ABSTRACT
This study was conducted at Marine Science Center, Basrah University, Basrah, Iraq during the period of 2015 to 2016. In the present work, we used NaCl and polyethylene glycol (PEG) to induced abiotic stress in vitro. Results show that high shoot numbers (24) were produced on MS (solid) medium enriched with 0.8 g/L NaCl in addition to cytokinins while, inclusion of drought agent PEG to proliferation medium (solid and liquid) produced a minimum shoots of 5 and 11 respectively. Root growth and development were also investigated in this study. It was found that high root development was observed on MS (solid or liquid) medium free of hormone (control) and MS (solid or liquid) medium containing 0.8 g/L NaCl 26.67, 25, 21.67 and 18 respectively whereas, less root development was obtained on MS medium (solid or liquid) enriched with NaCl and 26 g/L PEG. In this study it was found that inclusion of the amino acid proline to the stressed media did not improve the growth of shoots or roots. Histological analysis of multiplied shoots under abiotic stress agents showed accumulation of insoluble starch granules in the parenchyma cells of the cortex layer and this may be a part of protective tolerate mechanism used by medicinal hyssop (Bacopa monnieri) against abiotic stress.
Key words: Bacopa monnieri, medicinal hyssop, Brbin, micropropagation, salt stress, polyethylene glycol (PEG), proline.
Bacopa monnieri (L.) Wettst a water hyssop, known as Brahmi, or Brbin belonging to the family Plantaginaceae is widely distributed in warmer parts of Asia, Australia, and America. It is an important medicinal plant used in the Ayurvedic system for centuries. This creeping herb with a light purple flower is used for various ailment but is best known as a brain tonic, a nerve tonic for enhancing memory, improves intellectual and cognitive functions, anti-inflammatory, analgesic, antipyretic, sedative and antiepileptic agent. In India and Pakistan this plant has been used as a cardiac tonic, digestive aid and improves the respiratory system (Bammidi et al., 2011; Charoenphon et al., 2016). It also used in medical therapy for insomnia, asthma, hoarseness, snake bite, rheumatism, leprosy, eczema, water retention, blood cleaning and insanity (Banerjee and Modi, 2010).
Environmental stress and in particular salinity is one of the important factor that limits the distribution, crop productivity, morphogenesis and the formation of secondary metabolites of the glycophytic plant (Liu and Cheng, 2008; Charu et al., 2015; Vibhuti et al., 2015; Awasthi et al., 2016). However, plants have developed complex mechanisms to adapt to osmotic, ionic and oxidative stresses induced by salt stress; for instance, the accumulation of proline (Debnath, 2008; Bargali and Bargali, 2016). Tissue culture is used to evaluate the impact of abiotic stress on cell metabolism morphologically, anatomically and physiologically; it also opens a new path for the production of secondary metabolites (Debnath, 2008; Naik and Al-Khayri, 2015). Tissue culture is a novel technology used to investigate plants' tolerance to different abiotic stresses factors. So, the present study aimed to investigate the effect of salinity (NaCl) and drought agent polyethyleneglycol (PEG) on morphogenetic potential and histological aspect of in vitro grown shoots of Bacopa monnieri. (L.) Wettst.
Source of the plant materials and surface sterilization
This project was conducted at Marine Science Center, Basrah University, Basrah, Iraq during the period of 2015 to 2016. A plant was collected from different parts of Shatt- Al-Arab coast. Young and healthy shoots were selected as a material for in vitro culture use. Shoots were treated with 10% (v/v) liquid detergent solution for 5 to 10 min, followed by rinsing under running tap water for 15 to 20 min. Shoot tips of 1 to 2 cm were detached from the young shoots and immersed directly in antioxidant solution consisting of 100 mg/L ascorbic acid and 150 mg/L citric acid until the time of sterilization. The clean shoot tip explants were treated with 20% (v/v) bleach solution containing two drops of Tween-20 per 100 ml as a surfactant for 10 min under aseptic conditions (Laminar flow air cabinet). Shoots were rinsed three times with sterile distilled water to remove the traces of bleach solution.
In vitro culture establishment
Surface disinfectant apical buds were inoculated onto Murashige and Skoog (MS) medium (Murashige and Skoog, 1962) having 3% sucrose and supplemented with 0.5 mg/L benzyl adenine (BA) + 0.5 mg/L kinetin (AL-Aradi et al., 2016), and MS vitamins. pH of the medium was adjusted to 5.8. Then, 0.6% agar was added. The medium was then autoclaved at 121ºC for 20 min. In vitro generated healthy shoots were maintained by regular subculturing of propagules of four shoots each after every 8 weeks of culture. Shoots explants obtained from the in vitro cultures were used as inoculum and subjected to different abiotic stress treatments.
In vitro abiotic stress treatments
To determine the abiotic stress tolerance of medicinal plant B. monnirri by examining morphological and histological changes following water stress, the multiplied shoots were transferred to same proliferating MS (liquid and solid) medium containing different abiotic stress as follow: NaCl (0.8 g/L) and NaCl 0.8 g/L with 26 g/L polyethyleneglycol or 50 mg/L proline. Cultures were grown in a growth chamber at 27 ± 2°C with photoperiod of 16 h day/dark of 10 μmol m-2s-1.
To study the effect of abiotic stress on root development, multiplied shoot mass was transferred to hormone free MS (solid and liquid) medium fortified with different abiotic stress as follow: NaCl (0.8 g/L) and NaCl 0.8 g/L with 26 g/L polyethyleneglycol or 50 mg/L proline medium. Cultures were grown in a growth chamber at 27 ± 2°C with photoperiod 16 h day/dark of 10 μmol m-2 s-1.
Regeneration potential development
Shoots and plantlets from all stressed media were transferred to medium free of abiotic stress agents for shoot and root regeneration. Cultures were maintained at temperature 27 ± 2°C and 16 h photoperiod of 10 μmol m-2 s-1.
Data recording and statistical analysis
Experiments for shoots multiplication and rooting stress under normal and abiotic stress condition in vitro were repeated thrice. For each treatment, five tests tubes or jars were used. The data with respect to shoot and root numbers were recorded for each treatment after one and two months. Same date recording used for shoot and root regeneration after stress removed. All data were subjected to Analysis of Variance (ANOVA) for a completely randomized design (CRD). The differences among the treatment means were tested by Duncan’s new multiple range test (DMRT) (Gomez and Gomez, 1984).
Microscopic study
A comparative histological study was conducted on the shoot for different treatments mentioned above in addition to control. Specimens were killed and fixed in (formalin: acetic acid: alcohol 90:5:5) (FAA) for 24 h, then dehydrated in ethanol series using 50, 70, 80, 90% and absolute 100% concentrations (Johanson, 1940). Specimens were then embedded in paraffin (solidification point about 57 to 60°C) and sectioned to thickness 10-micron sections. The sections were double-stained with safranin and fast green. Sections cleared with xylene and mounted in Canada balsam (Yilun et al., 1992). Slides were microscopy examined.
Bud induction and shoot proliferation
It was observed that the addition of cytokinins to MS medium was essential for bud development and proliferation. Apical bud grown on hormone free medium showed less response and bud later died (data not shown in this paper). Proliferated shoots were transferred to same fresh medium after every eight weeks. Similar results were obtained by Binita et al. (2005), Sharma et al. (2010), Sundriyal et al. (2013) and Jain et al. (2014).
Table 1 shows that the highest shoot numbers were obtained on liquid MS medium supplemented with 0.5 mg/L BA + 0.5 mg/L kinetin and free of salinity and drought agents after one and two months; 33.33 and 42.2 respectively (Figure 1A and B). These results are in line with those of Tiwari et al. (2000), who proposed an efficient and rapid method for B. monnieri by using a liquid medium. Moreover, the MS medium fortified with 0.5 mg/L BA + 0.5 mg/L kinetin liquid or solid medium showed no rosette clump; so it has been used for this study. In vitro shoots explants from control cultures were used as inoculums and subjected to salinity (NaCl) and drought (PEG) stress.
In vitro stress treatments
Concerning the number of shoots, the results illustrated in Table 1 shows that maximum shoot numbers rate (24 culture) were produced on MS (solid) medium supplemented with 0.8 g/L NaCl after two months. The most harmful effects of multiplied shoots were recorded on MS solid medium containing 0.8 g/L NaCl +26 g/L PEG, where, the less average shoot numbers (5 shoot/ culture) were recorded in this medium after two months respectively (Table 1). Results also recorded some yellowing on multiplied shoots grown on MS media containing stress agents and no necrosis was observed (Figure 2A and C). But, in spite of a long-term exposure to salinity and drought stress for two months, shoot cultures were still viable. These findings indicated that B. monnirri can tolerate both NaCl and other water stress agents (Figure 2A to C). Table 1 also shows that cultures resumed their growth when stress relieved, and the shoots number increased distinctly compared with cultures subjected to stress. An identical observation was reported in same species B. monnirri, where plants are able to tolerate water stress for 45 days (Debnath, 2008).

Growth reduction in tissues grown in stressful media in vitro is the usual phenomenon, but stress metabolism resistance is really interrupted (Misra et al., 1997, Debnath, 2008). So, the response of shoots multiplication or roots formation in salinity and drought stress were different (Table 1).
NaCl stress involves both osmotic and ionic stress agents but the PEG is a high molecular weight osmotic agents. However, salinity and drought-induced production of active oxygen species (AOS) often cause oxidative stress (Sgherri et al., 2000; Khan and Panda, 2008). However, plants have a specific protective mechanism in various degree for defending themselves against activated oxygen (Sudhakar et al., 2001). In the present study, a significant statistically difference between shoots numbers of control and others of water stress treatments were recorded (Table 1 and Figure 2C) but, all the stressed plants showed the non-significant decrease in shoot numbers after one and two months. In the presence of both salinity and drought agent (PEG) in a liquid medium, a less shoots number was obtained; 3 and 5 shoots after 1 to 2 months because the (PEG) restrict the water translocation through plant inhibiting leaf expansion and plant growth (Yeo and Flowers, 1994). This restriction may affect shoot growth and proliferation by reducing the media uptake by shoot explants.
Concerning root growth and development, the impact of different abiotic stress represented in Table 1 shows that the best root numbers were obtained on MS solid and liquid medium free of hormone and abiotic stress agents after one and two months were 18, 24, 26.67 and 25, respectively. The result also revealed that addition of salinity agent (NaCl, 0.8 g/L) to the medium did not decrease the root numbers. Instead, a high root number (21.67 and 18) was recorded from shoots grown on MS (solid and liquid) medium containing salinity agent (NaCl, 0.8 g/L) after two months. However, on the rooting medium, the multiplied shoot on various stress media showed no significant increase in shoot growth after 1 to 2 months, and instead of that, it morphologically shifted from shoot proliferation to root formation (Table 1). This result is in accordance with that of Debnath (2008) who observed same phenomena on the B. monnieri hyssop cultured in vitro under stress agents while, other researchers (Ehsanpour and Amini, 2003; Misra et al., 1990) suggested that increasing the root length and branching is one way of tolerance mechanism against drought and salinity stress.
Results also revealed that combined PEG to the culture media (solid and liquid) decreased the roots numbers to 10 and 7.67 respectively. This finding is in agreement with those of Said et al. (2015) who found that root growth parameters markedly decreased in Banana plantlet cultured in vitro when PEG was included in the media. Results also shows that adding the proline to the MS medium containing salinity agent alone or in combination with drought agent (PEG) did not improve the shoot multiplication or root development (Table 1).
After stress, relieved numbers of roots showed insignificance difference between control and other stress treatments (Table 1).
Histological study
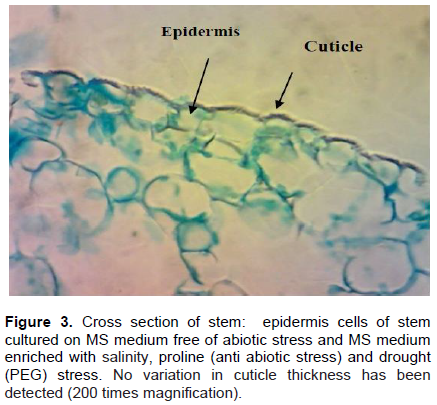
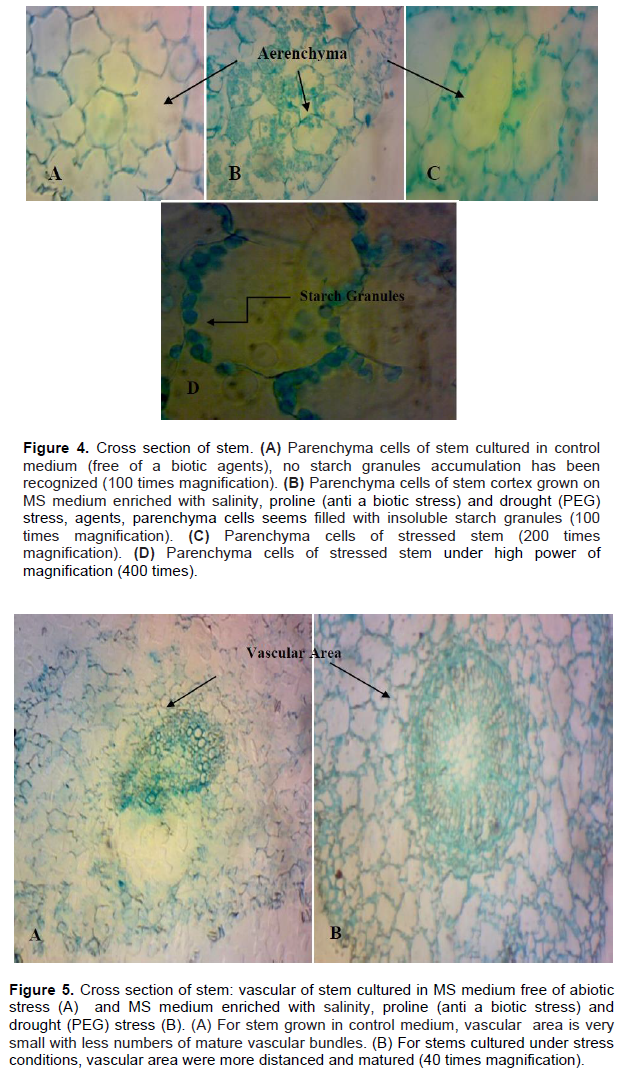
Anatomical studies of plants under stress are very important because they show adaptive features of plants under these conditions (Younis et al., 2013). Histological study of B. monnieri stem subjected to various stress treatments was conducted to describe its response to different abiotic stress in vitro. It was noticed that the epidermis of stems cultured in various salinity and drought stress agents, as well as control media, consist of one layer with thin- walled cells, no variation in cuticle thickness has been recognized (Figure 3). Stems grown in different abiotic stress agents’ media showed high insoluble starch granules accumulation, in parenchyma cells of the cortex (parenchyma) (Figure 4B, C and D); whereas, no starch granules were recognized in stem grown in control medium (stress-free medium) (Figure 4A). The vascular area (phloem and xylem) of stems subjected to various abiotic stresses is wide, distinct and contained high numbers of vascular bundles in compared with control (Figure 5B). For the control, the vascular area is very small; fewer numbers of mature vascular bundles was detected (Figure 5A). So, anatomical results indicated that starch accumulation, as well as wide vascular area, are a possible productive tolerate mechanism use for salinity or drought stress in glycophytic hyssop B. monnieri. However, no phenolics or damaged parenchyma cells have been recognized in all sections stems subjected to drought or salinity stress treatments. These findings are paralleled to those of Theerawitaya et al. (2015) study, who demonstrated that the accumulation of soluble and insoluble starch granules was detected in rice seedling cultivars IR29 and Pokkalid, depending on the salts stress exposure times and plant organs.
Salinity and drought stress are the most important environmental factors that restrict glycophytic plant growth. In this study we conclude that the deleterious effect on shoot multiplication and root development comes when PEG-containing medium was used. No adverse effects were recognized on shoot or plantlets grown on MS medium containing 0.8 g/L NaCl. Histological study revealed that the shoots grown under abiotic stress had a mature vascular area compared with the control. These preliminary results open the door to use a low salinity agent (0.8 g/L NaCl) instead of PEG and paclobutrazole to promote in vitro hardening.
The authors have not declared any conflict of interests.
REFERENCES
|
Al-Aradi HJ, Ansam MS, Khaun AM (2016). Propagation of water hyssop Bacopa monnieri L. in vitro, Iraqi J. Aquacul, (In press).
|
|
|
|
Awasthi P, Himani K, Bargali VK, Bargali SS (2016). Germination and Seedling Growth of Pulse Crop (Vigna Spp.) as affected by soil salt stress. Curr. Agri. Res. 4(2):159-170.
Crossref
|
|
|
|
|
Bammidi SR, Volluri SS, Chippada SC, Avanigadda S, Vangalapati MA (2011). Review on pharmacological studies of Bacopa monniera. J. Chem. Biol. Phy. Sci.1(2):250-259.
|
|
|
|
|
Banerjee M, Modi P (2010). Micropropagation of Bacopa monnieri using cyanobacterial liquid medium. Plant Tiss. Cult. Biotechnol. 20(2): 225-231.
|
|
|
|
|
Bargali K, Bargali SS (2016). Germination capacity of seeds of leguminous plants under water deficit conditions: implication for restoration of degraded lands in Kumaun Himalaya. Trop. Ecol. 57(3):445-453.
|
|
|
|
|
Binita BC, Ashok MD, Yogesh TJ (2005). Bacopa monnieri (L.) Pennell: A rapid, efficient and cost effective micropropagation. Plant Tiss. Cult. Biotechnol. 15(2):167-175.
|
|
|
|
|
Charoenphon N, Anandsongvit1 N, Kosai P, Sirisidthi K, Kangwanrangsan N, Jiraungkoorskul W (2016). Brahmi (Bacopa monnieri): Up-to-date of memory boosting medicinal plant: A review. Indian J. Agric. Res., 50 (1):1-7.
Crossref
|
|
|
|
|
Debnath M (2008). Responses of Bacopa monnieri to salinity and drought stress in vitro. J. Med. Plan. Res. 2(11):347-351.
|
|
|
|
|
Ehsanpour AA, Amini F (2003). Effect of salt and drought stress on acid phosphatase activities in alfalfa (Medicago sativa L.) explants under in vitro culture. Afr. J. Biotechnol. 2:133-135.
Crossref
|
|
|
|
|
Gomez KA, Gomez AA (1984). Statistical procedure for agricultural research, A Wiley Interscience Publication, New York, USA. Pp. 680.
|
|
|
|
|
Jain A, Pandey K, Benjamin D, Meena AK, Singh RK (2014). In vitro approach of medicinal herb: Bacopa monnieri. Int. J. Innov. Res. Sci. Eng. Technol. 3(5):12088-12093.
|
|
|
|
|
Johanson DA (1940). Plant Microtechnique. McGraw-Hill Book company Inc. London, P 523.
|
|
|
|
|
Khan MH, Panda SK (2008). Alterations in root lipid peroxidation and antioxidative responses in two rice cultivars under NaCl-salinity stress. Acta Physiol. Plant. 30: 81-89.
Crossref
|
|
|
|
|
Liu CZ, Cheng XY (2008). Enhancement of phenylethanoid glycosides biosynthesis in cell cultures of Cistanche deserticola by osmotic stress. Plant Cell Rep. 27(2):357-62.
Crossref
|
|
|
|
|
Misra AN, Misra M, Das N (1990). Plant responses to salinity: Metabolic changes and the use of plant tissue culture - a perspective. In: Environmental Concern and Tissue Injury, Part-I (Prakash R and Choubey S M, eds.), Jagmandir Books, New Delhi, Pp. 77-84.
|
|
|
|
|
Misra AN, Sahu S. Misra M, Mohapatra P, Merra I, Das N (1997). Sodium chloride induced changes in leaf growth, and pigment and protein contents in two rice cultivars. Biol. Plant. 39:257-262.
Crossref
|
|
|
|
|
Naik, PM, Al-Khayri JM (2015). Impact of Abiotic Elicitors on In vitro Production of Plant Secondary Metabolites: A Review. J. Adv. Res. Biotech. 1(2):1-7.
|
|
|
|
|
Said EM, Mahmoud RA, AlAkshar R, Safwat G, (2015). Drought Stress Tolerance and Enhancement of Banana Plantlets In Vitro. Austin. J. Biotechnol. Bioeng. 2 (2): 1-7.
|
|
|
|
|
Sgherri C, Maffel, M, Navari-Izzo F (2000). Antioxidative enzymes in wheat subjected to increasing water deficit and re-watering. J. Plant Physiol. 157:273-279.
Crossref
|
|
|
|
|
Shahi C, Vibhuti, Kiran B, Bargali SS (2015). Influence of seed size and salt stress on seed germination and seedling growth of wheat (Triticum aestivum L.). Indian J. Agri. Sci. 85(9):1134-1137.
|
|
|
|
|
Sharma S, Kamal B, Rathi N, Chauhan S, Jadon V, Vats N, Gehlot A, Arya S (2010). In vitro rapid and mass multiplication of highly valuable medicinal plant Bacopa monnieri (L.) Wettst. Afr. J. Biotechnol. 9(49):8318-8322.
|
|
|
|
|
Sudhakar C, Lakshmi A, Giridarakumar S (2001). Changes in the antioxidant enzyme efficacy in two high yielding genotypes of mulberry (Morus alba L.) under NaCl salinity. Plant Sci. 161:613–619.
Crossref
|
|
|
|
|
Sundriyal A, Rawat DS, Singh, AK (2013). Tissue Culture, Phytochemical & Pharmacological Study of Bacopa monnieri. Asian J. Biochem. Pharm. Res. 3(1):243-260.
|
|
|
|
|
Tiwari V, Tiwari KN, Singh BD (2000). Comparative studies of cytokinin on in vitro propagation of Bacopa monnieri. Plant Cel. Tissue Organ. Cult. 66(1):9-16.
Crossref
|
|
|
|
|
Theerawitaya C, Yamada N, Samphumphuang T, Cha-um S, Kirdmanee C, Takabe T (2015). Evaluation of Na+ enrichment and expression of some carbohydrate related genes in indica rice seedlings under salt stress. P.J. Omics 8(2):130-140.
|
|
|
|
|
Vibhuti, Shahi C, Bargali K, Bargali SS (2015). Seed germination and seedling growth parameters of rice (Oryza sativa L.) varieties as affected by salt and water stress. Indian J. Agric. Sci. 85(1):102-108.
|
|
|
|
|
Yao A, Flowers T (1994). Non-osmotic effect of polyethylene glycols upon sodium transport and sodium potassium selectivity by rice roots. Plant Physiol. 75:298-303.
Crossref
|
|
|
|
|
Yilun M, Sawhneym VK, Steeves TA (1992). Staining of paraffin embedded plant material in safranin and fastgreen without prior removal of the paraffin. Can. J. Bot. 71:996-999.
|
|
|
|
|
Younis A, Riaz A, Ikram S, Nawaz T, Hameed M (2013). Salinity-induced structural and functional changes in 3 cultivars of Alternanthera bettzickiana (Regel) G. Nichloson. Turk. J. Agric. For. 37:674-687.
Crossref
|
|