane and Muthukrishnan, 2010; Su et al., 2016). However, the six cysteine residues in RfCht1 gene were arranged as follows: 1Cx9-2Cx5-3Cx9-4Cx12-5Cx10-6C. It is obvious that the spacing between the first and the second conserved cysteines in RfCht1 CBD is only nine amino acids, that is, the spacing is shorter in RfCht1 compared to the chitinase genes isolated from the RPW and from other insects. However, the spacing between the remaining conserved cysteines in the five RfChts investigated here is within the range commonly found in other insect chitinases. These linker regions can be heavily glycosylated and are believed to increase the stability of chitinases when present in a protease-rich environment such as the gut or the molting fluid (Abdel-Banat and Koga, 2002; Arakane et al., 2003; Arakane and Muthukrishnan, 2010).
Notable observations were found in the conserved region II (FDGxDLDWEYP) where the conserved tyrosine (Y) was replaced by phenylalanine (F) in RfCht5 and the conserved glutamate residue (E) in the second catalytic domain of RfCht4 was replaced by asparagine (N). These replacements were also observed in CpCht10 from Culex pipiens and PhcCht10 from Pediculus humanus corporis (Arakane and Muthukrishnan, 2010). Generally, glutamate residue (E) is the most critical in the conserved motif II and is believed to be the proton donor required for cleavage of the glycosidic bond as evidenced by the replacement of the residue (E) by a glutamine (Q) or with an aspartic acid (D) which resulted in complete loss of enzyme activity (Lu et al., 2002). Structurally, the identified RfChts typically resemble other known chitinases that identified from other insect species, especially in their physical and multi-domain architectures (Pan et al., 2012; Huang et al., 2012).
Insect chitinases and chitinase-like proteins are categorized into distinct groups according to phylogenetic kinships. Group I chitinases are secreted proteins that are the most abundant enzymes in molting fluid and/or integument, and represent the prototype enzyme of GH18, with a single copy each of the catalytic domain and chitin-binding domain (CBD) connected by linker polypeptide rich with S/T residues (Arakane and Muthukrishnan, 2010; Khajuria et al., 2010; Tetreau et al., 2015). Previous studies reported that group II chitinases are larger-sized secreted proteins with multiple catalytic domains and CBDs (Zhu et al., 2008c; Tetreau et al., 2015). Group III chitinases contain two catalytic domains and are predicted as membrane-anchored proteins. Group IV chitinases are the most divergent. They usually lack a CBD and/or an S/T-rich linker domain and are predicted to be secreted proteins found in the gut or fat body. Group V proteins include the putative chitinase-like imaginal disc growth factors (IDGFs). In T. castaneum and D. melanogaster genomes, multiple genes were found to encode groups IV and group V chitinase-like proteins. In contrast, groups I, II, and III are each represented by only a single gene in each species (Zhu et al., 2008c; Tetreau et al., 2015). Group VI chitinases resemble group I chitinases; however, the C-terminal S/T-rich linker extends the molecular mass of proteins in this group. Group VII chitinases structurally resemble group IV but phylogenetically are placed as an outlier of group II (Merzendorfer, 2013). The group has an N-terminal signal peptide and a GH18 catalytic domain, but it lacks a CBD. The expression patterns of RfChts are similar to the expression patterns of a chitinase-like gene cluster (AgCht5) from the African malaria mosquito, A. gambiae (Diptera: Culicidae), where the five genes in the cluster showed different expression patterns at different developmental stages (Zhang et al., 2011a).
It has been reported that expression of insect chitinases is time controlled and released only during molting. Chitinase transcripts are not detectable in cuticular tissue until after the cessation of feeding in each larval instar and appear immediately prior to pupation before dropping to undetectable levels 1 to 2 days into the molt cycle (Muthukrishnan et al., 2016). In contrast, our study showed that transcripts of RfChts were detectable in all stages at earlier times before the larvae had commenced the molting. Similar expression patterns were reported in N. lugens chitinase-like gene family (Xi et al., 2015). This suggests that at least some RPW chitinases may have been enrolled in functions other than molting. Due to the concealed living behavior of the weevil within the trunk of the host plant, a place where many species of microbes survive and propagate, it is probable that some of the chitinases might be involved in the immune defense to protect the weevil from microbial infection. Molecular characterization and phylogenetic studies of five R. ferrugineus chitinases revealed RPW conserved the functional domain consensus commonly found in all identified chitinases from insects belonging to different orders. However, the expression pattern of some chitinases in the tissues of R. ferrugineus and developmental stages is unique. Expression of RPW chitinases varies from being in short time and in specific patterns usually found in other insects to an unusual constitutive expression throughout the larval developmental stages. This might be related to the living habitat of this insect pest, where an array of microbial inhabitants exists in the same habitation. Further studies are necessary to understand the specific roles of the constitutively expressed chitinases in R. ferrugineus.
The authors have not declared any conflict of interests.
The authors thank Ibrahim A. Bou-Khowh for technical assistance and Dr. Mark S. Hoddle at University of California, Riverside, for critically reading and editing the manuscript. Financial support was provided by the Deanship of Scientific Research (grant number 150221) and by the Date Palm Research Center of Excellence (grant number DPRC-2(2015), King Faisal University, Saudi Arabia.
REFERENCES
|
Abdel-Banat BMA, Koga D (2002). Alternative splicing of the primary transcript generates heterogeneity within the products of the gene for Bombyx mori chitinase. J. Biol. Chem. 277:30524-30534.
Crossref
|
|
|
|
Al-Ayedh H, Rizwan-ul-Haq M, Hussain A, Aljabr AM (2016). Insecticidal potency of RNAi-based catalase knockdown in Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae). Pest Manag. Sci. 72: 2118-2127.
Crossref
|
|
|
|
|
Al-Dosary NMN, Al-Dobai S, Faleiro JR (2016). Review on the management of red palm weevil Rhynchophorus ferrugineus Olivier in date palm Phoenix dactylifera L. Emir. J. Food Agric. 28:34-44.
Crossref
|
|
|
|
|
Arakane Y, Muthukrishnan S (2010). Insect chitinase and chitinase-like proteins. Cell. Mol. Life Sci. 67:201-216.
Crossref
|
|
|
|
|
Arakane Y, Zhu Q, Matsumiya M, Muthukrishnan S, Kramer KJ (2003). Properties of catalytic, linker and chitin-binding domains of insect chitinase. Insect Biochem. Mol. Biol. 33:631-648.
Crossref
|
|
|
|
|
Cao B, Bao W, Wuriyanghan H (2017). Silencing of target chitinase genes via oral delivery of dsRNA caused lethal phenotypic effects in Mythimna separata (Lepidoptera: Noctuidae). Appl. Biochem. Biotechnol 181:860-866.
Crossref
|
|
|
|
|
Dembilio O, Tapia GV, Téllez MM, Jacas JA (2012). Lower temperature thresholds for oviposition and egg hatching for the red palm weevil, Rhynchophorus ferrugineus (Coleoptera: Curculionidae), in a Mediterranean climate. Bull. Entomol. Res. 102:97-102.
Crossref
|
|
|
|
|
El-Shafie HAF, Faleiro JR, Abo-El-Saad MM, Aleid SM (2013). A meridic diet for laboratory rearing of red palm weevil, Rhynchophorus ferrugineus (Coleoptera: Curculionidae). Sci. Res. Essays 8:1924-1932.
|
|
|
|
|
Faleiro JR, Ben Abdullah A, El-Bellaj M, Al-Ajlan AM, Oihabi A (2012). Threat of red palm weevil, Rhynchophorus ferrugineus (Olivier) to date palm plantations in North Africa. Arab J. Plant Prot. 30:274-280.
|
|
|
|
|
Fiaboe KKM, Peterson AT, Kairo MTK, Roda AL (2012). Predicting the potential worldwide distribution of the red palm weevil Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae) using ecological niche modeling. Fla. Entomol. 95:559-673.
Crossref
|
|
|
|
|
Hirose T, Sunazuka T, Omura S (2010). Recent development of two chitinase inhibitors, Argifin and Argadin, produced by soil microorganisms. Proc. Jpn. Acad. B Phys. Biol. Sci. 86:85-102.
|
|
|
|
|
Hoddle MS, Al-Abbad AH, El-Shafie HAF, Faleiro JR, Sallam AA, Hoddle CD (2013). Assessing the impact of areawide pheromone trapping, pesticide applications, and eradication of infested date palms for Rhynchophorus ferrugineus (Coleoptera: Curculionidae) management in Al Ghowaybah, Saudi Arabia. Crop Prot. 53:152-160.
Crossref
|
|
|
|
|
Huang QS, Xie XL, Liang G, Gong F, Wang Y, Wei XQ Wang Q, Ji Z-L, Chen Q-X (2012). GH18 family of chitinases: Their domain architectures, function and evolutions. Glycobiology 22:23-34.
Crossref
|
|
|
|
|
Kawamura K, Shibata T, Saget O, Peel D, Bryant PJ (1999). A new family of growth factors produced by the fat body and active on Drosophila imaginal disc cells. Development 126:211-219.
|
|
|
|
|
Keeling CI, Yuen MM, Liao NY, Docking TR, Chan SK, Taylor GA, Palmquist DL, Jackman SD, Nguyen A, Li M, Henderson H, Janes JK, Zhao Y, Pandoh P, Moore R, Sperling FAH, Huber DPW, Birol I, Jones SJM, Bohlmann J (2013). Draft genome of the mountain pine beetle, Dendroctonus ponderosae Hopkins, a major forest pest. Genome Biol. 14:R27.
Crossref
|
|
|
|
|
Khajuria C, Buschman LL, Chen MS, Muthukrishnan S, Zhu KY (2010). A gut-specific chitinase gene essential for regulation of chitin content of peritrophic matrix and growth of Ostrinia nubilalis larvae. Insect Biochem. Mol. Biol. 40:621-629.
Crossref
|
|
|
|
|
Koga D, Funakoshi T, Mizuki K, Ide A, Kramer KJ, Zen KC, Choi H, Muthukrishnan S. (1992). Immunoblot analysis of chitinolytic enzymes in integument and molting fluid of the silkworm, Bombyx mori, and the tobacco hornworm, Manduca sexta. Insect Biochem. Mol. Biol. 22:305-311.
Crossref
|
|
|
|
|
Kramer KJ, Dziadik-Turner C, Koga D (1985). Chitin metabolism in insects. In Comprehensive Insect Physiology, Biochemistry and Pharmacology (Edited by Kerkut G. A. and Gilbert L. I.), Pergamon Press, New York. 3:75-115.
Crossref
|
|
|
|
|
Kramer KJ, Muthukrishnan S (1997). Insect chitinases: molecular biology and potential use as biopesticides. Insect Biochem. Mol. Biol. 27:887-900.
Crossref
|
|
|
|
|
Kumar S, Stecher G, Tamura K (2016). MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33:1870-1874.
Crossref
|
|
|
|
|
Li H, Greene LH (2010). Sequence and structural analysis of the chitinase insertion domain reveals two conserved motifs involved in chitin-binding. PLoS One 5:e8654.
Crossref
|
|
|
|
|
Llácer E, Dembilio O, Jacas JA (2010). Evaluation of the efficacy of an insecticidal paint based on chlorpyrifos and pyriproxyfen in a microencapsulated formulation against Rhynchophorus ferrugineus (Coleoptera: Curculionidae). J. Econ. Entomol. 103:402-408.
Crossref
|
|
|
|
|
Lu Y, Zen KC, Muthukrishnan S, Kramer KJ (2002). Site-directed mutagenesis and functional analysis of active site acidic amino acid residues D142, D144 and E146 in Manduca sexta (tobacco hornworm) chitinase. Insect Biochem. Mol. Biol. 32:1369-1382.
Crossref
|
|
|
|
|
Mazza G, Arizza V, Baracchi D, Barzanti GP, Benvenuti C, Francardi V, Frandi A, Gherardi F, Longo S, Manachini B, Perito B, Rumine P, Schillaci D, Turillazzi S, Cervo R (2011). Antimicrobial activity of the red palm weevil Rhynchophorus ferrugineus. Bull. Insectology 64:33-41.
|
|
|
|
|
Merzendorfer H, Zimoch L (2003). Chitin metabolism in insects: structure, function and regulation of chitin synthases and chitinases. J. Exp. Biol. 206:4393-4412.
Crossref
|
|
|
|
|
Merzendorfer H (2013). Insect-derived chitinases. Adv. Biochem. Eng. Biotechnol. 136:19-50.
Crossref
|
|
|
|
|
Murphy ST, Briscoe BR (1999). The red palm weevil as an alien invasive: biology and the prospects for biological control as a component of IPM. Biocontrol News and Information 20:35N-46N.
|
|
|
|
|
Muthukrishnan S, Merzendorfer H, Arakane Y, Yang Q (2016). Chitin metabolic pathways in insects and their regulation. In Extracellular Composite Matrices in Arthropods; Cohen E., Moussian B., Eds.; Springer International Publishing: Switzerland. pp. 31-65.
Crossref
|
|
|
|
|
Nakabachi A, Shigenobu S, Miyagishima S (2010). Chitinase-like proteins encoded in the genome of the pea aphid, Acyrthosiphon pisum. Insect Mol. Biol. 19:175-185.
Crossref
|
|
|
|
|
Niblett CL, Bailey AM (2012). Potential applications of gene silencing or RNA interference (RNAi) to control disease and insect pests of date palm. Emir. J. Food Agric. 24:462-469.
|
|
|
|
|
Pan Y, Lü P, Wang Y, Yin L, Ma H, Ma G, Chen K, He Y (2012). In silico identification of novel chitinase-like proteins in the silkworm, Bombyx mori, genome. J. Insect Sci. 12:150.
Crossref
|
|
|
|
|
Petersen TN, Brunak S, Heijne G, Nielsen H (2011). SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat. Methods 8:785-786.
Crossref
|
|
|
|
|
Qu M, Ma L, Chen P, Yang Q (2014). Proteomic analysis of insect molting fluid with a focus on enzymes involved in chitin degradation. J. Proteome Res. 13:2931-2940.
Crossref
|
|
|
|
|
Royer V, Fraichard S, Bouhin H (2002). A novel putative insect chitinase with multiple catalytic domains: hormonal regulation during metamorphosis. Biochem. J. 366:921-928.
Crossref
|
|
|
|
|
Sambrook J, Fritsch EF, Maniatis T (1989). Molecular Cloning: A Laboratory Manual, 2nd ed. Cold SpringHarbor Laboratory Press, Cold Spring Harbor, NY, USA.
|
|
|
|
|
Shen Z, Jacobs-Lorena M (1997). Characterization of a novel gut-specific chitinase gene from the human malaria vector Anopheles gambiae. J. Biol. Chem. 272:28895-28900.
Crossref
|
|
|
|
|
Su C, Tu G, Huang S, Yang Q, Shahzad MF, Li F (2016). Genome-wide analysis of chitinase genes and their varied functions in larval moult, pupation and eclosion in the rice striped stem borer, Chilo suppressalis. Insect Mol. Biol. 25:401-412.
Crossref
|
|
|
|
|
Tetreau G, Cao XL, Chen YR, Muthukrishnan S, Jiang H, Blissard, Kanost MR, Wang P (2015). Overview of chitin metabolism enzymes in Manduca sexta: identification, domain organization, phylogenetic analysis and gene expression. Insect Biochem. Mol. Biol. 62:114-126.
Crossref
|
|
|
|
|
Wang L, Zhang X-W, Pan L-L, Liu W-F, Wang D-P, Zhang G-Y, Yin Y-X, Yin A, Jia S-G, Yu X-G, Sun G-Y, Hu S-N, Al-Mssallem IS, Yu J (2013). A large-scale gene discovery for the red palm weevil Rhynchophorus ferrugineus (Coleoptera: Curculionidae). Insect Sci. 20:689-702.
Crossref
|
|
|
|
|
Zhang J, Zhang X, Arakane Y, Muthukrishnan S, Kramer KJ, Ma E, Zhu KY (2011a). Identification and characterization of a novel chitinase-like gene cluster (AgCht5) possibly derived from tandem duplications in the African malaria mosquito, Anopheles gambiae. Insect Biochem. Mol. Biol. 41:521-528.
Crossref
|
|
|
|
|
Zhang J, Zhang X, Arakane Y, Muthukrishnan S, Kramer KJ, Ma E, Zhu KY (2011b). Comparative genomic analysis of chitinase and chitinase-like genes in the African malaria mosquito (Anopheles gambiae). PLoS One 6: e19899.
Crossref
|
|
|
|
|
Zhu Q, Arakane Y, Beeman RW, Kramer KJ, Muthukrishnan S (2008a). Functional specialization among insect chitinase family genes revealed by RNA interference. Proc. Natl. Acad. Sci. USA. 105:6650-6655.
Crossref
|
|
|
|
|
Zhu Q, Arakane Y, Banerjee D, Beeman RW, Kramer KJ, Muthukrishnan S (2008b). Domain organization and phylogenetic analysis of the chitinase-like family of proteins in three species of insects. Insect Biochem. Mol. Biol. 38:452-466.
Crossref
|
|
|
|
|
Zhu Q, Arakane Y, Beeman RW, Kramer KJ, Muthukrishnan S (2008c). Characterization of recombinant chitinase-like proteins of Drosophila melanogaster and Tribolium castaneum. Insect Biochem. Mol. Biol. 38:467-477.
Crossref
|
|
|
|
|
Xi Y, Pan PL, Ye YX, Yu B, Xu HJ, Zhang CX (2015). Chitinase-like gene family in the brown planthopper, Nilaparvata lugens. Insect Mol. Biol. 24:29-40.
Crossref
|
|