ABSTRACT
A glasshouse experiment was conducted to compare the performance of a stay-green QTL introgression line (RSG 03123) and its parental lines (B35, a stay-green and R16, a high yielding cultivar with rapid senescence) after flowering, when water-limited (WL) or well-watered (WW). Flowering time and plant height were similar in RSG 03123 and the recurrent parent, R16. Under WW conditions, retention of green leaf area (GLA) and chlorophyll was similar in RSG 03123 and R16, whereas under WL conditions RSG 03123 retained significantly (P<0.05) more GLA. The rate of senescence was also lower in RSG 03123 as compared to R16 under WL conditions. B35 displayed a delayed onset of senescence under both WW and WL conditions while both R16 and RSG 01323 displayed an earlier onset of senescence under WL conditions. Photochemical efficiency of photosystem II (ΦPSII), gas exchange characteristics, and total soluble carbohydrates contents, in both leaf and stem, were higher in RSG 03123 than in R16 under WL conditions. These parameters were enhanced in RSG 03123 due to higher retention of chloroplast proteins, such as light-harvesting chlorophyll binding protein complex of PSII (LHCPII), oxygen-evolving complex 33 kDa (OEC33), phosphoenolpyruvate carboxylase (PEPC) and the large subunit of ribulose bisphosphate carboxylase-oxygenase (Rubisco). The stay-green characteristics of RSG 03123 were intermediate between R16 and B35; nonetheless, the results show that stay-green QTL are functional during senescence and improve tolerance to water limitation after flowering.
Key words: Stay-green QTL, introgression, drought, photosynthesis.
Feeding the increasing population is a challenge to all nations (Foley et al., 2011), especially those in the drought-prone agro-ecological regions. Sorghum is an important crop grown for food, feed and fibre as well as fuel (Paterson et al., 2009a, b; Boyles et al., 2019) and is able to withstand drought conditions and give a good yield (Borrell et al., 2014a; Lasky et al., 2015). However, post-flowering drought stress results in rapid senescence and premature death, stem collapse, lodging and rot which results in reduced grain yield in senescent cultivars (Xu et al., 2000b; Badigannavar et al., 2018).
Stay-green (SG) genotypes, on the other hand, do not display this drought-induced senescence and retain their green leaves longer resulting in improved grain yield under stress (Rosenow, 1994; Duvick et al., 2004; Jagadish et al., 2015; Badigannavar et al., 2018). Senescence is characterised by chlorophyll loss and a progressive decline in photosynthesis. Hence, early onset of senescence, consequently, affects assimilation and grain-filling (Xu et al., 2000b). Stay-green genotypes are either functional or cosmetic (Thomas and Howarth, 2000). Functional stay-green genotypes possess an increased duration of both green leaf area and of photosynthesis, whereas cosmetic stay-greens remain green due to defective chlorophyll breakdown but photosynthetic competence is lost (Thomas and Howarth, 2000).
Therefore, stay green can be viewed at a cell (Johnson et al., 2015), leaf, whole plant, crop and system level (Borrel et al., 2014a, b; Blümmel et al., 2015; Badigannavar et al., 2018). Sorghum genotypes possessing the SG trait retain green stems and upper leaves for longer periods than those not possessing the trait; thus, they are able to fill their grain normally under post-flowering drought conditions (Subudhi et al., 2000; Tao et al., 2000; Borrell et al., 2001). Since SG leaves are maintained after physiological maturity (Sanchez et al., 2002), the need for translocation of photosynthates from the stem during grain filling is reduced (Van Oosterom et al., 1996). Therefore, grain yield in sorghum under post-flowering drought stress correlates positively with green leaf area at mid-grain-filling (Borrell et al., 1999) and green leaf area at maturity (Borrell et al., 2000). However, some SG genotypes efficiently remobilize assimilates during grain filling, which results in maintenance of grain weight, quality and nutrient efficiency (Jagadish et al., 2015).
The longevity of leaves in the stay-green sorghum might be promoted by a combination of several biochemical factors, which interact to regulate nitrogen (N) remobilisation (Borrell and Hammer, 2000) and chlorophyll turnover, maintain the integrity of the photosynthetic apparatus as well as enzyme activity (Oh et al., 2003), particularly those involved in carbon and N assimilation (Hortensteiner and Feller, 2002).
Both grain and stover yields are dependent on photosynthesis, which, in turn, is influenced by green leaf area (GLA) and green leaf area duration (GLAD), especially under drought stress. Photosynthesis is also affected by stomatal conductance (gs), nutrient availability and enzyme activity. The efficient use of water for carbon assimilation, which is affected again by gs, transpiration and the capacity for CO2 assimilation, might be related to yield maintenance and drought resistance. Hence, analyses of GLA, GLAD, leaf chlorophyll retention rate, leaf gas exchange and their effects on grain yield would evaluate the functioning of the SG QTL in maintaining yield under drought stress. Such findings, in the long-term, would enhance the selection and breeding for improved genotypes.
Retention of chloroplast proteins are maintained in sorghum containing the KS19 source of SG (de Villiers et al., 1993), which explains, in part, the ability of these plants to maintain photosynthesis for longer periods than their senescent counterparts. However, these observations were made under non-drought stress conditions, whereas in sorghum the trait is fully apparent under severe drought stress. Therefore, investigating changes in chloroplast proteins and leaf N under both well-watered and water-limiting conditions should enhance understanding of the biochemical basis of the trait. In addition, assessment of changes in the carbohydrate metabolism in the leaf and stem, in SG genotypes, would enhance our understanding of the different mechanisms by which yield is maintained under drought stress.
B35 is a dwarf stay-green BC1 selection from IS12555
durra sorghum, a land race from Ethiopia (Rosenow et al., 1983) and has been released as BTx642 (Rosenow et al., 2002). B35 is the stay-green parent used in several quantitative loci (QTL) mapping studies of drought tolerance in sorghum (Walulu et al., 1994; Tuinstra et al., 1996, 1997, 1998; Crasta


et al., 1999; Xu et al., 2000b; Tao et al., 2000; Subudhi et al., 2000; Sanchez et al., 2002). R16 is a released variety that is high yielding but highly senescent under post-flowering drought stress conditions (Blümmel et al., 2015). Four major QTL associated with stay-green (SG), namely:
Stg1 on chromosome SBI-03 (LG 03)
, Stg2 on chromosome SBI-03 (LG 03),
Stg3 on chromosome SBI-01 (LG 01) and
Stg4 on chromosome SBI-05 (LG05) have been identified to be consistent in a range of genetic backgrounds and environments. These QTL account for up to 53.5% of the phenotypic variance and co-locate with the QTL for chlorophyll content at physiological maturity
(Xu et al., 2000b; Sanchez et al., 2002).
Stg2 was identified as the most important in contributing to the SG phenotype, followed by
Stg1,
Stg3 and
Stg4 (Xu et al., 2000b).
The SG trait has been used extensively in the breeding of sorghum for regions where post-flowering drought is prevalent because Sg genotypes are tolerant to both drought and heat (Pinto et al., 2016). A number of breeding programmes are using marker-assisted selection to incorporate these SG QTL in advanced breeding lines (Hash et al., 2003; Harris et al., 2007; Kassahun et al., 2010). Individual QTL have been assessed and found to influence canopy development and, consequently, crop water use efficiency and grain yield (Borrell et al., 2014a). However, some SG QTL (e.g., StgB) have been found not to have any concomitant improvement in stover and grain yield in R16 background, indicating the functioning of such QTL might be background-dependent (Blümmel et al., 2015). Since the genetic background of SG QTL can influence their expression, it is important to examine the functioning of SG QTL in a senescent background in comparison to the parental lines, such as R16 and B35. Findings from such studies should provide the basis for recommending appropriate SG introgression lines for farmers’ use. The objective of this study was to evaluate the physiological functioning of RSG 03123, a marker-assisted backcross (MABC) derived stay-green QTL introgression line along with the donor parent (B35) and recurrent parent (R16) under well-watered and water-limiting conditions. Specifically, green leaf area retention, chlorophyll fluorescence, photosynthesis, carbohydrate, nitrogen and protein metabolism were evaluated.
Plant culture and treatment
Seeds of B35, R16 and RSG 03123 were obtained from ICRISAT, India. RSG 03123 is a BC1F5 selection developed after marker-assisted backcrossing using markers flanking Stg1, Stg2, Stg3 and Stg4 from B35 as described in Kassahun et al. (2010). The recurrent parent, R16, is a high-yielding cultivar from Maharashtra, India, which has a very rapid rate of leaf senescence (Van Oosterom et al., 1996). Confirmation of the genotype of RSG 03123 is described in Galyuon et al. (2016). This indicated that Stg1, Stg3 and Stg4 had been successfully transferred from B35 but that Stg2 was not present in RSG 03123.
Twenty (20) plants per genotype were raised in plastic pots with perforated bottoms filled with a mixture of black soil, peat, grit and perlite in a ratio of 3:3:3:1, respectively. They were grown in a glasshouse in Aberystwyth with daily maximum and minimum temperature of 35°C (day) and 18°C (night). Plants were exposed to a 12-h supplementary light to simulate the conditions in sorghum growing areas in Africa and Asia.
At flowering, when at least 50% of the plants for each genotype had visible anthers at the top section of the panicle, half of the plants remained well-watered (WW), by adding 1000 ml of water daily. The other 50% were exposed to water-limited (WL) conditions by supplying them with 250 ml of water daily. For the stay-green trait to be expressed sufficiently to be used for selection, a prolonged drought period is required during grain-filling enough to accelerate senescence but not sufficient to cause premature death of plants (Mahalakshmi and Bidinger, 2002). Preliminary studies indicated that the drought treatment used in this study enabled the trait to be expressed and prevented premature plant death.
Measurement of plant height
Plant height was measured at flowering from the soil surface to the collar of the flag leaf, and, at physiological maturity, it wasmeasured from the soil surface to the tip of the panicle.
Determination of green leaf area (GLA)
Green leaf area (GLA) per plant was determined weekly as described by Wolfe et al. (1988). The length of each leaf (from the collar to the tip) along the midrib was measured and the width determined at the middle, the widest part of ensiform leaves (Doggett, 1988). At flowering, leaves were harvested from four plants of each cultivar and leaf area per plant measured using a Delta-T Area Meter (MK II). The linear relationship between the product of the length and width and the size measured using the area meter was determined. The relationship between the product of the length × width and the size measured using the area meter from 180 leaves was determined as y = 0.95 + 0.75x with an R2 = 94.7% (P<0.001), where y is the area measured using the area meter and x the product of the measured length and width for each leaf. This relationship was then used to determine the area for each leaf. The area of each leaf was corrected for senescence by subtracting the area of the lamina lost to senescence defined by visible yellowing using a percent score visually.
Determination of chlorophyll levels
A chlorophyll meter (Minolta Chlorophyll Meter SPAD-502, Minolta Camera Co., Ltd., Japan) was used to measure leaf chlorophyll levels weekly beginning at flowering. Each leaf was divided into three sections, base, middle and top. Within each section, six readings were taken, three on either side of the midrib and averaged.
Measurement of leaf gas exchange
Gas exchange was measured weekly beginning at flowering on the middle section of the fourth leaf, the flag leaf. CO2 assimilation rates (A), leaf conductance (gL) and transpiration rates (E) were measured on 5.6 cm2 of leaf lamina using an open portable gas exchange system (CIRAS-1, PP Systems, Hitchin Herts, UK). Cuvettes were maintained at 30°C, with an ambient CO2 concentration of 350 µL.L-1 and exposed to photosynthetically active radiation (PAR) of 1100 µmol m-2 s-1 from a LED lamp fitted and connected to light meter. Measurements were taken between 8:00 and 13.00 h. Photosynthetic water use efficiency (WUEL) was calculated by dividing CO2 assimilation rate (A) by the transpiration rate (E) for each leaf (that is, A/E).
Measurement of chlorophyll fluorescence
Chlorophyll fluorescence was measured weekly on the same leaves used for gas exchange. An EARS Plant Photosynthetic Measurement (PPM) System (EARS Earth Environment Monitoring B.V. Kanaalweg 1, 2628EB Delft, The Netherlands) was used to measure the quantum yield of photosynthetic electron transport of photosystem II (ΦPSII) as described by Maxwell and Johnson (2000) in light-adapted leaves. Three readings were taken between the collar and tip of the leaf at equal spacing and averaged.
Analysis of basal soluble carbohydrates (SC)
At flowering and physiological maturity, samples were taken from the middle section of the leaf, and fresh weight, length and width were recorded. Stem samples were taken from the fourth internode above the soil surface. Sugars were extracted using 80% ethanol according to a method described by Cairns et al. (2002). Total SC (TSC) extracts for both leaf and stem samples were freeze-dried for 48 h, re-dissolved in 500 µl sorbitol (40 µg/µl; internal standard) sucrose, glucose and fructose contents in the samples were analysed using an HPLC system with an Autosampler (DIONEX ASI-100), a carbohydrate analysis column [Bio-Rad, Aminex HPX87C column (125-0095)], a guard column (Bio-Rad, MicroGuard Carbo-C) and an Alltech (UK) Rheodyne Filter (3 mm). The system was run by DIONEX Chromeleon software (version 6.10) at a flow rate of 0.6 ml/min for 25 min for each sample using membrane-filtered [Nitrocellulose (0.42 µm), Whatman Filters] distilled water (82°C) as eluant.
Analysis of leaf nitrogen (N) content
Leaf samples were taken from the top four leaves of each plant, at flowering and physiological maturity, and ground into fine powder using a Retsch MM300 Mixer mill. The percentage of N in a known weight of the ground sample was determined by mass spectrometry. The amount of N (µg) per mg of dry weight (DW) of leaf sample was calculated along with the specific leaf nitrogen which is the quantity of N per unit leaf area.
Protein extraction, analysis of chloroplast proteins and photosynthetic enzymes
Total protein was extracted as described by Mae et al. (1993) at flowering and physiological maturity. Tissue was homogenised with ice-cold buffer (50 mM lithium phosphate pH 7.2 containing 120 mM 2-mercaptoethanol, 1 mM sodium monoiodoacetate, 1 mM phenylmethylsulfonyl-fluoride and 5% v/v glycerol) in the proportion of 5 ml g-1 fresh weight using a pestle and mortar. Lithium dodecyl sulphate was added to a final concentration of 2% (w/v) and the mixture incubated for 5 min at 100°C. The extract was centrifuged for 10 min at 13000 g and aliquots of the supernatant were stored at -80°C until analysed by electrophoresis.
One-dimensional sodium dodecyl-sulphate polyacrylamide gel electrophoresis (SDS-PAGE) was performed according to the method of Laemmli (1970) using a 12.5% running gel in a Bio-Rad Mini-protean system. Gels were loaded with equal volumes of homogenate, equivalent to equal fresh weights of tissue. Protein standards ranging from 113 to 20.9 kDa (Bio-Rad) were run alongside the samples on each gel.
Western analysis was conducted following the electrotransfer of proteins onto nitrocellulose paper and specific proteins detected using antibodies. The blots were then developed chemiluminescently using BM Chemiluminescence Blotting Substrate (POD) (Roche Applied Science, Germany) and visualised on x-ray film. The following publications give the sources of antibodies used in this study: Hilditch et al. (1989), Bachmann et al. (1994) and Borland et al. (1998).
Data analysis
The data were checked by calculating the means and standard errors and then subjected to analysis of variance (ANOVA) using MINITAB (Release 13) statistical software. Data for all cultivars were combined and analysed to find out if there were any interactions between genotypes, drought stress and duration of drought stress. Further, differences between means of treatment combinations were analysed for each sampling date. Differences between means were separated by Fisher’s least significance difference (LSD) at 5% level of significance.
Plant height and flowering
RSG 03123 plants were 198.1 and 192.8 cm tall at physiological maturity under WW and WL conditions, respectively. Similarly, R16 plants were 172.4 and 178.6 cm tall, while B35 pants were 118.8 and 92.4 cm tall at physiological maturity under WW and WL conditions. Within genotypes there were no significant (P>0.05) differences between the WW and WL plants. The differences between RSG 03123 and R16 plants were also not significant (P>0.05). However, compared to B35, both RSG 03123 and R16 were significantly (P<0.05) taller under WW and WL indicating that the introgression of the SG QTL from B35 did not change plant height. Days to flowering were the same in R16 and RSG (67 days after seedling emergence) whereas flowering was eight days later in B35.
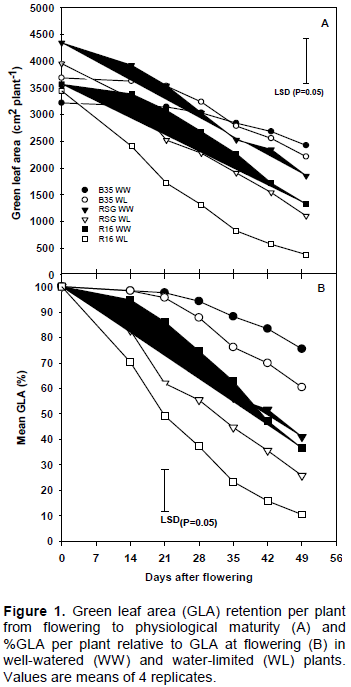
GLA retention
Water limitation reduced GLA compared to the WW plants in all genotypes with the greatest reduction in R16 and the smallest reduction in B35 (Figure 1A). The difference between the WW and WL plants was not significant (P>0.05) for either B35 or RSG 03123. In R16, GLA was significantly (P<0.05) reduced in the WL plants compared to the WW from 14 DAF. GLA retention (GLA as percent of the total leaf area at flowering per plant or %GLA) was also lower under WL conditions. B35 plants had the highest %GLA under both WW and WL conditions throughout the experiment (Figure 1B). The stay-green trait was clearly expressed in B35 with GLA declining after 21 DAF although the rate of loss was slightly greater in WL plants. By physiological maturity GLA in the WL B35 plants was 91% of that in the WW. The WW plants of R16 and RSG retained similar GLA. However, under WL conditions, retention of GLA was significantly (P<0.05) reduced in the R16 plants compared with the WW. At physiological maturity, the WL R16 plants retained 29% GLA compared to the WW. In the RSG plants, even though %GLA was lower under WL conditions, the differences were not significant (P>0.05) compared with the WW and at physiological maturity GLA in the WL was 60% of that in the WW.
Differences were also apparent in the onset and rate of senescence both between genotypes and watering regimes. A split linear regression was conducted on the %GLA data and from this the duration to 95% GLA (onset of senescence) and the rate of senescence were calculated. Under WW conditions, days to 95% GLA were 29.60, 9.50 and 6.33, respectively, in B35, R16 and RSG 03123 indicating that for both R16 and RSG, onset of senescence was much earlier than in B35. The values for the WL plants were 27.10 for B35, 2.47 for R16 and 3.99 for RSG 03123, indicating that in all 3 genotypes WL conditions resulted in an earlier onset of senescence. The onset of senescence was similar in RSG and R16. The rates of senescence per day were 0.87, 1.71 and 1.39 for B35, R16 and RSG 03123, respectively, under WW conditions, while under WL conditions the corresponding values were 1.26, 2.22 and 1.51. Thus, once senescence had started, the rate of senescence was greater in all three genotypes under WL conditions but the rate of leaf loss was slower in RSG 03123 than in R16.
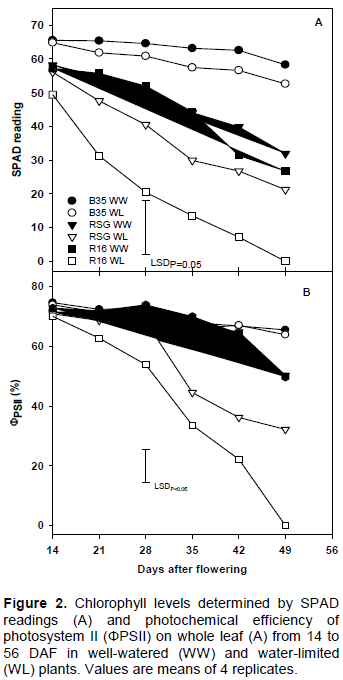
Chlorophyll retention
Chlorophyll retention in the fourth leaf from the top, as measured by SPAD, was highest in B35 followed by RSG 03123 and the lowest in R16 under WL conditions (Figure 2A). Chlorophyll levels were significantly (P<0.05) higher in B35 compared with RSG 03123 or R16 under both WW and WL conditions. RSG 03123 also had significantly (P<0.05) higher chlorophyll levels than R16 from 21 DAF under stress conditions, whereas there were no differences between RSG 03123 and R16 when well-watered. In addition, chlorophyll levels in R16 were significantly (P<0.05) reduced by drought stress whereas in both B35 and RSG 03123, the differences between the WW and WL plants were not significant (P>0.05). Under WL conditions, the rate of chlorophyll loss also was more gradual in B35 and RSG 03123 compared to R16.
Quantum efficiency of photosystem II (ΦPSII)
ΦPSII was reduced in the WL plants of R16 and RSG 03123 (Figure 2B). The onset was earlier and magnitude of reduction greater in the WL R16 plants, where ΦPSII continually fell from 14 DAF and the reduction was significant (P<0.05) compared to the WW. In the WL RSG 03123 plants, ΦPSII began reducing from 28 DAF and by 35 DAF the reduction (33%) was significant (P<0.05) compared with the WW plants. In contrast, in B35 there were no differences between the WL and WW plants. ΦPSII in the B35 plants was higher than in either RSG or R16 under WL conditions. Under WW conditions, however, there were no differences between genotypes, except at physiological maturity when ΦPSII was significantly (P<0.05) higher in B35. Hence, for both absolute values and in comparison with the WW plants, ΦPSII in B35 was the highest under both WW and WL conditions, RSG 03123 was intermediate and R16 was worst, particularly, under WL conditions.
Leaf gas exchange
Carbon dioxide (CO2) assimilation rate
CO2 assimilation rate (A) was reduced in all WL plants and the lowest in R16 (Figure 3A). Cultivar × treatment, and cultivar differences were significant (P<0.05). Overall, A in the WL R16 plants was reduced by 70% compared to the WW, whereas in the WL RSG 03123 and B35 plants the reductions in A were 32% and 19%, respectively. A was significantly (P<0.05) reduced in the WL R16 plants compared to the WW from 14 to 49 DAF. Indeed by 42 DAF A in R16 was reduced to zero. The reduction of A in the WL plants of both RSG 03123 and B35 was not significant (P>0.05) compared to their respective WW ones. For R16 A was significantly (P<0.05) reduced in the WL plants compared to the WW. Among the WW plants, A was similar, except at 14 and 49 DAF. The higher A in both B35 and RSG 03123 plants is consistent with the higher ΦPSII observed in these plants than in R16 under WL conditions. This confirms that under WL conditions photosynthesis was improved in the RSG 03123 plants as compared to R16.
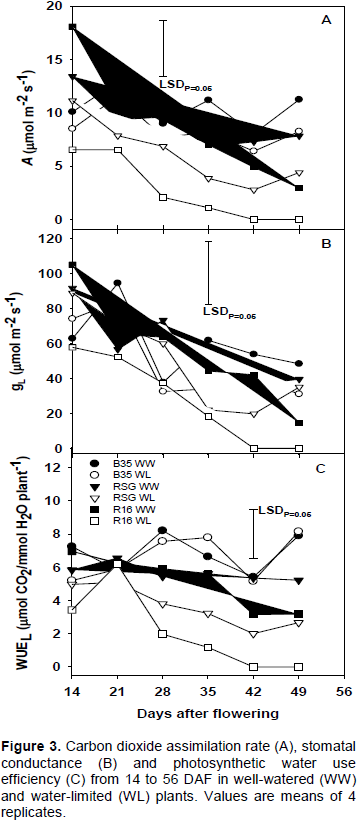
Leaf conductance (gL)
Overall, gL was reduced by 50, 14 and 15% in the WL plants of R16, RSG 03123 and B35 compared with the WW. In the WL R16 plants gL continually declined from 14 to 42 DAF when it became zero (Figure 3B). In RSG 03123, the rate of decline was similar to R16, but gL stayed higher and the decline stopped at 35 DAF. For most sampling dates, gL in B35 and RSG 03123 were higher than in R16 under drought stress and this increased conductance could partially be responsible for the higher A in RSG 03123 and B35. However, the differences were not significant (P>0.05) when compared with the WW in all genotypes. Overall differences between genotypes were significant (P<0.05) with the highest gL in B35 followed by RSG 03123 and the lowest in R16. This indicates that small differences in gL can result in large differences in A, particularly under WL conditions.
Transpiration water use efficiency (WUEL)
WUEL, computed as A/E, was reduced by drought stress, except in B35 and the reduction was greatest in the WL R16 plants (Figure 3C). It was significantly (P<0.05) reduced in the WL R16 plants compared to the WW from 28 DAF. For B35, WUELin both WW and WL plants were similar at all sampling dates. Even though WUEL in RSG 03123 was lower in the WL plants than in the WW, the differences were not significant (P>0.05). It was also higher in both RSG 03123 and B35 than in R16 under WL conditions.
These findings also indicate that WUEL was improved in the RSG 03123 plants compared to R16 under WL conditions. Also, for both B35 and RSG 03123, WUEL was high due to a much higher A values over E rather than reduced E caused by reduced gL. In R16, however, reductions in both A and E and, consequently, WUEL followed a reduction in gL. Changes of WUEL as those in the WL RSG 03123 and B35 plants should have beneficial effects for grain yield under drought stress, since water would be economically used and photosynthates made available for grain filling.
Changes in leaf and stem total soluble carbohydrates (TSC)
At flowering and physiological maturity, leaf TSC was highest in B35 followed by RSG 03123 and then R16 under both WW and WL conditions (Figure 4A). At physiological maturity, leaf TSC was reduced by 36% in R16 when grown under WL conditions as compared to WW, whereas in RSG 03123 and B35 the reduction was just 12% and 4%, respectively, between the WW and WL plants.
TSC in the stem increased in all genotypes between flowering and physiological maturity (Figure 4B). This increase was greatest in B35 under both WW and WL conditions. The amounts of TSC in the stem were unaffected by limitation of water supply in both B35 and RSG 03123, whereas in R16 they were reduced by 34% in the WL plants compared to WW.
Specific leaf nitrogen (SLN)
SLN was higher in both RSG 03123 and R16 compared to B35 at flowering (Figure 5). At physiological maturity under both WW and WL conditions, B35 maintained a much higher SLN than both RSG and R16. Under WW conditions, both R16 and RSG had a SLN of 45% at flowering. However, under WL conditions, SLN was greatly reduced in R16 (61% as compared to that in WW conditions), whereas in both B35 and RSG 03123, SLN was 88% of the WW value.
Changes in photosynthetic proteins
For most of the proteins examined, all four replicates had similar detectable amounts in any given genotype at flowering (Figure 6). There were considerable differences between genotypes at physiological maturity. The light-harvesting chlorophyll-binding proteins of photosystem II (LHCPII) did not differ much in the amounts at flowering and physiological maturity in B35 for all 4 leaves in the WW and WL plants. A similar result was found for RSG 03123. In R16, LHCPII abundance was reduced at physiological maturity and the reduction was greater in the WL plants, especially in leaf 4.
The amounts of the 33 kDa oxygen-evolving complex of PSII (OEC33) at flowering were greater in B35 and RSG 03123 than in R16. At physiological maturity the amounts were reduced in all genotypes but the reductions were greater in R16 under either WW or WL conditions. Under WW conditions the amounts of OEC33 were higher in all leaves of RSG 03123 compared to B35, while there were no differences between them under WL conditions. For R16, the reductions were much greater in the WL plants with barely detectable amounts in leaves 3 and 4.
B35 had the highest amounts of phosphoenolpyruvate carboxylase (PEPC) at flowering and physiological maturity followed by RSG 03123. PEPC did not change much between flowering and physiological maturity in B35, except in leaf 4 of the WL plants. At physiological maturity the bands of PEPC in B35 were about two times as large as in RSG 03123 for the same leaves under both WW and WL conditions. Similarly, RSG 03123 plants had about two times the amounts in R16 under WW conditions and much more than R16 under WL conditions. Leaves 3 and 4 of the WL R16 plants had no detectable bands and the intensity of the bands for leaves 1 and 2 was very low compared to those for RSG 03123.
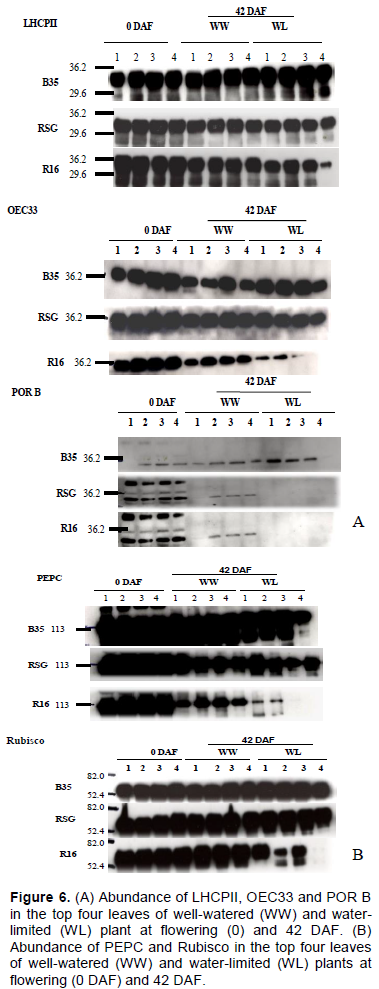
The amounts of the large subunit of Rubisco did not change much between flowering and physiological maturity in both B35 and RSG 03123 in either WW or WL conditions or for any leaf sampled with very little differences between them (Figure 6B). R16 plants had similar amounts of Rubisco as in RSG 03123 at flowering; however, at physiological maturity, the amount in the WL plants of R16 was highly reduced compared to the WW. Indeed, in leaves 3 and 4 of the WL plants of R16 there were no bands for Rubisco. Thus, the introgressed QTL from B35 improved the retention of PEPC and Rubisco also in RSG 03123. This could, partially, explain the maintenance of higher CO2 assimilation rates in B35 and RSG 03123 compared to R16 as stated earlier.
The amounts of protochlorophyllide-oxidoreductase B (POR B) at flowering were similar in R16 and RSG 03123 in all 4 leaves. However, at physiological maturity the level of POR B was reduced in all leaves of RSG 03123 and R16 while it was increased in B35. In B35 the WL plants had the higher amounts, except for leaf 4, whilst in RSG 03123 and R16 the amounts were greater in the WW plants. Since POR B is involved in chlorophyll synthesis, these findings thus confirm the higher chlorophyll levels in B35 and RSG 03123 than R16.
Plant height and flowering
Slight changes in the genetic code, which include simple sequence repeats (SSRs) and single nucleotide polymorphisms (SNPs), are directly linked to phenotypic differences (Barth et al., 2016). It is important that introgression of SG QTL does not have adverse effects on other aspects of the phenotype. The height and flowering time of RSG 03123 plants were not significantly different from the recurrent R16 parent, which is much taller than B35 at physiological maturity. Thus, in regions where stover is as important economically as the grain, introgression of SG QTL from a dwarf genotype, such as B35, could enhance survival in a tall genotype without affecting stover production.
Green leaf area duration and chlorophyll retention
Senescence is an endogenously controlled degenerative biochemical and physiological set of events comprising the final stages of leaf development from the mature fully expanded state to death (Verma et al., 2003). Drought stress during grain-filling hastens leaf senescence leading to premature death (Rosenow and Clark, 1981). However, stay-green sorghum genotypes retain more green leaf area than do genotypes not possessing this trait, and they also continue to fill grain normally under drought conditions (Rosenow et al., 1983; Borrell et al., 2014a,b). It has been argued that the extent of the effect of SG QTL on canopy development varies with environmental and management conditions experience by the crop (Borrell et al., 2014b). Green leaf area (GLA) at physiological maturity has proved to be an excellent indicator of stay-green, and has been successfully used to select drought-resistant sorghum genotypes in the USA (Rosenow et al., 1983) and in Australia (Henzell et al., 1992). Key components determining GLA at physiological maturity are: (i) maximum green leaf area at flowering, (ii) the timing of the onset of senescence, and (iii) rate of leaf senescence (Borrell et al., 2000). Genetic variation in the inheritance of all three components was reported (Van Oosterom et al., 1996; Badigannavar et al., 2018; Boyles et al., 2019). Van Oosterom et al. (1996) for instance concluded that the inheritance of the onset of senescence was additive, whereas the inheritance of the rate of senescence was completely dominant for a slow rate. Therefore, the relative green leaf area duration, being the sum of an additively and a dominantly inherited trait, displayed partial dominance for a large green leaf area duration.

The current results confirm the stay-green nature of B35 and the senescent nature of the recurrent parent, R16 (Blümmel et al., 2015). In the absence of stress, RSG 03123 displayed a similar senescence pattern to R16. However, under WL conditions, RSG 03123 retained significantly more GLA than the R16 parental line. This confirms that marker-assisted transfer of QTL identified for delayed-leaf-senescence from B35 to R16 was able to improve the retention of GLA in a senescent background. Thomas and Howarth (2000) have described how a stay-green phenotype could arise in a number of fundamentally distinct ways. In type A stay-greens, senescence is initiated late but then proceeds at a normal rate. Type B stay-greens initiate senescence on schedule but thereafter senesce comparatively slowly. This could arise from different genes being involved in the onset of senescence and in the regulation of its rate of progress. B35 displayed a delayed onset of senescence (Type A stay-green) under both WW and WL conditions. The onset of senescence in RSG 03123, however, was much earlier than in B35 under both WW and WL conditions suggesting that either the region(s) of the genome controlling this aspect of senescence was not introgressed from B35 or that it was not functional for some reason in RSG 03123. The onset of senescence in RSG 03123 was more similar to the recurrent parent, R16, but the rate of senescence was slower than R16 suggesting that RSG 03123 behaved like a type B stay-green. The differences in GLA discussed earlier were partly due to differences in chlorophyll loss. Hence, genotypes with reduced chlorophyll loss also had high GLA and/or retained higher proportion of GLA. This also confirms the improvement of GLA retention in RSG 03123 over R16. The findings of this study complements that of Harris et al. (2007) in which the retention of GLA under post-flowering drought conditions in the field was determined using near isogenic lines (NILs) developed by marker-assisted introgression of B35 SG QTL into the senescent RTx7000 background. Harris et al. (2007) found that Stg1, Stg2, Stg3 or Stg4 individually contributed to the SG phenotype but that NILs containing B35 DNA corresponding to Stg2 retained more green leaf area at maturity under terminal drought stress conditions than the recurrent parent RTx7000 of the other RTx7000 NILs.
The stay-green parent, B35 was not included in the study of Harris et al. (2007); but, in a previous study, it was shown that B35 exhibited delayed onset of senescence (Borrell et al., 2000). Xu et al. (2000a) also reported that under post-flowering drought stress conditions, B35 showed a much higher retention of chlorophyll content than that of the senescent parent Tx7000 at physiological maturity. A similar study carried out by Kassahun et al. (2010) found majority of the introgression lines with stay-green QTL from B35 having higher levels of leaf chlorophyll, which is a distinctive trait of the donor parent B35, than the senescent parent, R16. They also observed that the introgression lines also had a greater percentage GLA during the part of grain-filling than did the R16 recurrent parent. The present findings further confirm the findings of Kassahun et al. (2010) that none of the QTL introgression lines achieved the same level of stay-green as B35.
Visual scoring though easy and quick, particularly, when dealing with large numbers of plants in the field, is subject to individual biases and differences in ratings among observers (Rosenow, 1984). A visual rating of stay-green is limited by an inability to distinguish among the various mechanisms that ultimately determine the phenotype. Loss of chlorophyll is a prominent feature in post-flowering drought-induced senescence in leaves and stalks of sorghum. Thus, measurement of chlorophyll levels with time is an alternative method of indicating the onset and rate of senescence in leaves. SPAD values for sorghum have been found to be highly correlated with total leaf chlorophyll determined by spectrophotometry (Xu et al., 2000a). Apart from being non-destructive, SPAD measurements are easy and quick, especially for assessing large number of plants in the field. Chlorophyll concentration is also correlated with leaf nitrogen, which, in turn, is correlated with leaf longevity as well as grain yield (Blackmer and Schepers, 1995; Borrell et al., 2001). Hence, a SPAD meter can be used in the field for easy quantification of stay-green. Therefore, the present results also indicate that, not only is green leaf area duration extended in the RSG 03123, leaf nitrogen remobilization was also delayed compared to the R16 parent.
Chlorophyll fluorescence
Stay-green genotypes continue to fill grain normally under drought conditions (Rosenow et al., 1983). This suggests that they also maintain more photosynthetically active leaves under post-flowering drought stress and this is the first direct confirmation that the stay-green photosynthetic advantage can be transferred to a highly productive but senescent (R16) background. Chlorophyll fluorescence can be used as a tool for assessing the effect of stress on leaf photosynthesis. The reduction of Fv/Fm and ΦPSII indicates a reduction in photochemical efficiency of photosystem II (Maxwell and Johnson, 2000). ΦPSII in the RSG 03123 line was higher than in R16, but lower than in B35, for all sections of the leaf under drought stress, while there were no differences between RSG 03123 and R16 under WW conditions. Efficiency of the PSII antennae (Fv'/Fm') (Lu and Zhang, 1999) and ΦPSII (Lu and Zhang, 1999; Martinez et al., 2003) were reduced in drought-stressed wheat plants, whereas Fv/Fm was unaffected (Lu and Zhang, 1999). They also found that the reductions in ΦPSII and Fv'/Fm' were greater under severe drought stress. Furthermore, the decline of ΦPSII sets in earlier and is faster in the non-stay-green genotypes of wheat than in the stay-green genotypes (Spano et al., 2003). Similar findings were observed in the WL R16 in this study, where ΦPSII declined at a faster rate than in either RSG 03123 or B35.
Improved photosynthesis leads to better drought tolerance (Vadez et al., 2013). Photosynthesis depends on the functioning of light-harvesting and electron transport systems of the chloroplast, which is indicated by the photochemical efficiency of ΦPSII. Loss of efficiency of PSII can be attributed to the breakdown of proteins of PSII and photosynthetic enzymes and the destruction of membranes by lipid degradation (Thomas, 1987). Thus, in plants such as B35 and RSG 03123, photosynthetic efficiency was retained longer than in R16. This is indicative of higher CO2 assimilation in RSG 03123 and B35 compared to R16 plants under WL conditions.
Leaf gas exchange
Thomas and Howarth (2000) have described five types of the stay-green trait, three being cosmetic and two being functional. Maintenance of green leaf area at physiological maturity in sorghum correlates positively with grain yield and negatively with senescence (Borrell et al., 2000, Kassahun et al., 2010). Such findings indicate that the trait in sorghum is functional; however, actual CO2 assimilation rates have not previously been reported. Drought stress causes drastic reduction in photosynthesis and growth due to stomatal closure and associated changes in carbon and nitrogen mobilization (Badigannavar et al., 2018). CO2 assimilation rate (A) declines at moderate drought stress or even before leaf water status is changed in response to a reduction in soil water potential (Gollan et al., 1986). The limitation of leaf conductance (gL) to A depends on the severity of drought stress (Yordanov et al., 2003). Reduction of gL is the primary regulator of A under mild drought stress (Cornic and Briantais, 1991). A and gL were generally lower in RSG 03123 compared to B35 under both WW and WL conditions, however, the differences were not significant (P>0.05). Furthermore, just as in B35, the differences between the WW and WL plants were not significant (P<0.05) in RSG 03123 either. Both A and gL were also higher in RSG 03123 than in R16 and the rate of decline was slower in RSG 03123 under WL conditions, but under WW conditions there were no differences between them. The reductions in A were partially due to reductions in gL, as well as leaf senescence or chlorophyll loss since A was reduced with a decline in gL and an increase in senescence. The current findings are similar to those reported for wheat mutants with the stay-green trait, which had longer photosynthetic activity than the non-stay-green wild types (Spano et al., 2003).
Increased water use efficiency is of great interest to growers so that yields are maximised for available water supply in each growing season (Sinclair and Muchow, 2001). In water-limited environments, such as the arid and semi-arid regions, plant productivity is determined by the amount of water available and efficiency of its use by the plant (Xu and Hsiao, 2004). A reduction in WUEL with stomatal closure indicates that carbon metabolism and stomata limit photosynthesis as has been observed in sweet sorghum (Massacci et al., 1996). WUEL in RSG 03123 and R16 did not differ under WW conditions, but under WL conditions it was higher in RSG 03123. In these plants WUEL also declined with reductions in stomatal conductance and transpiration rate indicating that both carbon metabolism and stomata limited A. WUEL is higher in drought tolerant plants than in susceptible ones (Yordanov et al., 2001), indicating that B35 was drought tolerant. Similarly, RSG 03123 was more tolerant to WL conditions than R16. Differential gene expressions could account for the differences in tolerance to water-limited conditions. For instance, delta-1-proline-5-carboxylate synthase 2 (P5CS2) is highly expressed in B35 compared to R16, which correlated with high proline level in the SG line (Johnson et al., 2015). This may be responsible for a better tolerance to drought by SG genotypes than the senescent one. The enhanced water use efficiency conferred by SG QTL may be due to roots exploiting a larger volume of soil, which could enhance better uptake of water (Manschadi et al., 2006). Sorghum, maize and rice plants can reduce water loss by enhancing water uptake through profuse root proliferation (Badigannavar et al., 2018). Indeed, evidence exists that, in particular, the Stg4 SG QTL in sorghum co-locates with qRA1_5, a QTL for nodal root angle, indicating that root architecture can be a component of increased water use as found in stay-green near-isogenic lines (Mace et al., 2012; Borrell et al., 2014b). The current study did not look at root characteristics since root growth is restricted in pot experiments. Changes in WUEL reflect changes in stomatal conductance as well as the internal capacity for CO2 fixation, which is affected by enzyme activity (von Caemmerer et al., 1997) and nutrient status (Payne et al., 1992). In pearl millet, WUEL was found to increase with high soil phosphorus (P), but did not change with low P under drought stress (Brück et al., 2000). In this study, however, growing plants in the same growth medium eliminated differences in soil nutrients, even though differences in nutrient requirements and extraction could have led to differences in nutrient depletion in the growth medium. The differences in WUEL observed in this study appeared to have been influenced by differences in stomatal movements, rate of senescence and, maybe, enzyme activity (discussed later). The results show clearly that RSG 03123 was intermediate between B35 and R16 with improved performance compared to R16.
Metabolism of total soluble carbohydrates (TSC) in the leaf
Moderate drought stress can lead to increased concentration of TSC in the leaf, while severe drought stress can result in a constant concentration of SC in leaves, in spite of low A, because of the concomitant limitation of growth and export (Pinheiro et al., 2001). TSC in the leaf was reduced by more than a third in R16, whereas the reduction in RSG 03123 was one-eighth due to limitation of water availability. In B35 there was no difference between the WL and WW plants. These results indicate that in R16, the reduction in A due to limited water availability also resulted in a decrease in TSC. Similarly, TSC in RSG 03123 was lower than in B35 which had a higher A under WL conditions. TSC in the leaves of the plants grown under WL conditions reflected the reduction in photosynthesis in those leaves.
TSC metabolism in the stem
The principal non-structural carbohydrate stored by sorghum in the stem is sucrose, which accumulates after anthesis (McBee and Miller, 1982). Non-senescent sorghum cultivars accumulate more non-structural carbohydrates in the stem than senescent cultivars after anthesis (McBee et al., 1983; Vietor et al., 1990; McBee and Miller, 1993). The reduction in current assimilation due to leaf senescence during grain-filling in senescent cultivars has the potential to induce a greater stem reserve mobilization to, and utilization by, the grain; thus, one might expect higher non-structural carbohydrates in the stem of stay-green cultivars than in senescent ones. In the current study, TSC in the stem increased between flowering and physiological maturity. In B35 and RSG 03123, there was no significant difference between the amounts of TSC at physiological maturity in plants grown under WW or WL conditions. However, in R16 plants grown under WL conditions, TSC was reduced by 34% compared to the WW. This finding corroborates the earlier observations reported earlier. The introgression of the stay-green QTL from B35 into R16 thus reduced the necessity of remobilisation of stem reserves for grain-filling as observed in RSG 03123. The reduction in R16 was mainly due to a reduction in sucrose (results not shown), indicating that there was remobilisation of stem reserves. Stem reserves are increasingly recognized as an important source for grain filling when current photosynthesis is inhibited by stress.
Nitrogen
The longevity of a leaf is intimately related to its nitrogen (N) status (Thomas and Rogers, 1990). Stay-green trait is characterised by delayed-leaf-senescence and thus can also be viewed as a consequence of a balance between supply and demand for N during grain-filling (Borrell and Hammer, 2000). Under conditions of abiotic stress or N deficiency, remobilisation from vegetative parts becomes important for grain development (Ta and Weiland, 1992). Delayed remobilisation of N from leaves maintains photosynthetic activity for longer periods and can result in higher grain yield (Borrell and Hammer, 2000). Borrell and Hammer (2000) found that stay-green genotypes had more N than senescent genotypes under drought stress. N retention in RSG 03123 and R16 were similar under WW conditions, whereas under WL conditions the retention was better in RSG 03123. However, B35 retained more leaf N content (LNC) and specific leaf N (SLN) than RSG 03123 under both WW and WL conditions. High SLN allows more carbon and N to be allocated to roots of stay-green hybrids during grain-filling, thus maintaining a greater capacity to extract N from the soil compared to senescent genotypes (Borrell et al., 2001). As shown earlier, photosynthesis was prolonged in RSG 03123, just as in B35, under WL conditions. This condition probably made carbohydrates available for amino acid synthesis and, thus, protein synthesis. The continual protein synthesis could have enhanced protein turnover and thus maintained the photosynthetic apparatus, replenished the carbon and N assimilating enzymes, which enabled photosynthesis to continue in RSG 03123 and B35 under water limitation.
Specific proteins by Western Analysis
Chloroplast proteins: LHCPII and OEC33
Chloroplasts are dismantled during the early phase of senescence (Hortensteiner and Feller, 2002), indicating that chloroplast proteins are degraded. In genetic variants with stay-green trait, deconstruction of the photosynthetic apparatus during leaf senescence is partially or completely prevented (Thomas and Donnison, 2000). LHCPII is a major contributor to the overall loss of protein during leaf senescence (Matile, 1992). However, in stay-greens, where chlorophyll catabolism is blocked, LHCPII remains stabilised and proteolytic cleavage is restricted due to a small N-terminal that protrudes into the stroma (Thomas and Donnison, 2000). In R16, the WL plants were more affected, particularly in leaf 4. The changes in RSG 03123 were similar to those in B35, with no difference between the WW and WL plants.
OEC33 (the 33 kDa oxygen-evolving complex protein) is involved in photosynthetic electron transport (Zhang et al., 1998). The OEC33 subunit is known to stabilise the catalytic manganese (Mn) cluster, which is essential for water oxidation (Zhang et al., 1998). The release of OEC33 results in paramagnetic uncoupling and dissociation of two of the four Mn cations from PSII unless more than 100 mM Cl- is present (Zhang et al., 1998), thus confirming that it is involved in the stabilisation of the Mn cluster. Hence, a reduction in or degradation of OEC33, as a result of drought, would lead to a decline in CO2 assimilation. The changes in band intensities of OEC33 in B35 and RSG 03123 were similar with little differences between the bands at flowering and physiological maturity. On the other hand, OEC33 was highly reduced in the WL R16 plants. The reduction in OEC33 might have resulted, in part, in the low photosynthesis in WL R16 plants.
POR catalyses the light-dependent reduction of protochlorophyllide a to chlorophyllide a (Von Wettstein et al., 1995). Most angiosperms have isozymes referred to as POR A and POR B. POR A is dominant in etiolated plants and disappears rapidly when plants are exposed to light, whereas POR B is constitutively expressed and the only remaining POR in light-grown plants (Vavilin and Vermaas, 2002). The amounts of POR B in RSG 03123 were intermediate between those for B35 and R16. PORB might have been involved in the continuous replacement of chlorophyll in mature leaves of B35 and RSG 03123, which enabled these plants to remain photosynthetically active for longer periods compared to the senescent R16 under WL conditions.
Photosynthetic enzymes: PEPC and Rubisco
When the stress is severe, photosynthesis may be more controlled by the capacity of the chloroplast to fix CO2 than by reduced gL (Faver et al., 1996). Photosynthesis in C4 plants, including sorghum, involves the enzymes PEPCK, PEPC and Rubisco among others. PEPC occupies a key position as the initial CO2-fixing enzyme of the C4 pathway and is considered to be a major control point in this pathway (Dever et al., 1997). The band intensities of PEPC for B35 changed very little at physiological maturity, whereas in R16, it was drastically reduced at physiological maturity compared to the band intensities at flowering. Indeed, in the WL R16 it was barely detectable in leaves 1 and 2 and completely degraded in leaves 3 and 4. The retention of PEPC in RSG 03123 was also better than in R16 with sharp bands at physiological maturity, particularly under WL conditions, indicating an improvement in the retention of PEPC in RSG 03123. This could contribute to maintaining higher gas exchange rates in RSG 03123 compared to R16 under WL conditions.
Rubisco catalyses the fixation of CO2 in the bundle sheath cells (Edwards and Walker, 1983; Hatch, 1987). The abundance of Rubisco in leaves is controlled by the rate of its synthesis and degradation (Parry et al., 2002). In R16, Rubisco was reduced in the WL plants at physiological maturity. Indeed, in these plants what remained in leaves 1 and 2 was being degraded (just as for PEPC). In B35 and RSG 03123, the high amounts of Rubisco retained in the WL plants enabled CO2 assimilation to continue for longer periods than in the senescent cultivars. Reductions in the large subunit of Rubisco by drought stress have been reported for maize (Prakash and Rao, 1996) and wheat (Martinez et al., 2003) and in the transcripts of the small subunit in tomato (Bartholomew et al., 1991), and rice (Vu et al., 1999), indicating that its degradation was increased and synthesis reduced by water limitation. This is contrary to the arguments that Rubisco is not a primary target of drought stress (Holaday et al., 1992) and that limitation to CO2 assimilation in drought-stressed leaves is rather caused by a reduction in the supply of CO2 to Rubisco (Lal et al., 1996). In R16, on other hand, Rubisco was reduced just as in other plants like maize stated earlier. In RSG 03123, Rubisco was unaffected by drought stress confirming the findings by Holaday et al. (1992). Thus, the effect of drought stress on Rubisco in sorghum could be genotype dependent, having less effect in plants possessing the stay-green QTL as demonstrated in RSG 03123 and B35.
In the current study, we have proved that the introgression of the 3 putative SG QTL (Stg1, Stg3 and Stg4) from B35 into the senescent R16 background (RSG 03123) resulted in enhanced retention of GLA, chlorophyll and chloroplast enzymes. This enabled these plants to maintain photosynthesis for longer periods compared to R16. It would, therefore, be interesting to find out if the improvement observed in this study would translate into higher grain yield in RSG 03123 over R16 under field conditions. Furthermore, such improvement could enhance the nutritional contents of these plants, thus providing quality fodder for feeding livestock, particularly, for subsistence farmers. However, RSG 03123 may still require some improvement since its functioning was intermediate between B35 and R16. This study also proved that GLA retention and photosynthesis were enhanced by the SG QTL introgressed into RSG 03123. The maintenance of photosynthesis was due to the retention of chloroplast proteins and enzymes involved in photosynthesis for longer periods compared to the senescent R16 parent. The improvement in these parameters, as observed in RSG 03123 over R16, could be attributed to the SG QTL from B35 introgressed into R16, indicating that SG QTL can function in a senescent background to improve tolerance to water limitation or drought.
The authors have not declared any conflict of interests.
Funding from the Association of Commonwealth Universities, arranged through the University of Cape Coast, Ghana, and Institute of Biological, Environmental and Rural Sciences (IBERS), Aberystwyth University, Wales, UK for carrying out this work is gratefully acknowledged.
REFERENCES
|
Bachmann MF, Kündig TM, Hengartner H, Zinkernagel RM (1994). Regulation of IgG antibody titers by the amount persisting of immuneâ€complexed antigen. European Journal of Immunology 24(10):2567-2570.
Crossref
|
|
|
|
Badigannavar A, Teme N, Costa de Oliveira A, Li G, Vaksmann M, Viana VE, Ganapathi TR (2018). Physiological, genetic and molecular basis of drought resilience in sorghum (Sorghum bicolor (L.) Moench). Indian Journal of Plant Physiology 23(4):670-68.
Crossref
|
|
|
|
|
Barth S, Jankowska JM, Hodkinson TR, Vellani T, Klaas M (2016). Variation in sequences containing microsatellite motifs in the perennial biomass and forage grass, Phalaris arundinacea (Poaceae). BMC Research Notes 9:184.
Crossref
|
|
|
|
|
Bartholomew DM, Bartley GE, Scolnik PA (1991). Abscisic-acid control of rbcS and cab transcription in tomato leaves. Plant Physiology 96:291-296.
Crossref
|
|
|
|
|
Blackmer TM, Schepers JS (1995). Use of a chlorophyll meter to monitor nitrogen status and schedule fertilisation for corn. Journal of Production Agriculture 8:56-60.
Crossref
|
|
|
|
|
Blümmel M, Deshpande S, Khlova J, Vadez V (2015). Introgression of staygreen QTL's for concomitant improvement of food and fodder traits in Sorghum bicolor. Field Crops Research 180:228-237.
Crossref
|
|
|
|
|
Borland AM, Tecsi L, Leegood RC, Walker RP (1998). Inducibility of crassulacean acid metabolism (CAM) in Clusia species; physiological/ biochemical characterisation and intercellular localisation of carboxylation processes in three species which show different degrees of CAM. Planta 205:342-351.
Crossref
|
|
|
|
|
Borrell AK, Bidinger FR, Tao YZ, McIntyre CL, Douglas ACL (2001). Physiological and molecular aspects of stay-green in sorghum. In: A. K. Borrell and R.G. Henzell (eds). Proceedings from the Fourth Australian Sorghum Conference, Kooralbyn, 5-8 February 2001. CD-ROM format. Range Media Pty Ltd. ISBN: 0-7242-2163-8.
|
|
|
|
|
Borrell AK, Bidinger FR, Sunitha K (1999). Stay-green trait associated with yield in recombinant inbred sorghum lines varying in rate of leaf senescence. International Sorghum and Millets. Newsletter 40:31-34.
Crossref
|
|
|
|
|
Borrell AK, Hammer GL (2000). Nitrogen dynamics and the physiological basis of stay-green in sorghum. Crop Science 40:1295-1307.
Crossref
|
|
|
|
|
Borrell AK, Hammer GL, Henzell RO (2000). Does maintaining green leaf area in sorghum improve yield under drought? II. Dry matter production and yield. Crop Science 40:1037-1048.
Crossref
|
|
|
|
|
Borrell AK, Van Oosterom EJ, Mullet JE, George-Jaeggli B, Jordan DR, Klein PE, Hammer GL (2014a). Stay-green alleles individually enhance grain yield in sorghum under drought by modifying canopy development and water uptake patterns. New Phytololgy 203:817-830.
Crossref
|
|
|
|
|
Borrell AK, Mullet JE, George-Jaeggli B, van Oosterom EJ, Hammer GL, Klein PE, Jordan DR (2014b). Drought adaptation of stay-green sorghum is associated with canopy development, leaf anatomy, root growth, and water uptake. Journal of Experimental Botany 65:6251-6263.
Crossref
|
|
|
|
|
Boyles RE, Brenton ZW, Kresovich, S (2019). Genetic and genomic resources of sorghumto connect genotype with phenotype in contrasting environments. The Plant Journal 97:19-39.
Crossref
|
|
|
|
|
Brück H, Payne WA, Sattelmacher B (2000). Effects of phosphorus and water supply on yield, transpirational water-use efficiency and carbon isotope discrimination of pearl millet. Crop Science 40:120-125.
Crossref
|
|
|
|
|
Cairns A.J, Cookson A, Thomas BJ, Turner LB (2002). Starch metabolism in the fructan-grasses: patterns of starch accumulation in excised leaves of Lolium temulentum L. Journal of Plant Physiology 159 293-305.
Crossref
|
|
|
|
|
Cornic G, Briantais J-M (1991). Partitioning of photosynthetic electron flow between CO2 and O2 reduction in a C3 leaf (Phaseolus vulgaris L.) at different CO2 concentration and during drought stress. Planta 183:178-184.
Crossref
|
|
|
|
|
Crasta OR, Xu WW, Rosenow DT, Mullet JE, Nguyen HT (1999). Mapping of post-flowering drought resistance traits in sorghum: association between QTLs influencing premature senescence and maturity. Molecular and General Genetics 262:579-588.
Crossref
|
|
|
|
|
de Villiers L, Turk K, Thomas H, Howarth C (1993). Analysis and exploitation of the stay-green character in sorghum. Overseas Development Administration Project R4885 Annual Report.
|
|
|
|
|
Dever, LV, Bailey KJ, Leegood RC, Lea PJ (1997). Control of photosynthesis in Amaranthus edulis mutants with reduced amounts of PEP carboxylase. Australian Journal of Plant Physiology 24:469-476.
Crossref
|
|
|
|
|
Doggett H (1988). Sorghum, 2nd Edition. John Wiley & Sons: New York. pp. 165-199. Duncan RR, Bockholt AJ Miller FR (1981). Descriptive comparison of senescent and nonsenescent sorghum genotypes. Agronomy Journal 73:849-853.
Crossref
|
|
|
|
|
Duvick DN, Smith JSC, Cooper M (2004). Long-term selection in a commercial hybrid maize breeding program. Plant Breeding Reviews 24:109-152.
Crossref
|
|
|
|
|
Edwards GE, Walker DA (1983). C3, C4: Mechanisms and cellular and environmental regulation of photosynthesis. Los Angeles, CA: University of California Press.
|
|
|
|
|
Faver KL, Gerik TJ Thaxton PM, El-Zik KM (1996). Late season water stress in cotton: II. Leaf gas exchange and assimilation capacity. Crop Science 36:922-928.
Crossref
|
|
|
|
|
Foley JA, Ramankutty N, Braumann KA, Cassidy E, Gerber JS, Johnston M, Mueller ND, O'Connell C, Deepak KR, Zaks DPM (2011). Solutions for a cultivated planet. Nature 478:337-342.
Crossref
|
|
|
|
|
Galyuon IKA, Madhusudhana R, Borrell AK, Hash TC, Howarth CJ (2016). Genetic diversity of stay-green sorghums and their derivatives revealed by microsattelites. African Journal of Biotechnology 15(25):1363-1374.
Crossref
|
|
|
|
|
Gollan T, Passioura JB, Munns R (1986). Soil water status affects the stomatal conductance of fully turgid wheat and sunflower leaves. Australian Journal of Plant Physiology 13:459-464.
Crossref
|
|
|
|
|
Harris K, Subudhi PK, Borrell AK, Jordan D, Rosenow D, Nguyen H, Klein P, Klein R, Mullet J (2007). Sorghum stay-green QTL individually reduce post-flowering drought-induced leaf senescence. Journal of Experimental Botany 58:327-338.
Crossref
|
|
|
|
|
Hash CT, Bhasker Raj AG, Lindup S, Sharma A, Beniwal CR, Folkertsma RT, Mahalakshmi V, Zerbini E, Blümmel M (2003). Opportunities for marker-assisted selection (MAS) to improve the feed quality of crop residues in pearl millet and sorghum. Filed Crops Research 84:79-88.
Crossref
|
|
|
|
|
Hatch MD (1987). C4 photosynthesis: a unique blend of modified biochemistry, anatomy and ultrastructure. Biochimica et Biophysica Acta 985:81-106.
Crossref
|
|
|
|
|
Henzell RG, Brengman DS, Fletcher McCosker AN (1992). Relationships between yield and non-senescence (stay-green) in some grain sorghum hybrids grown under terminal drought stress. In: Foale, M. A., Henzell RG, Vance PN (eds) Proceedings of the second Australia Sorghum Conference Gatton, 4th-6th February, 1992. Australian Institute of Agricultural Science, Melbourne pp 355-358.
|
|
|
|
|
Hilditch PI, Thomas H, Thomas BJ, Rogers LJ (1989). Leaf senescence in non-yellowing mutant of Festuca pratensis: proteins of photosystem II. Planta 177:265-272.
Crossref
|
|
|
|
|
Holaday AS, Ritchie SW, Nguyen HT (1992). Effect of water deficit on gas exchange parameters and ribulose-1,5-bisphospahte carboxylase activation in wheat. Environmental and Experimental Botany 32:403-409.
Crossref
|
|
|
|
|
Hortensteiner S, Feller U (2002). Nitrogen metabolism and remobilization during senescence. Journal of Experimental Botany 53:927-937.
Crossref
|
|
|
|
|
Jagadish KSV, Kavi Kishor PB, Bahuguna RN, von Wiren N, Sreenivasulu N (2015). Staying alive or going to die during terminal senescence - An enigma surrounding yield stability. Frontiers in Plant Science 6:1070.
Crossref
|
|
|
|
|
Johnson SM, Cummins I, Lim FL, Slabas AR, Knight MR (2015). Transcriptomic analysis comparing stay-green and senescent Sorghum bicolor lines identifies a role for proline biosynthesis in the stay-green trait. Journal of Experimental Botany 66(22):7061-7073.
Crossref
|
|
|
|
|
Kassahun B, Bidinger FR, Hash CT, Kuruvinshetti MS (2010). Stay-green expression in early generation sorghum (Sorghum bicolor (L.) Moench) QTL introgression lines. Euphytica 172:351-362.
Crossref
|
|
|
|
|
Laemmli UK (1970). Cleavage of structural proteins during the assembly of the head of the bacteriophage T4. Nature 227:680-685.
Crossref
|
|
|
|
|
Lal A, Ku MSB, Edwards GE (1996). Analysis of inhibition of photosynthesis due to water stress in C3 species Hordeum vulgare and Vicia faba: electron transport, CO2 fixation and carboxylation capacity. Photosynthesis Research 49:57-69.
Crossref
|
|
|
|
|
Lasky JR, Upadhyaya HD, Ramu P, Deshpande S, Hash CT, Bonnette J, Juenger TE, Hyma K, Acharya C, Mitchell SE, Buckler ES, Brenton Z, Kresovich S, Morris GP (2015). Genome-environment associations in sorghum landraces predict additive traits. Science Advances 1:1-13.
https://doi.org/10.1126/sciadv.1400218
|
|
|
|
|
Lu C, Zhang J (1999). Effects of water stress on photosystem II photochemistry and its thermostability in wheat plants. Journal of Experimental Botany 50:1199-1206.
Crossref
|
|
|
|
|
Mace E, Singh V, Van Oosterom E, Hammer G, Hunt C, Jordan D (2012). QTL for nodal angle in sorghum (Sorghum bicolor (L.) Moench) co-locate with QTL for traits associated with drought adaptation. Theoretical and Applied Genetics 124:97-109.
Crossref
|
|
|
|
|
Mae T, Thomas H, Gay AP, Makino A, Hidoma J (1993). Leaf development in Lolium temulentum: photosynthesis and photosynthetic proteins in leaves senescing under different irradiances. Cell Physiology 34:391-399.
|
|
|
|
|
Martinez DE, Luquez VM, Bartoli CG, Guiamet JJ (2003). Persistence of photosynthetic components and photochemical efficiency in ears of water-stressed wheat (Triticum aestivum). Physiologia Plantarum 119:519-525.
Crossref
|
|
|
|
|
Mahalakshmi V, Bidinger FR (2002). Evaluation of the stay-green germplasm lines at ICRISAT. Crop Science 42:965-974.
Crossref
|
|
|
|
|
Manschadi AM, Christopher J, deVoil P, Hammer GL (2006). The role of root architectural traits in adaptation of wheat to water-limited environments. Functional Plant Biology 33(9):823-837.
Crossref
|
|
|
|
|
Massacci A, Battisetelli A, Loreto F (1996). Effect of drought stress on photosynthetic characteristics, growth and sugar accumulation of field-gron sweet sorghum. Australian Journal of Plant Physiology 23:331-340.
Crossref
|
|
|
|
|
Matile P (1992). Chloroplast senescence. In: Baker NR Thomas H (eds) Crop Photosynthesis: spatial and temporal determinants. Elsevier, Amsterdam pp. 413-440.
Crossref
|
|
|
|
|
Maxwell K, Johnson GN (2000). Chlorophyll fluorescence - a practical guide. Journal of Experimental Botany 51:659-668.
Crossref
|
|
|
|
|
McBee GG, Miller FR (1982). Carbohydrates in sorghum culms as influenced by cultivars, spacing and maturity over a diurnal period. Crop Science 22:381-385.
Crossref
|
|
|
|
|
McBee GG Miller FR (1993). Stem carbohydrates and lignin concentrations in sorghum hybrids at seven growth stages. Crop Science 33:530-534.
Crossref
|
|
|
|
|
McBee GG, Waskom III RM, Miller FR, Creelman RA (1983). Effect of senescence and nonsenescence on carbohydrates in sorghum during late kernel maturity states. Crop Science 23:372-376.
Crossref
|
|
|
|
|
Oh M-H, Kim Y-J, Lee C-H (2003). Increased stability of LCHIIby aggregate formation during dark-induced leaf senescence in Arabidopsis thaliana mutant ore10. Plant Cell Physiology 44:1368-1377.
Crossref
|
|
|
|
|
Parry MAJ, Andralojc PJ, Khan S, Lea PJ, Keys AJ (2002). Rubisco activity: Effects of drought stress. Annals of Botany 89:833-839.
Crossref
|
|
|
|
|
Paterson AH, Bowers JE, Bruggmann R, Dubchak I, Grimmwood J, Gundlach H, et al. (2009a). The Sorghum bicolor genome and the diversification of grasses. Nature 457:551-556.
Crossref
|
|
|
|
|
Paterson AH, Bowers JE, Feltus FA, Tang HB, Lin LF, Wang XY (2009b). Comparative genomics of grasses promises a bountiful harvest. Plant Physiology 149:125-131.
Crossref
|
|
|
|
|
Payne WA, Drew MC, Hossner LR, Lascano RJ, Onken AB, Wendt CW (1992). Soil phosphorus availability and pearl millet water use efficiency. Crop Science 32:1010-1015.
Crossref
|
|
|
|
|
Pinheiro C, Chaves MM, Ricardo CP (2001). Alterations in carbon and nitrogen metabolism induced by water deficit in the stems and leaves of Lupinus albus L. Journal of Experimental Botany 52:1063-1070.
Crossref
|
|
|
|
|
Pinto RS, Lopes MS, Collins NC, Reynolds MP (2016). Modelling and genetic dissection of staygreen under heat stress Theoretical and Applied Genetics 129(11):2055-2074.
Crossref
|
|
|
|
|
Prakash KR, Rao VS (1996). The altered activities of carbonic anhydrase, phosphoenolpyruvate carboxylase and ribulose bisphosphate carboxylase due to water stress and after its relief. Journal of Environmental Biology 17:39-42.
|
|
|
|
|
Rosenow DT (1984). Breeding for resistance to root and stalk rots in Texas. In: Mughogho LK
|
|
|
|
|
(ed) Sorghum root and stalk diseases, a critical review. Proceedings of the Consultative Group discussion of research needs and strategies for control of sorghum root and stalk diseases. Bellagio, Italy, 27th November to 2nd December, 1983, ICRISAT, Pantacheru Andrah Pradesh, India pp 209-217.
|
|
|
|
|
Rosenow, DT (1994). Evaluation for drought and disease resistance in sorghum for use molecular marker-assisted selection. In: Witcombe JR, Duncan RR (eds). Proceedings of the use of molecular markers in sorghum and pearl millet breeding for developing countries. Norwich. pp 27-31.
|
|
|
|
|
Rosenow DT, Clark LE (1981). Drought tolerance in sorghum. In: Loden HD and Wilkinson D (eds). Proceedings of the 36th Annual Corn and Sorghum Industry Research Conference. Chicago, Illinois, USA. 9th to 11th December, 1981. American Seed trade Association, Washington DC. pp. 18-31.
|
|
|
|
|
Rosenow DT, Clark LE, Dahlberg JA, Frederiksen RA, Odvody GN, Peterson GC, Schaefer K, Collins SD, Jones JW, Hamburger AJ (2002). Release of four A/B sorghum parental lines ATx642 through ATx645. International Sorghum and Millets. Newsletter 43:24-3
|
|
|
|
|
Rosenow DT, Quisenberry JE, Wendt CW, Clark LE (1983). Drought tolerant sorghum and cotton germplasm. Agriculture Water Managent 7:207-222.
Crossref
|
|
|
|
|
Sanchez AC, Subudhi PK, Rosenow DT, Nguyen HT (2002). Mapping QTLs associated with drought resistance in sorghum (Sorghum bicolour (L.) Moench). Plant Molecular Biology 48:713-726.
Crossref
|
|
|
|
|
Sinclair TR Muchow RC (2001). System analysis of plant traits to increase grain yield on limited water supplies. Agronomy Journal 93:263-270.
Crossref
|
|
|
|
|
Spano G, Di Fonzo N, Perrotta C, Platani C, Ronga G, Lawlor DW, Napier JA, Shewry PR (2003). Physiological characterization of 'stay green' mutants in durum wheat. Journal of Experimental Botany 54:1415-1420.
Crossref
|
|
|
|
|
Subudhi PK, Rosenow DT, Nguyen HT (2000). Quantitative trait loci for the stay-green trait in sorghum (Sorghum bicolor (L.) Moench): consistency across genetic backgrounds and environments. Theoretical and Applied Genetics 101:733-741.
Crossref
|
|
|
|
|
Ta CT, Weiland RT (1992). Nitrogen partitioning in maize during ear development. Crop Science 32:443-451.
Crossref
|
|
|
|
|
Tao YZ, Henzell RG, Jordan DR, Butler DG, Kelly AM, McIntyre CL (2000). Identification of genomic regions associated with stay-green in sorghum by testing RILs in multiple environments. Theoretical and Applied Genetics 100:1225-1232.
Crossref
|
|
|
|
|
Thomas H (1987). Sid: A Mendelian locus controlling thylakoid membrane disassembly in senescing leaves of Festuca pratensis. Theoretical and Applied Genetics 73:551-555.
Crossref
|
|
|
|
|
Thomas H, Donnison I (2000). Back from the brink: plant senescence and its reversibility. In: Bryant JA, Hughes SG Garland JM (eds) Programme cell death in animals and plants. Oxford: Bios pp 149-162.
|
|
|
|
|
Thomas H and Howarth, CJ (2000). Five ways to stay green. Journal of Experimental Botany 51:329-337.
Crossref
|
|
|
|
|
Thomas H, Rogers LJ (1990). Turning over an old leaf. University of Wales Reviews of Science and Technology 6:29-38.
|
|
|
|
|
Tuinstra MR, Ejeta G, Goldsborough PB (1998). Evaluation of near-isogenic sorghum lines for QTL markers associated with drought tolerance. Crop Science 38:835-842.
Crossref
|
|
|
|
|
Tuinsra MR, Grote EM, Goldsborough PB, Ejeta G (1996). Indentification of quantitative trait loci associated with pre-flowering drought tolerance in sorghum. Crop Science 36:1337-1344.
Crossref
|
|
|
|
|
Tuinstra MR, Grote EM, Goldsborough PB Ejeta G (1997). Genetic analysis of of post-flowering drought tolerance and components of grain development in Sorghum bicolor (L.) Moench. Molecular Breeding 3:439-448.
Crossref
|
|
|
|
|
Vadez V, Deshpande S, Kholova J, Ramu P, Hash CT (2013). Molecular breeding for stay-green: progress and challenges in sorghum. In:
Varshney R, Tuberosa R (eds) Genomic applications to crop breeding: Volume 2. Improvement for abiotic stress, quality and yield improvement. New York: Wiley pp. 125-141.
Crossref
|
|
|
|
|
Van Oosterom EJ, Jayachandran R, Bindinger F (1996). Diallel nalysis of stay-green trait and its components in sorghum. Crop Science 36:540-555.
Crossref
|
|
|
|
|
Vavilin DV, Vermaas FJ (2002). Regulation of the tetrapyrrole biosynthetic pathway leading to heme and chlorophyll in plants and cyanobacteria. Plant Physiology 115:9-24.
Crossref
|
|
|
|
|
Verma V, Foulkes MJ, Worland AJ, Sylvester-Bradley R, Caligari PDS, Snape JW (2003). Mapping quantitative trait loci for flag leaf senescence as yield determinant in winter wheat under optimal and drought-stressed environments. Euphytica 135:255-263.
Crossref
|
|
|
|
|
Vietor DM, Miller FR, Cralle HT (1990). Nonstructural carbohydrates in axillary branches and main stem of senescent and nonsenescent sorghum types. Crop Science 30:97-100.
Crossref
|
|
|
|
|
Von Caemmerer S, Ludwig M, Millgate A, Farquhar GD, Price D, Badger M, Furbank RT (1997). Carbon isotope discrimination during C4 photosynthesis: Insights from transgenic plants. Australian Journal of Plant Physiology 24:487-494.
Crossref
|
|
|
|
|
Von Wettstein D, Gough S, Kannangara CG (1995). Chlorophyll biosynthesis. Plant Cell 7:1039-1057.
Crossref
|
|
|
|
|
Vu JCV, Gesch RW, Allen LH, Boote KJ, Bowes G (1999). CO2 enrichment delays a rapid, drought-induced decrease in Rubisco small subunit transcript abundance. Journal Plant Physiology 155:139-142.
Crossref
|
|
|
|
|
Walulu RS, Rosenow DT, Wester DB, Nguyen HT (1994). Inheritance of stay-green trait in sorghum. Crop Science 34:970-972.
Crossref
|
|
|
|
|
Wolfe DW, Henderson DW, Hsiao TC Alvino A (1988). Interactive water and nitrogen effects on senescence of maize. I. Leaf area duration, nitrogen distribution and yield. Agronomy Journal 80:859-864.
Crossref
|
|
|
|
|
Xu L-K, Hsiao TC (2004). Predicted versus measured photosynthetic water-use efficiency of crop stands under dynamically changing field environments. Journal of Experimental Botany 55:2395-2411.
Crossref
|
|
|
|
|
Xu W, Rosenow DT, Nguyen HT (2000a). Stay-green trait in grain sorghum: relationship between visual rating and leaf chlorophyll concentration. Plant Breeding 119:365-367.
Crossref
|
|
|
|
|
Xu W, Subhudi PK, Crasta OR, Rosenow DT, Mullet JE, Nguyen HT (2000b). Molecular mapping of QTLs conferring stay-green in grain sorghum (Sorghum bicolor (L) Moench). Genome 43:461-469.
Crossref
|
|
|
|
|
Yordanov I, Tsonev T, Velikova V, Georgieva K, Ivanov P, Tsenov N, Petrova T (2001). Changes in CO2 assimilation, transpiration and stomatal resistance of different wheat cultivars experiencing drought under filed conditions. Bulgarian Journal of Plant Physiology 27:20-33.
|
|
|
|
|
Yordanov I, Velikova V, Tsonev T (2003). Plant responses to drought and stress tolerance. Bulgarian Journal of Plant Physiology Special pp. 187-206.
|
|
|
|
|
Zhang H, Ishikawa Y, Yamamoto Y, Carpentier R (1998). Secondary structure and thermal stability of the extrinsic 23 kDa protein of photosystem II studied by Fourier transform infrared spectroscopy. FEBS Letters 426:347-351.
Crossref
|
|