Full Length Research Paper
ABSTRACT
For production and preservation of traditional fermented foods, the genera, lactic acid bacteria (LAB) have been used. This study was carried out to determine the characteristics and the antimicrobial activities of lactic acid bacteria isolated from selected Nigerian traditional fermented foods. Changes in pH and titratable acidity (TA) of the samples were investigated for a period of four days (96 h). Eleven tentative LAB from fermented maize and cassava (Ogi and Fufu, respectively) were isolated and characterized. The spoilage organisms from fish were aseptically identified and the antimicrobial activity was determined by agar well diffusion method against three isolated food spoilage organisms (Pseudomonas aeruginosa, Enterobacter aerogene and Bacillus cereus). The isolates were selected and further identified as Lactobacillus amylolyticus strain L6, Lactobacillus plantarum strain ci-4w and Lactobacillus sakei strain MLS1 by the aide of genotypic characteristics (16S rRNA gene sequences). These strains were screened for their EPS producing activity, resistance to low pH and bile salts as well as bacteriocin activity. These strains can be used as starter culture or protective cultures to improve the hygiene, quality and increased safety of the food products by inhibiting the food borne pathogens and spoilage microorganisms.
Key words: Lactic acid bacteria, fermented foods, exopolysaccharides, antimicrobial activity, ogi, fufu.
INTRODUCTION
Over recent decades, lactic acid bacteria (LAB) have received much attention due to the health-promoting properties of certain strains, called probiotics. They are normal inhabitants of the healthy gut microbiota as they improve the balance of the microbial community in the intestine, confer protection against potential pathogenic bacteria, prevent or cure intestinal diseases and present in numerous fermented food products (Ngene et al., 2019; Rijkers et al., 2011; Brown and Valiere, 2004; Adak et al., 2002). LAB are used in a wide range of fermented food, they play a critical role in food processing and spontaneous fermentation (Elayaraja et al., 2014); also, they have been shown to be a major potential for use in biopreservation due to the fact that they are generally recognized as safe (GRAS) status (Salem, 2012; Vignolo et al., 2008; Radha and Padmavathi, 2015). They exert a strong antagonistic activity against many food contaminating microorganisms and these effects are mediated by production of antimicrobial metabolites such as organic acids (for example lactate, acetate, and butyrate), hydrogen peroxide, bacteriocins, and competition with harmful bacteria for nutrients or adhesion receptors (Maurya and Thakur, 2012; Wilson et al., 2011). LAB are among the most important microbes which are used in food fermentations, as well as in enhancing taste and texture in fermented food products (Van Geel-Schuttená et al., 1998; Hati et al., 2013).
One of the concerns in food industry is the contamination by food spoilage microorganisms and pathogens, which are frequent cause of food spoilage and food borne diseases. An important aspect of food contamination by microorganisms is the presence of potentially pathogenic species, which pose a great risk for the human and animal health (Broberg et al., 2007). Bacteria and various fungi are the cause of spoilage and can create serious consequences for the consumers and some troublesome spoilage microorganisms include aerobic psychrotrophic Gram-negative bacteria, yeasts, molds, heterofermentative lactobacilli, and spore-forming bacteria. They can cause extensive damage of the food such as unpleasant smell, taste or appearance as well as formation of harmful substances for the consumer’s health (Dinev et al., 2017; Garcia et al., 2010; Garcha, 2018).
In order to achieve improved food safety against such spoilage microorganisms, food industry makes use of chemical preservatives or physical treatments (e.g. high temperatures). Many drawbacks which include the proven toxicity of the chemical preservatives has been recorded for these preservation techniques (e.g. nitrites), the alteration of the organoleptic and nutritional properties of foods, and especially recent consumer demands for safe but minimally processed products without additives (Ananou et al., 2007; Sharma et al., 2006).
However, the increasing resistance of food spoilage microorganisms to current preservatives, the consumer’s high demand for safe, minimally processed foods, the alteration of the organoleptic and nutritional properties of foods and the hazards associated with the use of high doses of chemical preservatives have led to the need for finding safer alternatives in food preservation and disease prevention (Garcia et al., 2010; Nath et al., 2013).
Therefore, the need for alternatives to extend the shelf life of foods without changing their sensory properties and use in the treatment or prevention of gastrointestinal disease have launched research on probiotics and biopreservation technologies, which are based on the use of non-pathogenic microorganisms (lactic acid bacteria) or their metabolites to retard food spoilage or to improve food safety and confer health benefit (De Martinis et al., 2001; Ross et al., 2002). This research is designed to evaluate probiotics properties and to enhance the shelf-life of fermented food through assessing the biopreservation potency of lactic acid bacteria with the aim of developing starter/protective culture with predictable characteristics, for use in industrial application. The aim of this research is to analyze antimicrobial activities of exopolysaccharide lactic acid bacteria isolated from Ogi and Fufu on food spoilage organisms.
MATERIALS AND METHODS
Source and collection of samples
Maize grains (from two maize varieties: white and yellow) and freshly harvested cassava root tubers were purchased from a local market in Uyo, Akwa Ibom State, Nigeria. They were immediately processed and transported aseptically to the Post Graduate Microbiology laboratory, University of Uyo, for further analysis.
Sample preparation
The fermentation of maize grains and cassava tubers to produce Ogi and fufu, respectively, were carried out by simulating the traditional methods of processing (Figures 1 and 2). The fermentation of maize grains and cassava tubers to produce ogi and fufu, respectively, were carried out by simulating the traditional methods of processing.
Physicochemical analysis
Determination of pH
Samples of fermenting cassava mash (10 g) were homogenized in distilled water (100 ml) then the pH of the fermenting substrates was measured (Achi and Akomas, 2006), whereas pH of the fermentation liquor of cereal samples was determined directly (Anumudu et al., 2018; Nwachukwu and Ijeoma, 2010b) using a pH meter and the changes in pH of fermenting samples were monitored daily for 4 days (96 h).
Determination of titratable acidity (TA)
In order to determine the titratable acidity (TA) 10 ml of the samples were transferred to a 50 ml measuring flask and filled up to 50 ml with distilled water. After mixing, 10 ml of the diluted sample were titrated against 0.1 M sodium hydroxide (NaOH) using phenolphthalein as indicator until a pink colour appeared. Each ml of 0.1 M NaOH is equivalent to 90.08 mg of lactic acid (Nwachukwu and Ijeoma, 2010b).
TA of lactic acid (mg/ml) = (ml NaOH × M NaOH × M.E) / Volume of sample used
Where ml NaOH is the volume of NaOH used, M NaOH is the Molarity of NaOH used, M.E = Equivalent factor = 90.08 mg (Wakil and Ajayi, 2013; Nwachukwu and Ijeoma, 2010b).
Microbiological analysis
Isolation and characterization of lactic acid bacteria
For preliminary identification, lactic acid bacteria (LAB) were isolated and enumerated using the De Mann, Rogosa and Sharpe (MRS) agar. A 1 ml of sample was diluted serially in ten folds dilution blanks properly mixed with sterile glass rod and 0.1 ml of diluted sample was introduced into sterile plate and molten sterile agar medium was poured (Harrigan and McCance, 1996; Ibeabuchi et al., 2014). The inoculated plates were incubated at 37°C for 48 h and suspected LAB colonies were picked randomly, sub-cultured on MRS agar to obtain pure culture and thereafter, pure cultures were grown on agar slants and kept at 4°C for further analysis.
LAB strains were characterized on the basis of their morphological, biochemical and physiological properties. Each isolate was examined under light microscope using oil immersion objectives after Gram-staining and Spore staining for the purpose of identification. All strains were subjected to Catalase test, Oxidase test, Motility test, Sugar fermentation (Bukola and Abiodun, 2008; Ibeabuchi et al., 2014), adjusted pH range of 4, 6, and 8, respectively using diluted buffer solutions, growth at different temperatures of 15, 25, 35, and 45°C and growth in the presence of different concentrations of NaCl (2, 4, 6 and 8%). After 24 to 48 h of incubation their growth were determined by observing their turbidity (Karthikeyan and Santosh, 2009). Probable identities were confirmed using Bergey's Manual of Systematic Bacteriology (Holt et al., 1994).
Isolation and identification of test organisms
The test organisms were isolated from Fish. A 10 g of the sample was aseptically cut and thoroughly blended with 10 ml sterile water using sterile blender. A 1 ml aliquot volume of the blended sample was measured out and homogenized in 9 ml of buffered peptone water and diluted serially in ten folds dilution blanks (Eze et al., 2011). 0.1 ml of the diluted sample was plated onto sterile Nutrient agar (Oxoid, England), Cetrimide agar and Eosin Methylene Blue (EMB) agar and incubated at 37°C under aerobic condition for 24 h The isolated bacterial colonies were identified on the basis of their morphological, cultural and biochemical characters and probable identities were confirmed using Bergey's Manual of Systematic Bacteriology (Holt et al., 1994).
Antimicrobial assay of lactic acid bacteria
In the screening of the LAB cells for antagonistic activity, the agar-well diffusion method was employed. Indicator lawns were prepared with 40 mL of Mueller Hinton Agar (MHA) seeded with 100 μL of an overnight culture of each food spoilage organism (Pseudomonas aeruginosa, Enterobacter aerogene and Bacillus cereus). With a sterile 5 mm diameter cork borer, wells were cut into the agar. Each LAB isolate were placed into each well. The plates were incubated at 30°C for 24 to 48 h after which they were examined for probable clearing of zones (Bali et al., 2011; Tambekar and Bhutada, 2010).
Evaluation of in vitro probiotic potentials of the selected lab isolates
Screening of the LAB isolates for exopolysaccharide production
Screening of exopolysaccharide from the LAB isolates was done using EPS selection medium (ESM 90 g of skimmed milk, 3.5 g of yeast extract, 3.5 g of peptone, 10 g of glucose/L) as described by van den Berg et al. (1993) and Patel et al. (2012). The isolates were inoculated differently into sterile ESM medium and incubated at 30°C for 24 h. The colonies developed thereafter were examined for mucoid and glistening by visual examination. The ropiness of the culture was also determined by touching the colonies with sterile wireloop and measuring the strings for extension of 5 mm or more.
Acid tolerance test
The acid resistance was performed by the viable plate count method (Hassanzadazar et al., 2012; Sahadeva et al., 2011). Bacterial cells were inoculated to adjusted pH of 2.0 and 3.0 and samples were taken every 3 h, thereafter, the viable colony counts were enumerated on MRS agar after incubation at 37°C for 6 h simulating the acidic environment in the human stomach.
Bile salt tolerance test
The bile tolerance test was carried out by method of Walker and
Gilliland (1990). Briefly, cells of the selected strains were grown in MRS broth at 37°C overnight, and then subcultured in MRS broth containing different concentrations (0.1, 0.3, and 0.5%) of bile salts. The growth rate of each strain was determined by the viable plate count method after 24 h (Sahadeva et al., 2011).
Evaluation of biopreservation potential of lactic acid bacteria
Extraction of bacteriocins
Selected LAB isolates were grown in MRS broth at 37°C for 24 h. Cell free culture supernatant (CFCS) of each isolate was obtained by centrifugation at 3,000 rpm for 20 min. The supernatant was adjusted to pH 6.5 with 1M NaOH to neutralize any effect of acidity and inhibitory activity from hydrogen peroxide was eliminated by the addition of a 5 mg/ml catalase and subsequently filters sterilized through a 0.2 μm membrane filter (Onwuakor et al., 2014).
Detection of inhibitory activity of bacteriocin from selected isolates
The agar-well diffusion method was employed and 0.1 ml of test organisms (P. aeruginosa, E. aerogene and B. cereus) were plated using spread plate method on Mueller Hinton agar (MHA) plates. Wells were cut into the agar with a sterile 6 mm diameter cork borer. A 100 μL partially purified bacteriocins of each potential producer strain was placed into each well. The plates were then incubated at 30°C for 24 to 48 h after which they were examined for probable clearing zones (Bali et al., 2011).
Effect of pH on bacteriocin
Bacteriocin of the LAB isolates were adjusted to pH range of 4, 5, 6, 7, 8, and 9, respectively using diluted acid and base solutions and were allowed to stand at room temperature for 2 h. The residual bacteriocin activity of the cell free culture supernatant (CFCS) was then determined against the test organisms by well diffusion method and then measuring the diameter inhibition zone (Karthikeyan and Santosh, 2009).
Effect of temperature on crude bacteriocins
The isolated semi purified bacteriocins were subjected to different heat temperatures for 10 min at 40, 60, 80 and 100°C. Temperature stability was determined by measuring the residual antimicrobial activity of semi purified bacteriocins after treatments against the selected test organisms using the agar well diffusion assay (Karthikeyan and Santosh, 2009).
Effect of storage time on crude bacteriocin
The pH of cell free culture supernatant (CFCS) of the LAB isolates was adjusted and the effect of the organic acids and hydrogen peroxide (H2O2) was eliminated as stated earlier. 20 ml of the crude bacteriocin of LAB isolates was then stored at 37°C and 5ml of the crude bacteriocin was tested in well diffusion assay against the indicator organisms every 24 h for 4 days (96 h) using well diffusion method (Bali et al., 2011).
Molecular identification of lactic acid bacteria
DNA extraction
DNA extraction was conducted using the facilities of the Center for Molecular Biology and Biotechnology (CMBB), Michael Okpara University of Agriculture, Umudike (MOUAU), Nigeria. Genomic DNA from the isolated LAB was extracted with Zymo-Spin™ kits according to the manufacturer's instructions and the extracted DNA was separated on a 1% agarose gel electrophoresis.
PCR analysis
The 16S rRNA coding gene was amplified through polymerase chain reaction (PCR) using universal primers, 27F (5’-AGAGTTTGATCMTGGCTCAG-3’) and 907R (5’-CCGTCAATTCMTTTRAGTTT-3’). PCR amplifications were carried out in a thermal cycler (T Gradient model, Biometera, Germany) using the following steps: one cycle of denaturation for 5 min at 94°C followed by thirty-five cycles of 94°C for 30 s. Annealing was performed at 50°C for 30 s, extension at 68°C for 1 min and final extension was done at 68°C for 10 min. The PCR products were kept at 4°C and the integrity of the PCR amplicons were separated on a 1% agarose gel electrophoresis (CSL-AG500, Cleaver Scientific Ltd) and visualized by staining with EZ-vision® Bluelight DNA Dye.
Sequencing
PCR products were purified using ExoSAP Protocol and sequencing was done with the Applied Biosystems™ BigDye™ Terminator v3.1 Cycle Sequencing Kit (Catalogue No. 4337455) using ABI 3500XL Genetic Analyser by Inqaba Biotec, South Africa.
BLAST analysis
The resulting 16S rRNA gene sequences were analyzed in NCBI website (http://www.ncbi.nlm.nih.gov) using the Basic Local Alignment Search Tool (BLAST) Software in the GenBank nonredundant/nucleotide collection (nr/nt) to compare sequences and identification was performed on the basis of 16S rRNA sequence percentage similarity with the type strains.
Phylogenetic analysis
The sequences were aligned using ClustalW and the evolutionary history was inferred using the Neighbor-Joining method whereas the evolutionary distances were computed using the Maximum Composite Likelihood method. All ambiguous positions were removed for each sequence pair (pairwise deletion option) and the evolutionary analyses were conducted in MEGA X (Saitou and Nei, 1987; Kumar et al., 2018).
Statistical analysis
The experiments were carried out in replicate and results are given as the mean ± standard deviation. Data generated were subjected to One-Way analysis of variance (ANOVA), and Duncan Multiple range test was used for separation of mean, while P<0.05 was considered significant using Statistical Package for Social Sciences (SPSS) version 20.
RESULTS
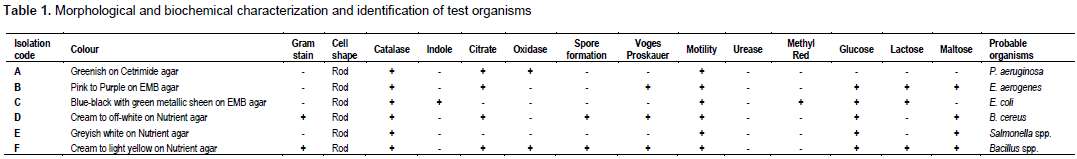
Table 1 shows identification of indicator isolates (food spoilage organisms) from spoiled meat. The food spoilage organisms were identified based on morphological, cultural and biochemical characteristics as P. aeruginosa, E. aerogenes, and B. cereus.
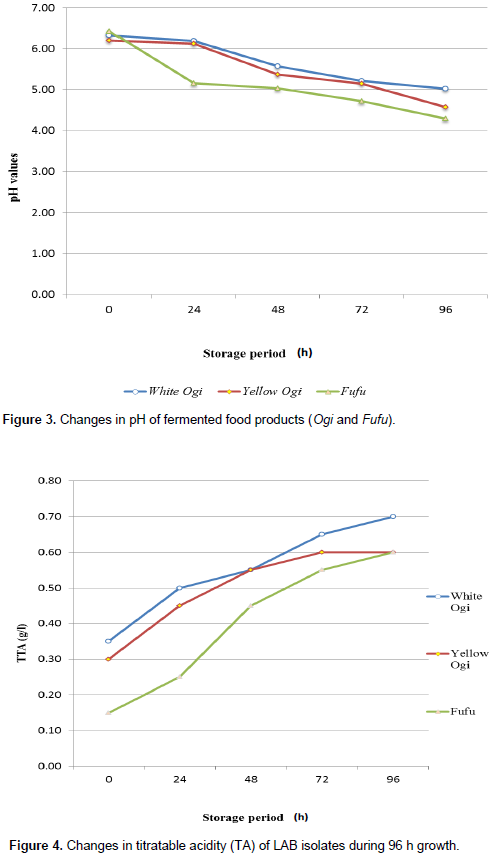
The changes in the pH and titratable acidity during the spontaneous fermentation of ogi and fufu are presented in Figures 3 and 4. However, there was an increase in titratable acidity with a reduction in pH of the traditionally fermented food samples (ogi and fufu). There was a general decrease in pH from 6.32 to 5.02 (White Ogi), 6.20 to 4.58 (Yellow Ogi) and 6.43 to 4.29 (Fufu) after 96 h whereas titratable acidity increased from 0.35 to 0.70 (White Ogi), 0.30 to 0.60 (Yellow Ogi) and 0.15 to 0.60 after 96 h.
Isolation and identification of 11 LAB isolates were preliminarily isolated from traditionally fermented food samples (Ogi and Fufu) collected from a local market in Uyo, Akwa Ibom State and six LAB strains that showed a strong antibacterial activity against the indicator organisms were selected and preserved for further analysis. Based on the morphological, physiological and biochemical characteristics, these isolates were Gram-positive, rods (bacilli)-cocci, non-sporulating, non-motile, acid-tolerant, catalase and oxidase negative which grew in both aerobic and anaerobic environments (Tables 2 and 3).

The antimicrobial activity of all the tentative Lactobacillus strains had between 7.00 ± 0.00 and 22.00 ± 0.71 mm inhibitory zones towards the food spoilage organisms (Table 4). Six isolates designated as OG1, W22, W12, Y11, F12 and F6 showed a strong antibacterial activity against P. aeruginosa, E. aerogene and B. cereus ranging from 11.25±1.06 to 22.00±0.71 mm. The highest diameters of 22.00±0.71 and 20.20±0.42 mm were recorded for isolates coded F12, and OG1, while no activity was recorded for OG6.
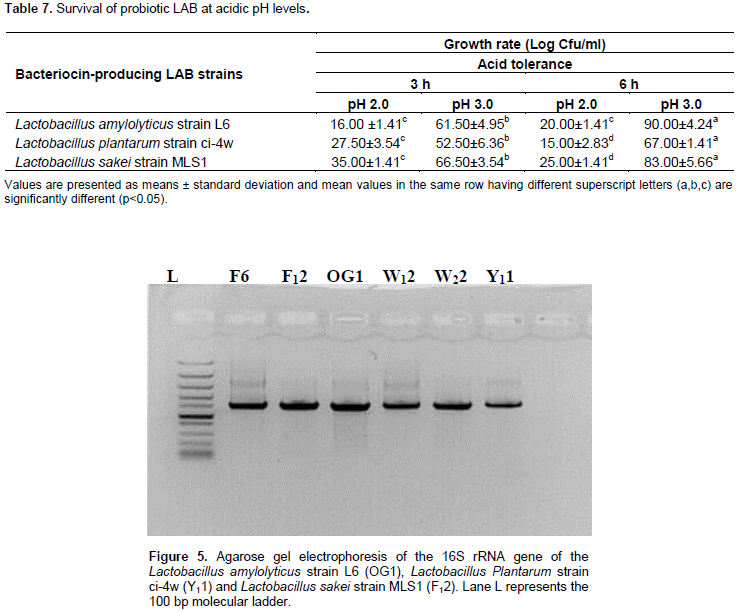
These isolates were identified on the basis of genotypic characteristics (16S rRNA gene sequences similarity with the type strains) during BLAST searches as Lactobacillus amylolyticus strain L6, Lactobacillus plantarum strain ci-4w and Lactobacillus sakei strain MLS1 (Table 5).


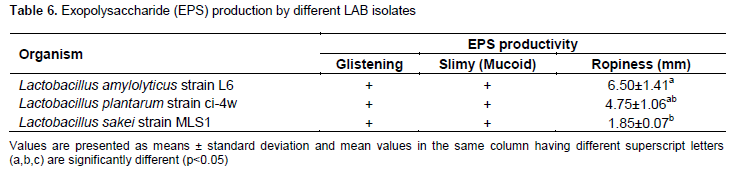
Table 6 shows the exopolysaccharide (EPS) production of different LAB isolates and the results revealed that L. amylolyticus strain L6 showed the highest ropy strand formation.
In Table 7, the result showed that these Lactobacillus strains tolerated pH 2 and 3 of the medium for 3 and 6 h of incubation. Generally, there is a reduction in probiotic count, as they were exposed to pH 2 after 6 h with lower count 15.00±2.83 to 25.00±1.41 CFU/ml compared similarly at pH 3.0 after 6 h which recorded higher count 67.00±1.41 to 90.00±4.24 CFU/ml. The survival rate of these Lactobacillus strains supplemented with bile shows gradual decline in viable count as the bile concentrations increased (Figure 5).
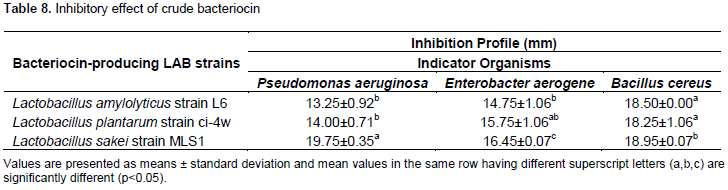
The antimicrobial activity of crude bacteriocin from different LAB-producing strains as depicted in Table 8 showed that bacteriocins produced by L. amylolyticus strain L6, L. plantarum strain ci-4w and L. sakei strain MLS1 have broad spectrum activity against Gram positive (Bacillus cereus) and Gram negative bacteria (P. aeruginosa and E. aerogene). It produced high inhibition zone diameters ranging from 13.25±0.92 to 19.75±0.35 mm against the food spoilage organisms.
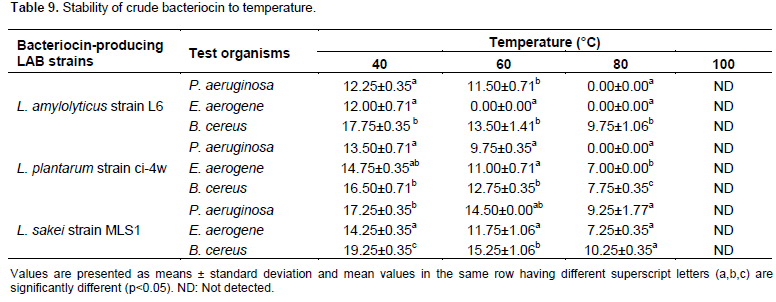
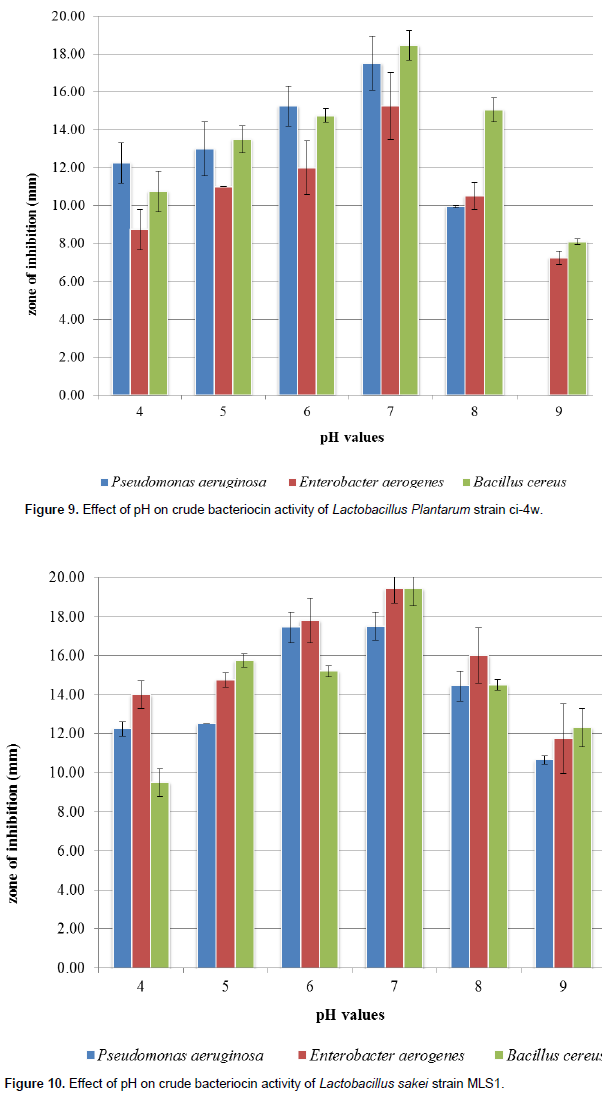
The effect of varied pH on inhibitory effects of bacteriocin indicates that bacteriocin activity was affected as different inhibition zones were observed among the various Lactobacillus strains against the indicator organisms (Figures 6 to 8). The highest antimicrobial productions were recorded mostly between pH 6.0 and 7.0, and inactivated at pH values above 8.0. The rate of antimicrobial production was indicated by increase in zones of inhibition (in diameter) from pH 5.0 to pH 7.0 followed by gradual decrease between pH 8.0 and 9.0.
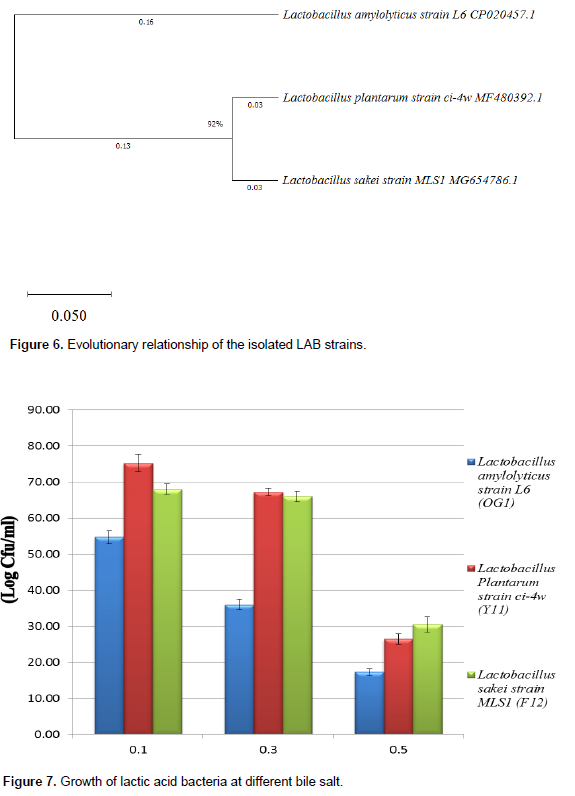
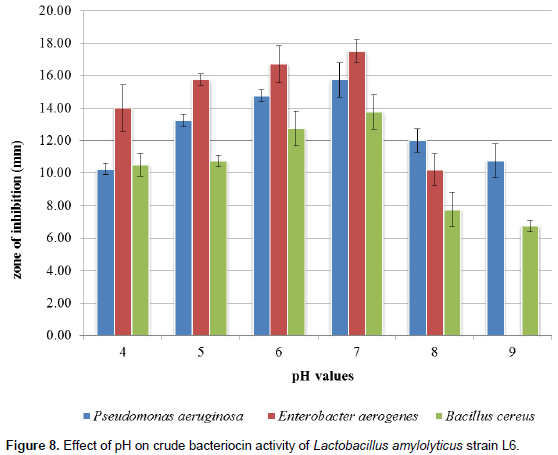
Table 9 shows the effect of temperature on the bacteriocin activity against P. aeruginosa, E. aerogenes and B. cereus. Among the bacteriocins produced by the isolated Lactobacilli, however, bacteriocin produced by L. sakei strain MLS1 showed thermostability over a wide range of temperature from 40 to 80°C for 10 min. Generally, as the temperature increases, the decreases of antibacterial activity of the crude bacteriocins occur. Result showed that the highest significant (P < 0.05) zone of inhibition was recorded against B. cereus (19.25±0.35a mm) at 40°C for 10 min.
The effect of varied storage time (h) on bacteriocin activity was noted as different zones of inhibition were observed among the various Lactobacillus strains against the indicator organisms (Table 10). The highest inhibitory effects were recorded at 24 and 48 h storage period. The rate of antimicrobial production was indicated by increase in diameter of zones of inhibition from 24 to 48 h followed by gradual decrease between the storage time of 72 and 96 h.
DISCUSSION
Based on preliminary data obtained in this study, a total of 11 LAB strains isolated from different fermented food samples (white Ogi, yellow Ogi and fufu) were screened for antimicrobial activity against three indicator organisms (P. aeruginosa, E. aerogenes and B. cereus). Out of these LAB isolates (n=11), six LAB isolates exhibited good antimicrobial activity with the highest significant (p < 0.05); they were selected on the basis of their antimicrobial activities against indicator strains and were designated as W22 , W12, Y11, OG1, F12 and F6 based on the source of their isolation with OG1 and F12 having the highest inhibitory diameter of 18.25±0.35 and 22.00±0.71 mm against Bacillus cereus, 20.20±0.42 and 18.75±0.35 mm against P. aeruginosa, and 12.50±0.71 and 15.25±1.77 mm against E. aerogene, respectively (Table 4). Values obtained for this test coincide for some strains with the work of Amara et al. (2019), where E. aerogenes and Citrobacter freundii strains were inhibited by all LAB with inhibition zones ranging between 13 and 21 mm in diameter and antagonism of Lactobacilli was also observed on B. cereus, Staphylococcus aureus ATCC 433005, Enterobacter cloacae, Escherichia coli ATCC 25922, P. aeruginosa ATCC 27853 and Klebsiella pneumonia. Due to several antagonistic characteristics of LAB which include: bacteriocins or hydrogen peroxide production, lowering of pH by production of organic acids in the medium, and nutrients competition, inhibits the spoilage organisms/pathogens (Bezkorvainy, 2001; Isolauri et al., 2004; Charlier et al., 2009; Merzoug et al., 2016, 2018). The maximum antibacterial potential shown by these lactobacillus strains against the food spoilage bacteria indicated that these strains could be used as probiotics and biopreservatives for extending the storage life and quality of food.
The LAB isolates were characterized and identified on the basis of morphological, physiological, biochemical and genotypic characteristics (16S rRNA gene sequences similarity with the type strains) (Tables 2, 3 and 5).
The identification carried out for representative Lactobacillus species from the traditional fermented food products (ogi and fufu) demonstrated the dominance of L. amylolyticus strain L6, L. plantarum strain ci-4w and L. sakei strain MLS1. Lactobacillus species have been reported to be predominant in fermented foods (Ogunshe et al., 2007). Nwokoro and Chukwu (2012) reported that the microbial composition of Akamu sample within the first 48 h of fermentation included Lactobacillus delbrueckii, L. plantarum, Lactobacillus fermentum and Lactobacillus amylovorus.
Changes in pH and titratable acidity during the ogi and fufu fermentation showed a marginal increase in titratable acidity with reduction in pH values (Figures 3 and 4).
However, fermentation caused a general decrease in pH from 6.32 to 5.02 (White Ogi), 6.20 to 4.58 (Yellow Ogi) and 6.43 to 4.29 (Fufu) after 96 h whereas titratable acidity increased from 0.35 to 0.70 (White Ogi), 0.30 to 0.60 (Yellow Ogi) and 0.15 to 0.60 during 96 h fermentation. The reduction of the pH values towards acidity was possibly due to fermentation by the LAB (Abegaz, 2007). According to Inyang and Idoko (2006), an increase in acidity as the fermentation progressed was because of the accelerated growth rate of LAB. The amount of acid produced during fermentation increased exponentially with decrease in pH is in agreement with the findings of Nwachukwu and Ijeoma (2010b) which indicated an increase in titratable acidity with a reduction in pH during fermentation of maize for Ogi. Also, Nwokoro and Chukwu (2012) observed pH decreased from 6.6 at the start of the Akamu fermentation to 3.9 after 72 h while titratable acidity increased from 0.48 at 0 h to 0.79 after 72 h. During the steeping of maize grains for Ogi production, the activities of lactic acid bacteria which is responsible for the production of lactic acid may result to decrease in pH (Odunfa and Adeyele, 2000). Whereas an increase in titratable acidity with declined pH during 96 h of fufu fermentation was in accordance with Achi and Akomas (2006) who demonstrated that the initial pH of retted cassava samples was 6.8, followed by subsequent decrease to 3.8 at the end of 96 h fermentation. Also, Oyedeji et al. (2013) observed that the pH of the fermenting cassava roots decreased from 5.6 to 3.7 during 72 h fermenting period while the total titratable acidity (in % lactic acid) increased from 0.07 ± 0.01 to 0.21 ± 0.01 and decreased to 0.09 by the end of 72 h fermentation period whereas the pH of fermenting maize grains dropped from 5.9 to 3.8 by the end of the 72 h fermentation period while the total titratable acidity increased from 0.13 ± 0.01 to 0.28 ± 0.01 after 24 h and then decreased to 0.14 by the end of fermentation period which is significant to this present study which confirms that there is a reduction in the pH with increase in the titratable acidity (production of organic acids) during ogi and fufu fermentation.
The screening for exopolysaccharide (EPS) producing Lactobacilli revealed that all studied lactic acid bacteria strains (n=3), showed slimy and ropy colonies, however, Lactobacillus amylolyticus strain L6 isolated from white Ogi showed highly ropy strand formation of 6.50±1.41 mm followed by L. plantarum strain ci-4w isolated from yellow Ogi with 4.75±1.06 mm (Table 6) and this is in conformity with the results of Mostefaoui et al. (2014) which revealed the presence of twelve (12) mucoid and ropy strains of Lactobacillus of twenty six (26) tested strains in culture collection. This finding is also similar to the investigation of Adebayo-Tayo and Onilude (2008) which showed that more than 64.29% of the studied L. plantarum strains were active producers of exopolysaccharide, also, the EPS produced by L. plantarum (LPWO11) strain isolated from “white Ogi” and L. casei ssp tolerans (LCN6) isolated from “fufu” had the highest while the strain isolated from “brown Ogi” (LPBOI4) had the lowest EPS activity. Moreover, Mostefaoui et al., (2014) has reported that microbial EPS are not consumed as an energy source by the producing microorganism, but are released to protect the producer organism during starvation conditions and also at extreme pH and temperature conditions (Mostefaoui et al., 2014). Therefore, exopolysaccharide production is considered an important probiotic attribute of lactic acid bacteria in the present search for human probiotic LAB.
Similarly, other important criteria to be a good source of probiotics are the tolerance to bile and high acid levels, which is present in the small intestine of the stomach (Dunne et al., 2001; Jena et al., 2013; Garcia et al., 2016). The three LAB strains, L. amylolyticus strain L6, L. plantarum strain ci-4w and L. sakei strain MLS1 showed acid tolerance at pH 2 and pH 3 (Table 7), and high bile salt tolerance at 0.3 and 0.5% (Figure 7) which is considered a prerequisite for colonization and metabolic activity of these LAB strains in the small intestine of the host. As cited by Sultana et al. (2000) and Chan and Zhang (2005), low pH environments are thought to inhibit the metabolism activity and growth of LAB, thus reducing the probiotics’ viability as aciduric members of LAB, as these cells were proven to be vulnerable at pH 2.0 and
below, where L. acidophilus could not survive in acidic pH environment. In this present study, L. amylolyticus strain L6, L. plantarum strain ci-4w and L. sakei strain MLS1 growth decreased with increasing duration of 6 h at pH 2 and remained constantly stable at higher pH 3 for 6 h which is in accordance with the study conducted by Mandal et al. (2006). This is also in agreement with the findings of Fernandez et al. (2003) who reported that good probiotic sources should withstand at least pH range of 3.0 but it contrasts the findings of Sahadeva et al. (2011) who reported that there was a reduction in probiotic count, as they were exposed to pH 1.5 and 3.0 and the count was fairly constant at pH 7.2 (control). The present finding indicates that there is no correlation between pH 2 and 3 during the growth period (h) of LAB strains because as viable count at pH 2 was decreasing, viable count at pH 3 was increasing.
The isolated probiotic strains proved to exhibit an excellent quality of bile tolerance. Data obtained from the acid tolerance study indicated that LAB strains that survive at high acidic pH 2 were also able to grow in the presence of the subsequent bile salt test. The findings in this study are in accordance to Leyer and Johnson (1993) who postulated that acid and bile have separate and combined effects on the growth of bacteria as bile stress takes place after pH stress in the stomach. The enhanced survival capabilities appeared to be due to the adaptation ability or acclimatization of the bacteria to the low pH environment, therefore minimizing the relative toxicity to glycoconjugates in the intestine (Begley et al., 2005; Martoni et al., 2007). Therefore, the results of the present study on acid and bile tolerance exhibit that these. Lactobacilli will reach the small intestine and colon and thus contribute in balancing the intestinal microflora. However, studies concerning bacteriocins produced by LAB have received an increasing interest because of the potential use of bacteriocins as food preservatives and bacteriocins are supposed to act only on closely related species, which limits their application as a good preservative (Cleveland et al., 2001). In contrast, this study revealed that bacteriocins produced by L. amylolyticus strain L6, L. plantarum strain ci-4w and L sakei strain MLS1 have broad spectrum activity against Gram positive (B. cereus) and Gram negative bacteria (P aeruginosa and E aerogenes). It showed a good antagonistic activity against these potent food spoilage organisms, which shows its efficacy and potential application as a natural food preservative. Adesokan et al. (2009) recorded a similar result against P. aeruginosa, S . aureus, and E. coli. Todorov et al. (2012) also obtained similar results in the case of bacteriocin produced by L. sakei ST22Ch which inhibited the growth of Pseudomonas and Staphylococcus species. However, several investigation revealed that L. plantarum strains produce a broad range of bacteriocins such as ST28MS, ST26MS, bacST202Ch, bacST216Ch, ST71KS, AMA-K, plantaricin B, D, G, K, etc., (Enan et al., 1996; Todorov and Dicks, 2005; Hata et al., 2010; Gong et al., 2010; Martinez et al., 2013). According to the report by Dinev et al. (2017), L. plantarum exerts inhibitory activity against a variety of potentially harmful Gram-positive bacteria including Listeria monocytogenes, S. aureus and some members of the genera Bacillus, Clostridium, and Enterococcus as well as being active against many Gram-negative pathogens and food spoilage microorganisms including Escherichia coli (including enteropathogenic, enterotoxigaenic, enteroinvasive, multidrug-resistant enteroaggregative E. coli and E. coli 0157:H7), P. aeruginosa, Yersinia enterocolitica, Campylobacter jejuni, Helicobacter pylori, Klebsiella, Salmonella, and Shigella species which is in conformity with the present study. Report by Ogunshe et al. (2007) reported that Lactobacillus strains produced a bacteriocin compounds which can inhibit several bacteria. Lactic acid bacteria have potentials to inhibit the growth of pathogenic and food spoilage bacteria and the possibilities exist for their use as probiotic LAB (Obi, 2018). Therefore, possession of bacteriocin in Lactobacilli reveals their probiotic and biopreservation potentials.
Bacteriocin production as well as its activity seemed to be influenced by some factors such as the pH of culture
medium, temperature and incubation conditions or storage time. Wang et al. (2010) reported that cell aggregation and medium composition can affect bacteriocin production by LAB. This recent pH optimization studies indicate that bacteriocins produced by L. amylolyticus strain L6, L. plantarum strain ci-4w and Lactobacillus sakei strain MLS1 proved to have high stability at pH 5, 6 and 7; the bacteriocin activity was found to be highest at pH 7 with highest significant (p < 0.05) zone of inhibition of 19.40±071 mm, whereas considerable decrease was observed at both acidic (pH 4) as well as alkaline (pH 8 to 9) (Figures 8 to 10) which cohere with the report of Tambekar and Bhutada (2010) that bacteriocin were stable in acidic to neutral range, that is, from pH 3.0 to 7.0 but it became inactive in alkaline range at pH 9.0. Holzapfel et al. (2010) also reported that L. plantarum excreted other compounds such as bacteriocins that inhibited the growth of pathogens. However, this present study also indicates the strong biopreservation potential of these bacteriocins which may be used to extend the shelf life of food and also implies that bacteriocin obtained from L. amylolyticus strain L6, L. plantarum strain ci-4w and L. sakei strain MLS1 will be effective against both Gram positive and Gram negative bacteria such as P. aeruginosa, E. aerogenes and Bacillus cereus with maximum activity at neutral pH. This is similar to the report by Adebayo and Famurewa (2002) who opined that bacteriocin of Lactobacillus were active over a wide range of pH 2 to 6 and it is the optimum pH range for good inhibitory activity of bacteriocin from Lactobacillus strains against a wide range of various pathogenic organisms, while inactivation occurred mostly at pH 12.
Exposure of the crude bacteriocin to heating temperature had significant effects (P<0.05) on the antibacterial activity of the crude bacteriocin in this present investigation. The result showed that the bacteriocin activity of L. amylolyticus strain L6 and L. plantarum strain ci-4w was most stable at 60°C for 10 min against all the indicator organisms although its resistance was observed up to 80°C for 10 min with partial loss of activity. This is in accordance with the work of Heredia-Castro et al. (2015) which showed that the antimicrobial activity of crude bacteriocin against S. aureus, E. coli, Salmonella Typhimurium, and Listeria innocua was stable at 65°C for 30 min but antimicrobial activity decreased to some extent at 100°C for 30 min and was most unstable at 121°C for 15 min. However, this present study exhibits that bacteriocin produced by L. sakei strain MLS1 showed thermo-stability over a wide range of temperature from 40 to 80°C for 10 min. This bacteriocin maintained full stability after heat treatment at 40, 60 and 80°C for 10 min but its efficacy decreased with the continuous increase in temperature, supporting the work of Padmavathi and Radha (2015). This result is in agreement with Veskovic-Moracanin et al. (2010) who stated that results of the examination of high temperatures on the activity of bacteriocin isolated from L. sakei I 154 implied its emphasized thermo resistance. Similar results by Ageni et al. (2017) and Ponce et al. (2008) reported that a number of bacteriocins produced by Lactobacillus strains were resistant at 100°C for 15 min.
The storage stability of crude bacteriocin by L. amylolyticus strain L6, L. plantarum strain ci-4w and L. sakei strain MLS1 was affected as different zones of inhibition were observed against the indicator organisms at varied storage time (h) (Table 10). Maximum activity was noted at 24 and 48 h storage period ranging from 9.75±1.06 to 18.50±0.71 mm against the food spoilage microorganisms. There were significant differences between the various storage times (at P<0.05). There were observable reduction bacteriocin activities as incubation time prolonged. This result was in total agreement with Onwuakor et al. (2014) who observed reduction of crude supernatant activities as incubation time dropped, they reported that optimum crude supernatant production was observed after 72 h judged by the zones of inhibition against the indicator. Whereas Tulini et al. (2011) observed a reduction of the bacteriocin production at 96 h when compared with the control incubation (24 h). This present result is in complete agreement with Obi (2015) and Elayaraja et al. (2014), who reported that the highest bacteriocin activity of Lactobacillus tucceti CECT 5920 was recorded within the first 1to 3 days against S. aureus NCTC 8325 and E. coli 0157:H7.
CONCLUSION
In this study, the selected fermented food products (Ogi and Fufu) contain several strains of Lactobacilli, which are capable of producing antimicrobial compounds such as bacteriocins and have potential for exopolysaccharide (EPS) production. Among the isolated LAB from Ogi and Fufu, L. amylolyticus strain L6, L. plantarum strain ci-4w and L. sakei strain MLS1 exhibit good in vitro probiotic and biopreservative capacities. These strains were found to have wide pH tolerance, varying bile salt tolerance and antibacterial activities. The antibacterial activity shown by these strains could be useful to control undesirable contaminations in food industries. However, elimination of acid and hydrogen peroxide effect in cell free supernatant did not have any effect on the inhibitory activity of the bacteriocin rather the partially purified bacteriocin produced from L. amylolyticus strain L6, L. plantarum strain ci-4w and L. sakei strain MLS1 were found to be active against Gram positive (B. cereus) and Gram-negative bacteria (P. aeruginosa and E aerogene) suggesting its broad spectrum of activity and when harnessed under different culture conditions showed resistance to acidic pH more than basic pH, heat stable and retain its activity for a longer period which in turn will increase the shelf life of food which is considered an important criteria to be used in the modern food industry as biopreservative.
Therefore, L. amylolyticus strain L6, L. plantarum strain ci-4w and L. sakei strain MLS1 may be recommended as potential bacteriocin producers in the pharmaceutical industries to replace conventional antibiotics in combating pathogens that are vastly acquiring antimicrobial resistance and in the food processing industries to enhance extension of shelf life of food products; also, to replace the use of chemical preservatives as many chemicals used for the inactivation of food spoilage organisms and pathogens in order to preserve food products for longer period is being declined due to undesirable effects such as alteration of organoleptic and nutritional properties of food and their adverse toxic effects on human body system. Moreover, these strains can be used as starter culture or protective cultures to improve the hygiene, quality and increased safety of the food products by inhibiting the food borne pathogens and spoilage microorganisms.
These results provided a basis for performing future studies on more purification steps of the bacteriocins for application as food preservative; also, assessment of in vivo probiotic characteristics of these potential Lactobacillus strains is encouraged.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
REFERENCES
|
Abegaz K (2007). Isolation, characterization and identification of lactic acid bacteria involved in traditional fermentation of borde, an Ethiopian cereal beverage. African Journal of Bacteriology 6(12):1469-1478. |
|
|
Achi OK, Akomas NS (2006). Comparative Assessment of Fermentation Techniques in the Processing of Fufu, a Traditional Fermented Cassava Product. Pakistan Journal of Nutrition 5(3):224-229. |
|
|
Adak K, Long S, O'Brien S (2002). Trends in indigenous foodborne disease and deaths. England and Wales: 1992 to 2000. Gut 51(6):832-841. |
|
|
Adebayo CO, Famurewa O (2002). Antimicrobial effectiveness of in-house-use concentration of disinfectants in a Nigerian hospital. I Technolscience 6(1):17-20. |
|
|
Adesokan IA, Odetoyinbo BB, Okanlawon BM (2009). Optimization of Lactic Acid Production by Lactic Acid Bacteria Isolated from Some Traditional Fermented Food in Nigeria. Pakistan Journal of Nutrition 8(5):611-615. |
|
|
Ageni LV, Ajibade GA, Yerima B, Appah J (2017). Shelf life extension study of ogi and fufu using bacteriocin isolated from Lactobacillus acidophilus of fermented dairy products. African Journal of Microbiology Research 11(32):1286-1293. |
|
|
Amara S, Zadi-Karam H, Karam N (2019). Selection of Lactobacillus strains newly isolated from Algerian camel and mare fermented milk for their in vitro probiotic and lipolytic potentials. African Journal of Biotechnology 18(30):882-894. |
|
|
Ananou S, Maqueda M, Martínez-Bueno M, Valdivia E (2007). Biopreservation, an ecological approach to improve the safety and shelf-life of foods In:.Communicating Current Research and Educational Topics and Trends in Applied Microbiology. A. Méndez-Vila (Ed.), Formatex. |
|
|
Anumudu CK, Omeje FI, Obinwa GN (2018). Microbial succession pattern in Ogi fermentation. International Journal of Advanced Research in Biological Sciences 5(7):247-251. |
|
|
Ayoade F, Paulina OA, Kellanny SA, Yeitarere AA, Titilayo OA, Scott OF, Onikepe F (2018). The predominant lactic acid microorganisms and proximate composition of spontaneously fermented gari and fufu, cassava food products. Annual Research and Review in Biology 1-12. |
|
|
Bali V, Panesar S, Bera B (2011). Isolation, Screening and Evaluation of Antimicrobial Activity of Potential Bacteriocin Producing Lactic Acid Bacteria Isolate. Microbiology Journal 1(3):113-119. |
|
|
Begley M, Gahan CGM, Hill C (2005). The interaction between bacteria and bile. FEMS Microbiology Reviews 29(4):625-651. |
|
|
Bezkorvainy A (2001). Probiotics: Determinants of survival and growth in the gut. The American Journal of Clinical Nutrition 73(2):399s-405s. |
|
|
Broberg A, Jacobsson K, Ström K, Schnürer J (2007). Metabolite profiles of lactic acid bacteria in grass silage. Applied and Environmental Microbiology 73(17):5547-5552. |
|
|
Brown AC, Valiere A (2004). "Probiotics and Medical Nutrition Therapy". Nutrition in clinical care: an official publication of Tufts University 7(2):56-68. |
|
|
Bukola CA, Abiodun AO (2008). Screening of Lactic Acid Bacteria Strains Isolated from Some Nigerian Fermented Foods for EPS Production. World Applied Sciences Journal 4(5):741-747. |
|
|
Chan ES, Zhang Z (2005). Bioencapsulation by compression coating of probiotic bacteria for their protection in an acidic medium. Process Biochemistry 40:3346-3351. |
|
|
Charlier CM, Cretenet S, Even Y, Le Loir Y (2009). Interactions between Staphylococcus aureus and lactic acid bacteria: An old story with new perspectives. International Journal of Food Microbiology 131(1):30-39. |
|
|
Cleveland J, Montville TJ, Nes IF, Chikindas ML (2001). Bacteriocins: safe, natural antimicrobials for food preservation. International Journal of Food Microbiology 71(1):1-20. |
|
|
De Martinis E, Bernadette D, Franco B (2001). Inhibition of Listeria Monocytogenesin a pork product by a Lactobacillus sake strain. International Journal of Food Microbiology 42(1-2):119-126. |
|
|
Dinev T, Beev G, Tzanova M, Denev S, Dermendzhieva D, Stoyanova A (2017). Antimicrobial activity of lactobacillus plantarum against pathogenic and food spoilage microorganisms: A Review. Bulgarian Journal of Veterinary Medicine 21(3). |
|
|
Dunne C, O'Mahony L, Murphy L, Thonton G, Morrissey D, O'Halloran S, Feeney M, Flynn S, Fitzgerald G, Daly C, Kiely B, O'Sullivan GC, Shanahan F, Collins JK (2001). In vitro selection criteria for probiotic bacteria of human origin: correlation with in vivo findings. The American Journal of Clinical Nutrition 73(2):386s-392s. |
|
|
Elayaraja S, Annamalai N, Mayavu P, Balasubramanian T (2014). Production, purification and characterization of bacteriocin from Lactobacillus murinus AU06 and its broad antibacterial spectrum. Asian Pacific Journal of Tropical Biomedicine 4:S305-S311. |
|
|
Enan G, El-Essawy A, Uyttendaele M, Debevere J (1996). Antibacterial activity of Lactobacillus plantarum isolated from dry sausage: characterization production and bactericidal action of plantaricin UG1. International Journal of Food Microbiology 30:189-215. |
|
|
Eze EI, Echezona BC, Uzodinma EC (2011). Isolation and identification of pathogenic bacteria associated with frozen mackerel fish (Scomber scombrus) in a humid tropical environment. African Journal of Agricultural Research 6(8):1947-1951. |
|
|
Garcha S (2018). Control of food spoilage molds using lactobacillus bacteriocins. Journal of Pure and Applied Microbiology 12(3):1365-1373. |
|
|
Garcia EF, Luciano WA, Xavier DE, da Costa WC, de Sousa OK, Franco OL (2016). Identification of lactic acid bacteria in fruit pulp processing byproducts and potential probiotic properties of selected Lactobacillus strains. Frontiers in Microbiology 7:1371. |
|
|
Garcia P, Rodriguez L, Rodriguez A, Martinez B (2010). Food biopreservation: Promising strategies using bacteriocins, bacteriophage and endolysins. Trends in Food Science and Technology 21(8):373-382. |
|
|
Gong H, Meng, X, Wang H (2010). Plantaricin MG active against Gram-negative bacteria produced by Lactobacillus plantarum KLDS1.0391 isolated from "Jiaoke", a traditional fermented cream from China. Food Control 21(1):89-96. |
|
|
Harrigan WF, McCance ME (1996). Laboratory methods in Microbiology. Academic press: London, p. 342. |
|
|
Hassanzadazar H, Ehsani A, Mardani K, Hesari J (2012). Investigation of antibacterial, acid and bile tolerance properties of lactobacilli isolated from Koozeh cheese. Veterinary Research Forum 3(3):181-185. |
|
|
Hata T, Tanaka R, Ohmomo S (2010). Isolation and characterization of plantaricin ASM1: A new bacteriocin produced by Lactobacillus plantarum A-1. International Journal of Food Microbiology 137(1):94-99. |
|
|
Hati S, Mandal S, Prajapati JB (2013). Novel starters for value added fermented dairy products. Current Research in Nutrition and Food Science Journal 1(1):83-91. |
|
|
Heredia-Castro PY, Méndez-Romero JI, Hernández-Mendoza A, Acedo-Félix E, González-Córdova AF, Vallejo-Cordoba B (2015). Antimicrobial activity and partial characterization of bacteriocin-like inhibitory substances produced by Lactobacillus spp. isolated from artisanal Mexican cheese. Journal of Dairy Science 98(12):8285-8293. |
|
|
Hleba L, Císarová M, Glinushkin A, Laishevtcev A, Derkanosova A, Igor P, Shariati MA (2021). Physicochemical, functional and sensory properties of acha-tamba based ogi enriched with hydrolysed soy peptides. Journal of Microbiology, Biotechnology and Food Sciences 9(4):823-830. |
|
|
Holt JG, Krieg NR, Sneath PH, Stalely JT, Williams ST (1994). Bergey's manual of determinative bacteriology. Bergey's Manual of Determinative Bacteriology. Williams and Wilkins. |
|
|
Holzapfel WH, Cho G, Huch M, Franz CMP (2010). Genetic Analysis of the Plantaricin Efl. Locus of Lactobacillus plantarum PCS20 reveals an unusual plantaricin E gene sequence as a result of mutation. International Journal of Food Microbiology 141:5117-S124. |
|
|
Ibeabuchi JC, Olawuni IA, Iheagwara MC, Ojukwu M, Ofoedu CE (2014). Microbiological Evaluation of 'Iru' and 'Ogiri-Isi' Used As Food Condiments. Journal of Environmental Science, Toxicology and Food Technology 8(8):45-50. |
|
|
Inyang CU, Idoko CA (2006). Assessment of the quality of Ogi made from malted millet. African Journal of Biotechnology 5(22):2334-2337. |
|
|
Isolauri E, Salminen S, Ouwehand A (2004). Microbial-gut interactions in health and disease, Probiotics. Best Practice and Research Clinical Gastroenterology 18(2):299-313. |
|
|
Jena PK, Trivedi D, Thakore K, Chaudhary H, Giri SS, Seshadri S (2013). Isolation and characterization of probiotic properties of lactobacilli isolated from rat fecal microbiota. Microbiology and Immunology 57(6):407-416. |
|
|
Karthikeyan V, Santosh SW (2009). Isolation and partial characterization of bacteriocin produced from Lactobacillus plantarum. African Journal of Microbiology Research 3(5):233-239. |
|
|
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018). MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Molecular Biology and Evolution 35(6):1547-1549. |
|
|
Leyer GL, Johnson EA (1993). Acid adaptation induces cross-protection against environmental stresses in Salmonella typhimurium. Applied and Environmental Microbiology 59(6):1842-1847. |
|
|
Mandal S, Puniya AK, Singh K (2006). Effect of alginate concentration on survival of encapsulated Lactobacillus casei NCDC-298. International Dairy Journal 16(10):1190-1195. |
|
|
Martinez R, Wachsman M, Torres N, LeBlanc J, Todorov S, de Melo Franco B (2013). Biochemical, antimicrobial and molecular characterization of a noncytotoxic bacteriocin produced by Lactobacillus plantarum ST71KS. Food Microbiology 34(2):376-381. |
|
|
Martoni C, Bhathena J, Jones ML, Urbanska AM, Chen HM, Prakash S (2007). Investigation of microencapsulated BSH active Lactobacillus in the simulated human GI tract. Journal of Biomedicine and Biotechnology 12:1-9. |
|
|
Maurya P, Thakur L (2012). Inhibition spectrum, purification and characterization of bacteriocin from Leuconostoc NT-1. Current Science 103(12):1405-1407. |
|
|
Merzoug M, Dalache F, Zadi Karam H, Karam NE (2016). Isolation and preliminary characterisation of bacteriocin, produced by Enterococcus faecium GHB21 isolated from Algerian paste of dates "ghars". Annals of Microbiology 66(2):795-805. |
|
|
Merzoug M, Mosbahi K, Walker D, Karam NE (2018). Screening of the Enterocin-Encoding Genes and Their Genetic Determinism in the Bacteriocinogenic Enterococcus faecium GHB21. Probiotics and Antimicrobial Proteins 11(1):325-331. |
|
|
Mostefaoui A, Hakem A, Yabrir B, Boutaiba S, Badis A (2014). Screening for exopolysaccharide producing strains of thermophilic lactic acid bacteria isolated from Algerian raw camel milk. African Journal of Microbiology Research 8(22): 2208-2214. |
|
|
Nath S, Chowdhury S, Sarkar S, Dora C (2013). Lactic Acid Bacteria - A Potential Biopreservative In Sea Food Industry. International Journal of Advanced Research 1(6):471-475. |
|
|
Ngene AC, Onwuakor CE, Aguiyi JC, Ifeanyi VO, Ohaegbu CG, Okwuchukwu CP, Egbere JO (2019). Screening of Some Lactic Acid Bacteria Isolated from Selected Nigerian Fermented Foods for Vitamin Production. Advances in Microbiology 9(11):943-955. |
|
|
Nwachukwu E, Ijeoma O (2010b). Isolation and characterization of Lactic Acid Bacteria associated with the fermentation of a cereal-based product for the development of a starter culture. Food 4(1):45-48. |
|
|
Nwokoro O, Chukwu BC (2012). Studies on Akamu, a traditional fermented maize food. Revista Chilena de Nutrición 39(4):180-184. |
|
|
Obi CN (2018). In vitro screening for human probiotic potentials of Lactobacillus tucceti CECT 5920 and Lactobacillus mindensis TMW isolated from Nigerian Fermented foods. The Bioscientist Journal 6(1):13-30. |
|
|
Odunfa SA, Adeyele S, (2000). "Preliminary Study of the Effect of Lactic Fermentation on the Rheology and pH of Ogi Porridge", Journal of Food Microbiology 20(25):71-75. |
|
|
Ogunshe AA, Omotosho MA, Adeyeye JA (2007). In vitro Antimicrobial Characteristic of Bacteriocin Producing Lactobacillus strains from Nigeria Indigenous Fermented Food. African Journal of Biotechnology 6(4):445-453. |
|
|
Onwuakor CE, Nwaugo VO, Nnadi CJ, Emetole JM (2014). Effect of Varied Culture Conditions on Crude Supernatant (Bacteriocin) Production from Four Lactobacillus Species Isolated from Locally Fermented Maize (Ogi). American Journal of Microbiological Research 2(5):125-130. |
|
|
Oyedeji O, Ogunbanwo ST, Onilude AA (2013). Predominant Lactic Acid Bacteria Involved in the Traditional Fermentation of Fufu and Ogi, Two Nigerian Fermented Food Products. Food and Nutrition Sciences 4(11):40-46. |
|
|
Padmavathi T, Radha KR (2015). Purification, characterization and application of bacteriocin for improving the shelf-life of sprouts: An approach to Bio preservation. Indicator 8(9):10. |
|
|
Patel A, Lindström C, Patel A, Prajapati JB, Holst O (2012). Probiotic properties of exopolysaccharide producing lactic acid bacteria isolated from vegetables and traditional Indian fermented foods. International Journal of Fermented Foods 1(1):87-101. |
|
|
Ponce AG, Moreira MR, Del Valle CE, Roura SI (2008). Preliminary characterization of bacteriocin-like substances from lactic acid bacteria isolated from organic leafy vegetables. LWT-Food Science and Technology 41(3):432-441. |
|
|
Radha KR, Padmavathi T (2015). Purification, characterization and application of bacteriocin for improving the shelf-life of sprouts: An approach to Bio preservation. International Journal of ChemTech Research 8(8):265-277. |
|
|
Rijkers GT, de Vos WM, Brummer RJ, Morelli L, Corthier G, Marteau P (2011). Health benefits and health claims of probiotics: Bridging science and marketing. British Journal of Nutrition 106 (9):1291-1296. |
|
|
Ross P, Morgan S, Hill C (2002). Preservation and fermentation: past, present and Future. International Journal of Food Microbiology 79(1-2):3-16. |
|
|
Sahadeva RPK, Leong SF, Chua KH, Tan CH, Chan HY, Tong EV, Wong SYW, Chan HK (2011). Survival of commercial probiotic strains to pH and bile. International Food Research Journal 18(4):1515-1522. |
|
|
Saitou N, Nei M (1987). The neighbor-joining method: A new method for reconstructing phylogenetic trees. Molecular Biology and Evolution 4(4):406-425. |
|
|
Salem AM (2012). Bio-Preservation Challenge for Shelf-Life and Safety Improvement of Minced Beef. Global Journal of Biotechnology and Biochemistry 7(2):50-60. |
|
|
Sharma N, Kapoor G, Neopaney B (2006).Characterization of a new bacteriocin produced from a novel isolated strain of Bacillus lentus NG121. Antonie Van Leeuwenhoek 89(3):337-343. |
|
|
Sultana K, Godward G, Reynolds N, Arumugaswamy R, Peiris P, Kailasapathy K (2000). Encapsulation of probiotic bacteria with alginate-starch and evaluation of survival in simulated gastrointestinal conditions and in yoghurt. International Journal of Food Microbiology 62(1-2)):47-55. |
|
|
Tambekar DH, Bhutada SA (2010). Acid and bile tolerance, antibacterial activity, antibiotic resistance and bacteriocins activity of probiotic lactobacillus species. Recent Research in Science and Technology 2(4):94-98. |
|
|
Todorov S, Dicks L (2005). Lactobacillus plantarum isolated from molasses produced bacteriocins active against Gram negative bacteria. Enzyme and Microbial Technology 36(2-3):318-326. |
|
|
Todorov SD, Oliviera PS, Vaz-Velho (2012). Media Optimization of Bacteriocin ST22Ch Production by Lactobacillus sakei ST22Ch Isolated from Salpicao, a Traditional Meat-a product from Portugal, Chemical Engineering Transactions 27:283-288. |
|
|
Tulini FL, Gomes BC, de Martinis EC (2011). Partial purification and characterization of a bacteriocin produced by Enterococcus faecium 130 isolated from mozzarella cheese. Food Science and Technology 31(1):155-159. |
|
|
Van den Berg DJC, Smith A, Pot B, Ledeboer AM, Kerstens K, Verbakel JMA, Verrips CT (1993). Isolation, screening and identification of lactic acid bacteria from traditional food fermentation processes and culture collections. Food Biotechnology 7(3):189-205. |
|
|
Van Geel-Schuttená GH, Flesch F, ten Brink B, Smith MR, Dijkhuizen L (1998). Screening and characterization of Lactobacillus strains producing large amounts of exopolysaccharides. Applied Microbiology and Biotechnology 50(6):697-703. |
|
|
Veskovic-Moracanin S, Obradovic, D, Velebit, Branka B, Marija S, Turubatovic L (2010). Antimicrobial properties of indigenous Lactobacillus sakei strain. Acta Veterinaria 60(1):59-66. |
|
|
Vignolo G, Castellano P, Belfiore C, Fadda S (2008). A review of bacteriocinogenic lactic acid bacteria used as bio-protective cultures in fresh meat produced in Argentina. Meat Science 79(3):483-499. |
|
|
Wakil SM, Ajayi OO (2013). Production of lactic acid from Starchy-based food substrates. Journal of Applied Biosciences 71:5673-5681. |
|
|
Wang Q, Cui Y, Lackeyram D, Yuan L, Xu J, Wang W, Li Xu L (2010). Effect of cultural components on antimicrobial activity of bacteriocin produced by bacteria isolated from gut of poultry. African Journal of Microbiology Research 4(19):1970-1980. |
|
|
Wilson BA, Thomas SM, Ho M (2011). The human vaginal microbiome. In: Nelson KE, editor. Metagenomics of the human body. Germany: Springer. pp. 91-115. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0