ABSTRACT
Spermidine (Spd) is one of the most important polyamines (PAs) showing important roles in growth, development and stress responses in plants. The role of Spd in regulation of a dominant glutathione S-transferase (GST, E.C.2.5.1.18) and ionic balance of Na+ and K+ in onion leaves were examined. Onion GSTs were separated from fresh bulb tissue using DEAE-cellulose chromatography. Three GSTs were eluted at 56, 120 and 169 mM of KCl. Among them, GST2 containing >60% of total activity was termed as dominant GST and it was subsequently purified using affinity chromatography S-hexylglutathione-agarose. This purified protein was used for western blotting analysis of the GST in leaves of two months onion seedlings imposed on NaCl induced 16 dSm-1 salinity with or without foliar spray of 100 µM Spd on 1st, 3rd, 5th and 7th days of stress. The results suggested that dominant GST accumulated by salinity in onion seedling with or without Spd spray. However, at early saline stress, accumulation was significantly stronger in leaves without Spd than in those with Spd. At 7th day, GST band was increased and apparently seemed to be intensified in Spd sprayed leaves than those without spray. The values of Na+/K+ suggested that Spd maintained ionic balance better at early stage of stress than late stage.
Key words: Onion glutathione S-transferases (GST), ionic balance, salt stress, spermidine.
Glutathione S-transferases (GSTs, E.C.2.5.1.18) are a multigene family of isozymes, known to catalyze conjugation of tripeptide glutathione (GSH) to wide variety of electrophilic and hydrophobic substrates. GSTs
are a famous super family of enzymes for their role in detoxification reactions. It is well established that GSTs conjugate GSH to a variety of electrophilic compounds of both exogenous and endogenous origins (Cummins et al., 2011). GSTs have been found in all the organisms including bacteria and fungi (Frova, 2006; Perperopoulou et al., 2017). Although, primarily, plant GSTs were comprehensively studied for herbicide detoxification; recently, specific members of this family have been reported to confer tolerance toward herbicide in many species (Chronopoulou et al., 2017). GSTs catalyze the conjugation of thiol group of GSH and electrophilic substrate. The conjugate is either sequestered into the vacuoles or transferred from the cells by ATP-dependent transporter. In addition to herbicide detoxification, GSTs are involved in hormone biosynthesis, tyrosine degradation, breakdown peroxide breakdown (Oakley, 2011), stress signaling proteins (Loyall et al., 2000), nodule formation (Dalton et al., 2009) as well as ligandins for flavonoid-binding proteins (Mueller et al., 2000). GSTs have also been reported in involvement of different biological processes such as modulation of cell signaling kinases, ion channels, redox homeostasis and post-translational glutathionylation of proteins (Dixon et al., 2010). Plant GSTs have been reported to increase tolerance in different plant species under abiotic stresses (Ding et al., 2017) including heavy metal (Zhang et al., 2013), ultra-violet (UV) radiations (Liu and Li, 2002), salinity (Rohman et al., 2016a) and drought (Rohman et al., 2016b).
Polyamines (PAs), including diamine putrescine (Put), triamine spermidine (Spd) and tetraamine spermine (Spm), are abundant low-molecular-weight aliphatic amines involved in different biological and physiological process of growth and development of plants (Liu et al., 2015) and are also known for their anti-stress and anti-senescence effects due to their acid neutralizing, antioxidant and cell membrane stabilizing activities (Zhao and Yang, 2008). Due to their cationic nature at physiological pH, PAs are known to interact with proteins, nucleic acids, membrane lipids and cell wall constituents resulting in stabilizing these molecules (Khare et al., 2018). Apart from these, PAs are reported to involve in biotic and abiotic stresses defenses (Alcázar et al., 2006). It has been shown that a higher PAs concentration in cell correlates with plant tolerance to a wide array of environmental stresses (Liu et al., 2015). Several biochemical and physiological effects were provoked by exogenously applied PAs including Spd under environmental stress. Exogenous Spd was effective in enhancing the activity of peroxidase under salinity stress and the salt-induced increase in reducing sugar and free proline level was further promoted by Spd in indica rice (Roychoudhury et al., 2011). Moreover, it has been shown that over expression of Spd synthase gene in transgenic Arabidopsis thaliana maintained higher levels of Spd content and enhanced tolerance to salinity, chilling, hyperosmosis and drought comparative to the wild-type plants suggesting important role of Spd in stress signaling pathway to enhance stress tolerance mechanism for plants (Kasukabe et al., 2004). Onion is a model crop for GST showing higher GSH dependent detoxification enzymatic activity than other vegetable crops (Rohman et al., 2009). In our previous study, Spd up-regulated glutathione and ascorbic dependent enzymatic antioxidants in onion leaves conferring tolerance to salinity (Islam et al., 2016). In that study, GST activity was induced substantially with or without Spd in salinity stressed onion seedlings. However, specific GST isozyme contributing the activity was known. Moreover, information on ionic regulation by Spd in plant cell under saline condition is still limited. Therefore, the onion GSTs were separated to examine the accumulation of highly expressed GST. At the same time, ionic balance of Na+ and K+ by Spd application in onion seedlings under salinity was also reported.
Seedlings of two months old (Allium cepa L. var BARI Piaj-3) were used as plant material. They were grown in plastic bucket (30 L), under green house of Bangladesh Agricultural Research Institute (BARI). Homogenous mixture of organic matter and soil were used as growing media in the buckets and 10 seedlings were allowed to grow. Two months old seedlings were imposed to salinity stress by adding NaCl saline solution (10 gL-1) to increase salinity of 16 dSm-1 at 50% field capacity. An EC meter (Hanna 993310) was used to measure salinity level. Spd at 100 μM concentration was sprayed twice daily. Soil surface of the bucket was sealed with rock and polythene to maintain the soil moisture. This condition was maintained for seven days. A control without salinity and Spd was maintained under same condition. Data were measured in fully expanded leaves at 1st, 3rd, 5th and 7th days of stress.
Extraction of soluble protein for Western blotting
Onion leaf tissue (0.5 g) was homogenized in 1 ml of 50 mM ice-cold potassium-phosphate (K-P) buffer (pH 7.0) by mortar and pestle containing 100 mM KCl, 1 mM ascorbate, 5 mM β-mercaptoethanol and 10% (w/v) glycerol. Homogenates were centrifuged at 11,500×g for 10 min and the supernatants were used for Western blotting. All procedures were performed below 4°C (Rohman et al., 2009)
Measurement of Na+/K+
The sap was extracted from leaves and put on compact Na+ ion meter (Horiba-731, Japan) and compact K+ ion meter (Horiba-722, Japan) to estimate the Na+ and K+ ions in leaves. The Na+/K+ ratio was calculated from the estimated values.
Determination of protein
The protein concentration in the leaf extracts was determined according to the method of Bradford (1976) using BSA as a protein standard.
Separation and purification of GST and production of polyclonal antibody
For GSTs separation and purification, onion bulb tissue was used as onion bulb showing higher GST activity than leaf (Rohman et al., 2010). For separation of GSTs, 150 g fresh onion bulb tissue was homogenized in an equal volume of 25 mM Tris-HCl buffer (pH 8.0) containing 1 mM EDTA, 1% (w/v) ascorbate and 10% (w/v) glycerol and the supernatant was precipitated with ammonium sulfate at 65% saturation. GSTs were separated by anion exchange column (1.77 cm i.d.´20 cm) of DEAE-cellulose (DE-52; Whatman, UK). The fractions corresponding to the high GST active peaks were combined as the GST pool which was further purified by S-hexylglutathione-agarose (Sigma, St. Louis, MO) eluting with 1.2 mM S-hexylglutathione. Purified protein was used to prepare polyclonal antibody.
Production of polyclonal antibodies against GST
A rabbit (weighing about 2.5 kg) received subcutaneous injections of a 0.5 mg of purified GST protein in Freund’s complete adjuvant at several sites. After two weeks, the rabbit was given a first booster injection of 0.5 mg of the purified GST protein in incomplete adjuvant, and then a second booster injection of 0.5 mg of the purified protein in incomplete adjuvant was given two weeks after the first booster injection. Blood was taken from the ear vein one week after the second booster injection. The blood serum was used as a polyclonal antibody (Rohman et al., 2009).
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting
SDS-PAGE was done in 12.5% (w/v) gel containing 0.1% (w/v) SDS by the method of Laemmli (1970). The western blot was done following the protocol of Perkin-Elmer Life Science Inc.
Statistical analysis
Data of Na+/K+ was analyzed by Statistix 10 statistical program following complete randomized design (CRD) and the mean differences were compared by Tukey’s tests. P values<0.05 were considered to be significant.
In application of soluble extract of onion bulb on a DEAE-cellulose chromatography, a total of 125 fractions, each containing 5 ml elution, were obtained (Figure 1). GTS activity toward CDNB and absorbance at 280 nm (A280) were measured and plotted as shown in Figure 1. These three GSTs were eluted through the anion exchange chromatography and were named as GST1, GST2 and GST3 (Figure 1).
The three GSTs were eluted at 56, 120 and 169 mM of KCl, respectively, while GST1, GST2 and GST3 contained 6.48, 63.38 and 30.14% of total activity, respectively (Table 1). As GST2 contained 63.38% of the total activity, it was termed as dominant GST, and it was further subjected to purification by affinity chromatography of S-hexyl glutathione-agarose.
The high active GST peaks were pooled and pass through the affinity chromatography. The GST was eluted with 1.2 mM S-hexyl glutathione. Total 28 fractions, each containing 2.5 ml, were collected. Among them, fractions 21 to 28 are S-hexyl glutathione eluted fractions (Figure 2). The GST activity and absorbance (A280) of the fractions showed that S-hexyl glutathione fractions are eluted with high GST activity.
The purity of highly active fractions (fraction 23, 24, 25, 26, 27 and 28) were tested by SDS-PAGE following silver staining (Figure 2). The silver staining of the active fraction revealed that the fraction 24 contained highly purified GST with intensified band. However, in this study, fractions 24 and 25 were pooled. The summary of the purification is shown in Table 2. The purified GST had specific activity of 16407.6 nmol min-1 mg-1 protein with recovery percentage and purification fold of 2.81 and 29.5, respectively. The purification process was repeated several times to produce necessary protein required for developing polyclonal antibody in rabbit antiserum.
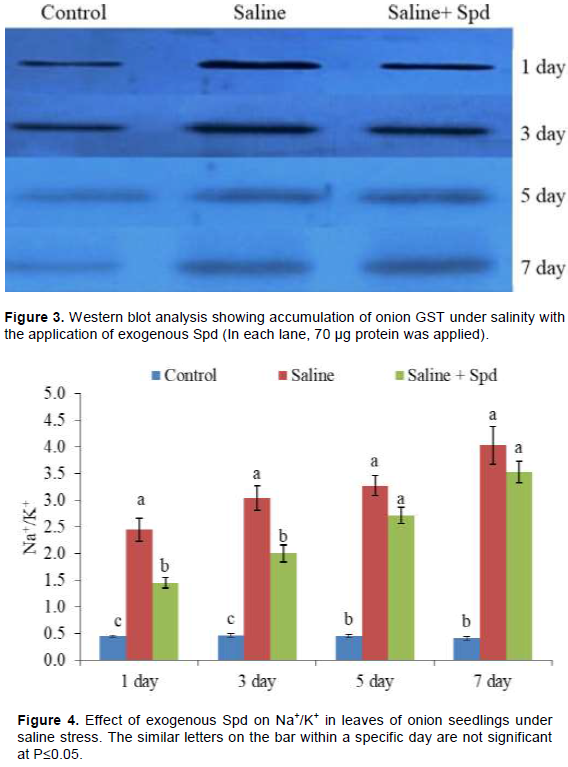
Western blot analysis of soluble extract of onion leaves is as shown in Figure 3. The protein bands showed significant accumulation of the dominant GST under salinity stress in the presence and absence of Spd (Figure 3).
The intensification of bands in Western blot clearly indicated the accumulation of the GST in onion leaf under salinity with or without Spd application (Figure 3). However, concentration of the GST was lower in the leaves with Spd than those without Spd. However, accumulation of the GST increased with stress duration, particularly in Spd sprayed leaves. Significant induction of GST could have significant biological role in stress mitigation onion seedlings under salinity. GSTs are an ancient and diverse group of multi-functional proteins that are widely distributed amongst living organisms. Early plant GST research is focused on the role of GSTs in herbicide resistance and vacuolar sequestration of anthocyanins (Edwards and Dixon, 2000). On one hand, the substantial accumulation of the GST under salinity might play important physiological role like vacuolar sequestration of flavonoids like quercetin (Fini et al., 2011) as quercetin is the most abundant physiological substrate in onion bulb (Rohman et al., 2009). On the other hand, high activity might be associated with recycling and stabilizing flavonoid (Dixon et al., 2011). GSTs have also been shown to possess GST activity towards 4-hydroxy-2-nonenal (HNE) (Gronwald and Plaisance, 1998), a naturally occurring lipid peroxidation product that causes oxidation and alkylation of proteins and DNA. In addition, GST activity allows GSTs to detoxify electrophilic compounds by catalyzing their conjugation to GSH, while GSH peroxidase (GPX) activity allows GSTs to directly detoxify lipid and DNA peroxidation products (Marrs, 1996). Therefore, it is also possible that the induced accumulation of GST in the present study as well as induced GST activity in our previous study (Islam et al., 2016) could detoxify HNE as well as MDA, another natural lipid peroxidation product, under saline stress condition. Moreover, GST may be involved in program cell death like leaf senescence (Kunieda et al., 2005). Therefore, the induced GST in the presence and absence of Spd can help in leaf senescence in onion under salinity stress. However, comparatively lower accumulation of GST in the presence of Spd is not clear. One of the possibilities may be that lessened stress of onion plants in the presence of Spd can decrease the synthesis of the enzyme. In our previous study, it was suggested that Spd could be useful for saline stress up to 3 days of stress, and in prolonged stress, bursts of ROS and MDA were found even in the presence of Spd (Islam et al., 2016; Rohman et al., 2017). Therefore, higher accumulation of GST at later stage could also be related to oxidative stress.
The value of Na+/K+ increased sharply and continuously in onion leaves with stress duration (Figure 4). Data also showed that application of Spd reduced Na+/K+ under saline stress while significant reduction was found at the 1st and 3rd days (Figure 4).
It is established that accumulation of Na+ and loss of K+ ions occurs under salinity stress. Previously, lower accumulation of Na+ in the presence of Spd treatment in rice was associated with prevention of electrolyte and amino acid leakage and chlorophyll loss under saline stress (Parvin et al., 2014; Duan et al., 2008). Spd is also reported to interact with phospholipids or other anionic groups of membranes, and thus, it stabilizes the membrane in stress environments (Amri et al., 2011).
Maintenance of cellular Na+/K+ is essential for growth and development of plants. Salinity causes the ion toxicity to increase oxidative damage (Gill and Tujeta, 2010). In this study, the ratio of Na+/K+ increased under salinity stress conditions and the values decreased in Spd sprayed seedlings (Figure 4). Under salinity, exogenous Spd has been reported to enhance the activities of stress mitigating enzymes like S-adenosylmethionine decarboxylase and diamine oxidase (DAO) in zoysia grass cultivars (Li et al., 2016) and reduced the activities of arginine decarboxylase. Exogenous Spd treatment regulates the metabolic status of PAs in tomato (Solanum lycopersicum) under salinity-alkalinity stress by providing tolerance (Hu et al., 2012). Spd has also been shown to inhibit the extent of salt-induced protein carbonylation. On the other hand, Spd promoted the synthesis of salt-induced anthocyanin, reducing sugar and proline levels in rice (Roychoudhury et al., 2011). These results suggested the stress tolerant role of SPd under salinity stress. In our previous study, Spd increased the activities of ployamine oxidase (PAO) and DAO along with ROS and MG detoxifying enzymatic and non-enzymatic antioxidants in onion (Islam et al., 2016; Rohman et al., 2017). It also maintained higher chlorophyll contents in onion under salinity stress. In another study, improved tolerance was found with higher PAO and DAO activities as well as antioxidant activities in maize seedlings by exogenous Spd (Akter et al., 2018). In the present study, application of Spd reduced the Na+/K+ (Figure 4) in the leaves of onion seedlings. This result suggested that use of Spd reduced Na+ ion toxicity as well as increase K+ uptake. Previously, reduced Na+ content and higher chlorophyll values, higher variable fluorescence/maximum fluorescence values and a higher net photosynthetic rate were reported in Spd applied wheat seedlings under saline stress (Gill and Tuteja, 2010). Similarly, reduced uptake of toxic Na+ ion concurrently with higher uptake of beneficial ions such as Ca2+ and Mg2+ by Spd treatment was also reported to attribute better salt tolerance in plants (Anjum, 2011).
In this study, three GST isozymes were found, and among them, the dominant GST contained 63.38% of the total activity. The specific activity of the purified GST was 16407.6 nmol min-1 mg-1 protein with recovery and purification fold of 2.81 and 29.5, respectively. The Western blotting analysis suggested the accumulation of the GST under salinity alone at early stage of stress while in the later stage in Spd treated seedlings. The ration of Na+/K+ indicated the importance of Spd in improving ionic balance Na+ and K+. Thus, these results along with our previous result suggested improved tolerance by Spd spray in onion seedlings under saline stress. However, the lower accumulation of the dominant GST at early stress by Spd thrusted more research.
The authors have not declared any conflict of interests.
REFERENCES
|
Akter S, Rasul MG, Zakaria M, Sarker M M, Nila IS, Dutta S, Haque MM, Rohman MM (2018). Effect of polyamine on pigmentation, reactive oxidative species and antioxidant under drought in maize (Zea mays L.) Turkish Journal of Agriculture - Food Science and Technology 6(7):799-811.
|
|
|
|
Alcázar R, Marco F, Cuevas JC, Patron M, Ferrando A, Carrasco P, Tiburcio AF, Altabella T (2006). Involvement of polyamines in plant response to abiotic stress. Biotechnology Letters 28:1867-1876.
Crossref
|
|
|
|
|
Amri E, Mirzaei M, Moradi M, Zare K (2011). The effects of spermidine and putrescine polyamines on growth of pomegranate (Punica granatum L. cv "Rabbab") in salinity circumstance. International Journal of Plant Physiology and Biochemistry 3:43-49.
|
|
|
|
|
Anjum MA (2011). Effect of exogenously applied spermidine on growth and physiology of citrus rootstock Troyer citrange under saline. Turkish Journal of Agriculture and Forestry 35:43-53.
|
|
|
|
|
Bradford MM (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Annals of Biochemistry 72:248-254.
Crossref
|
|
|
|
|
Chronopoulou E, Georgakis N, Nianiou-Obeidat I, Madesis P, Perperopoulou F, Pouliou F, Vasilopoulou E, Ioannou E, Ataya FS, Labrou NE (2017). "Plant glutathione transferases in abiotic stress response and herbicide resistance," in Glutathione in Plant Growth, Development, and Stress Tolerance, eds MA. Hossain, MG. Mostofa, P. Diaz-Vivancos, DJ. Burritt, M. Fujita, and LSP. Tran (Berlin: Springer), 215-233.
Crossref
|
|
|
|
|
Cummins I, Dixon DP, Freitag-Pohl S, Skipsey M, Edwards R (2011). Multiple roles for plant glutathione transferases in xenobiotic detoxification. Drug Metabolism Reviews 43:266-280.
Crossref
|
|
|
|
|
Dalton DA, Boniface C, Turner Z, Lindahl A, Kim HJ, Jelinek L, Govindarajulu M, Finger RE, Taylor CG (2009). Physiological roles of glutathione S-transferases in soybean root nodules. Plant Physiology 150:521-530.
Crossref
|
|
|
|
|
Ding N, Wang A, Zhang X, Wu Y, Wang R, Cui H, Huang R, Luo Y (2017). Identification and analysis of glutathione S-transferase gene family in sweet potato reveal divergent GST-mediated networks in aboveground and underground tissues in response to abiotic stresses. BMC Plant Biology 17:e225.
Crossref
|
|
|
|
|
Dixon DP, Skipsey M, Edwards R (2010). Roles for glutathione transferases in plant secondary metabolism. Phytochemistry 71:338-350.
Crossref
|
|
|
|
|
Dixon DP, Steel PG, Edwards R (2011). Roles for glutathione transferases in antioxidant recycling. Plant Signaling and Behavior 6(8):1223-1227.
Crossref
|
|
|
|
|
Duan J, Li J, Guo S, Kang Y (2008). Exogenous spermidine affects polyamine metabolism in salinity-stressed Cucumis sativus roots and enhances short-term salinity tolerance. Journal of Plant Physiology 165:1620-1635.
Crossref
|
|
|
|
|
Edwards R, Dixon DP (2000). The role of glutathione transferases in herbicide metabolism. In Herbicides and Their Mechanisms of Action (Cobb, A.H. and Kirkwood, R.C., eds). Sheffield: Sheffield Academic Press Ltd., pp. 8138-71.
|
|
|
|
|
Fini A, Brunetti C, Ferdinando MD, Ferrini F, Tattini (2011). Stress-induced flavonoid biosynthesis and the antioxidant machinery of plants. Plant Signaling and Behavior 6(5):709-711.
Crossref
|
|
|
|
|
Frova C (2006). Glutathione transferases in the genomics era: new insights and perspectives. Biomolecular Engineering 23:149-169.
Crossref
|
|
|
|
|
Gill SS, Tuteja N (2010). Polyamines and abiotic stress tolerance in plants. Plant Signaling and Behavior 5:26-33.
Crossref
|
|
|
|
|
Gronwald JW, Plaisance KL (1998). Isolation and characterization of glutathione S-transferase isozymes from sorghum. Plant Physiology 117(3):877-892.
Crossref
|
|
|
|
|
Hu XH, Zhang Y, Shi Y, Zhang Z, Zou ZR, Zhang H, Zhao JZ (2012). (2012) Effect of exogenous spermidine on polyamine content and metabolism in tomato exposed to salinity-alkalinity mixed stress. Plant Physiology and Biochemistry 57:200-209.
Crossref
|
|
|
|
|
Islam T, Hossain MI, Rahaman MS, Rohman MM (2016). Spermidine enhances activities of detoxification enzymes in onion (Allium cepa L.) seedlings under short term salinity. Cell Biology 4(3):18-23.
|
|
|
|
|
Kasukabe Y, He L, Nada K, Misawa S, Ihara I, Tachibana S (2004). Overexpression of spermidine synthase enhances tolerance to multiple environmental stresses and up-regulates the expression of various stress-regulated genes in transgenic Arabidopsis thaliana. Plant and Cell Physiology 45(6):712-722.
Crossref
|
|
|
|
|
Khare T, Srivastav A, Shaikh S, Kumar V (2018). Polyamines and their metabolic engineering for plant salinity stress tolerance. Kumar et al. (eds.). Salinity responses and tolerance in plants. Volume 1.
Crossref
|
|
|
|
|
Kunieda T, Fujiwara T, Amano T, Shioi Y (2005). Molecular cloning and characterization of a senescence-induced Tau-class glutathione S-transferase from barley leaves. Plant and Cell Physiology 46(9):1540-1548.
Crossref
|
|
|
|
|
Laemmli UK (1970). Cleavage of structural proteins during the assembly of head of bacteriophage T4. Nature 227:680-685.
Crossref
|
|
|
|
|
Li S, Jin H, Zhang Q (2016). The effect of exogenous spermidine concentration on polyamine metabolism and salt tolerance in zoysia grass (Zoysia japonica Steud) subjected to short-term salinity stress. Frontier in Plant Science 7:1221.
|
|
|
|
|
Liu XF, Li JY (2002). Characterization of an ultra-violet inducible gene that encodes glutathione S-transferase in Arabidopsis thaliana. Acta Genetica Sinica 29:458-460.
|
|
|
|
|
Liu J-H, Wang W, Wu H, Gong X and Moriguchi T (2015). Polyamines function in stress tolerance: from synthesis to regulation. Frontier in Plant Science 6:827.
Crossref
|
|
|
|
|
Loyall L, Uchida,K, Braun S, Furuya M, Frohnmeyer H (2000). Glutathione and a UV light–induced glutathione S-transferase are involved in signaling to chalcone synthase in cell cultures. Plant Cell 12:1939-1950.
Crossref
|
|
|
|
|
Marrs KA (1996). The functions and regulation of glutathione S-transferases in plants. Annual Review of Plant Physiology and Molecular Biology 47:127-158.
Crossref
|
|
|
|
|
Mueller LA, Goodman CD, Silady RA, Walbot V (2000). AN9, a petunia glutathione S-transferase required for anthocyanin sequestration, is a flavonoid-binding protein. Plant Physiology 123:1561-1570.
Crossref
|
|
|
|
|
Oakley AJ (2011). Glutathione transferases: a structural perspective. Drug Metabolism Reviews 43:138-151.
Crossref
|
|
|
|
|
Parvin S, Ran O, Sathiyaraj G, Khorolragchaa A, Kim YJ, Yang DC (2014). Spermidine alleviates the growth of saline-stressed ginseng seedlings through antioxidative defense system. Gene 537:70-78.
Crossref
|
|
|
|
|
Perperopoulou F, Pouliou F, Labrou NE (2017). Recent advances in protein engineering and biotechnological applications of glutathione transferases. Critical Review in Biotechnology 38:511-528.
Crossref
|
|
|
|
|
Rohman MM, Talukder MZA, Hossain MG, Uddin MS, Amiruzzaman MA, Biswas, Ahsan AFMS, Chowdhury MAZ (2016a). Saline sensitivity leads to oxidative stress and increases the antioxidants in presence of proline and betaine in maize (Zea mays L.) inbred. Plant omics 9(1):35-47.
|
|
|
|
|
Rohman MM, Begum S, Talukder MZA, Akhi AH, Amiruzzaman M, Ahsan AFMS, Hossain Z (2016b). Drought sensitive maize inbred shows more oxidative damage and higher ROS scavenging enzymes, but not glyoxalases than a tolerant one at seedling stage. Plant Omics 9(4):220-232.
Crossref
|
|
|
|
|
Rohman MM, Islam T, Mohi-Ud-Din M, Islam MR, Molla MR, Hossain MG, Chowdhury MAZ (2017). Use of spermidine reduced the oxidative damage in onion seedlings under salinity by modulating antioxidants. African Journal of Agricultural Research 12(46):3304-3314.
Crossref
|
|
|
|
|
Rohman MM, Hossain MD, Suzuki T, Takada G, Fujita M (2009). Quercetin-4'-glucoside: a physiological inhibitor of the activities of dominant glutathione S-transferases in onion (Allium cepa L.) bulb. Acta Physiologiae Plantarum 31(2):301-309.
Crossref
|
|
|
|
|
Rohman MM, Uddin S, Fujita M (2010). Up-regulation of onion bulb glutathione S-transferases (GSTs) by abiotic stresses: A comparative study between two differently sensitive GSTs to their physiological inhibitors. Plant Omics 3:28-34.
|
|
|
|
|
Roychoudhury A, Basu S, Sengupta DN (2011). Amelioration of salinity stress by exogenously applied spermidine or spermine in three varieties of indica rice differing in their level of salt tolerance. Journal of Plant Physiology 168:317-328.
Crossref
|
|
|
|
|
Zhang Y, Liu J, Zhou Y, Gong T, Wang J, Ge Y (2013). Enhanced phytoremediation of mixed heavy metal (mercury)-organic pollutants (trichloroethylene) with transgenic alfalfa co-expressing glutathione S-transferase and human P450 2E1. Journal of Hazardous Materials 260:1100-1107.
Crossref
|
|
|
|
|
Zhao H, Yang H (2008). Exogenous polyamines alleviate the lipid peroxidation induced by cadmium chloride stress in Malus hupehensis Rehd. Scientia Horticulturea 116:442-447.
Crossref
|
|