Full Length Research Paper
ABSTRACT
Bamboo plant is an important biodegradable raw material which can play important role in rejuvenation of Indian rural economy through positively impacting agricultural, industrial, energy and in environmental sectors. Traditional methods of vegetative propagation of this plant have insufficient multiplication rate. In the present investigation, in vitro bud break and aseptic culture establishment in relation to different seasons of Bihar were evaluated for large scale clonal propagation of two commercially important bamboo species, Bambusa tulda Roxb. and Dendrocalamus stocksii Munro. The experiment was conducted thrice using a completely randomized design with 10 replicate per treatment. Mean calculation was performed using Duncan’s Multiple Range Test (DMRT) at P<0.05. Summer climate (April-June) was the most congenial season for initiation and establishment of aseptic cultures. 6 Benzylaminopurine (BAP) without supplementation of any additive resulted in significant bud breakage in B. tulda, however, in case of D. stocksii, Thidiazurone (TDZ) with the additives resulted in comparatively better response. For multiplication, BAP (2.5 mg/l) showed maximum number of shoots (24±6.2) in D. stocksii. However, in B. tulda high multiplication rate with adequate shoot length was observed in semi solid media supplemented with BAP (1 mg/l). For both the species, the survival rate during hardening (primary and secondary) was maximum during monsoon season. The refined in vitro regeneration system of two commercially important bamboo species developed is efficient and will be an impetus to raising bamboo nurseries of elite germplasm for bamboo growing areas of Bihar.
Key words: MS media, clonal propagation, in vitro regeneration, phytohormones, 6benzylaminopurine, thidiazurone.
INTRODUCTION
Bamboo belongs to the highest evolved flowering family, Poaceae. It is a natural biodegradable raw material, which can play important role in rejuvenation of Indian rural economy through impacting in agricultural, industrial, energy and environmental sectors. Among the flowering plants, it is one of the highest carbon sequesters, environment friendly and suited to adapt and grow even on degraded lands (Hossain et al., 2015; Singh et al., 2020; Waghmare et al., 2021). This plant can grow very fast (0.9 to 1.2 m/day) within four to five years and can regrow after harvesting, without the need of replanting and thereby, making it a perennial renewable bio-resource. Due to its versatility, this plant is also known as green gold, poor man’s timber, friends of the people and also cradle to coffin timber (Sawarkar et al., 2020; Singh et al., 2021).
The green cover in Bihar had reduced to 9% after the bifurcation of Jharkhand from Bihar state in 2000. The population density of this state is quite high (1106 people/km2) and the average land holdings is quite low, 0.24 ha (2011 census) (India, 2021). In addition, farmers of this state also face extreme causalities both with floods and droughts every year. It was thereby, decided by the state Department of Environment, Forest and Climate Change (DEFCC) to increase the green cover from 15 to 17% and subsequently to 19% through massive development of plantations under ‘ Haryali Mission’. Bamboo is among one of such plants for integrating it into agriculture by planting on farm boundaries, farm-land (Agro forestry) and non-agricultural land (waste land, degraded lands and in homesteads). Bamboos can also provide farmers a perennial source of income (Hoogendoorn and Benton, 2014; Liese and Kohl, 2015).
Since time immemorial, bamboos have grown naturally but they are now also being cultivated. However, this plant has also witnessed serious depletion as is the case with other plants in the natural habitats. In order to conserve the depleting bamboo resource, the commercially important species need to be selected and propagated. However, the propagation of this plant is impeded by long flowering cycles that can last 40 to120 years, lack of seed availability and also short viability of seeds. Traditional methods of vegetative propagations (rhizome splitting, offset cuttings, branch and branch cuttings) have low multiplication rate. Also, these methods are season specific, being bulky and so inconvenient in handling and transport (Waghmare et al., 2021; Mustafa et al., 2021).
These gaps can be filled by micropropagation. Through tissue culture techniques, large scale mass clonal propagation of high quality germplasms can be made (Goyal and Sen, 2016). India possesses world’s largest bamboo resources, next to China, with 136 species and 23 genera spread over sixteen millions hectares of land (Forest Survey of India, 2019).
In this study, two economically important bamboo species (Bambusa tulda Roxb. and Dendrocalamus stocksii Munro.) were selected due to their adaptability to the local climatic conditions. D. stocksii is thorn less, solid stemmed, loosely spaced, erect bamboo growing upto 9m height with the diameter ranging from 30 to 50 mm. The leaves are lanceolate (10-20 cm long) and are most suited for agroforestry as it has low canopy coverage. It is utilized for furniture, basket making, poles, stakes, crafts and also in construction. B. tulda is another commercially important bamboo species, with the characteristics of being tall, quick growing and with the ability to grow up to 13 to 26 m in height. It also prevents soil erosion. This bamboo has high demand as it being one of the fastest growing plants and culms are used to manufacture many handicrafts, furniture, house construction, paper, and pulp production.
Earlier work on the development of protocols for micro clonal propagation of these two species of bamboos showed challenges with rhizogenesis and hardening of the seedlings (Waikhom and Louis, 2014; Sharma and Sharma, 2013; Somashekar et al., 2018; Rajput et al., 2019).
The main objective of the study was to evaluate in vitro bud break and aseptic culture establishment in relation to different seasons of Bihar for above two commercially important bamboo species. Effects of different PGRs and additives in various concentrations and combinations to initiate healthy multiple shoots under in vitro conditions were monitored. Influence of seasons on hardening (primary and secondary) of tissue culture raised seedlings with survival efficiencies was also observed.
MATERIALS AND METHODS
Source of explants
Explants were collected from actively growing shoots of four years old healthy culms of D. stocksii and B. tulda which were brought from Institute of Wood Science and Technology (IWST) Bengaluru and were planted and maintained at Plant Tissue Culture (
Surface sterilization
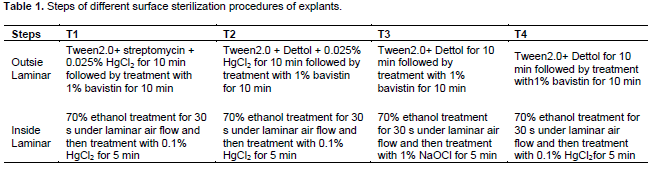
At first the shoots were surface sterilized with the help of cotton swab dipped in 70% ethanol in order to remove the dust and microorganisms. Shoots were then washed in running tap water Explants of the size of 1.5 to 3.0 cm in length were cut into pieces containing one node axillary buds. Surface sterilization procedures. of explants were also observed in order to monitor the bud breakage response and the levels of contamination. In the present investigation, four different procedures were applied for treatment of explants. Those were named as T1, T2, T3, and T4 (Table 1).
After each treatment, explants were rinsed with autoclaved distilled water for three to four times. Then the nodal segments were brought to the laminar flow chamber under aseptic conditions and were sterilized by treating buds with 70% ethanol (for 30 to 35 sec) followed by three rinses with autoclaved distilled water. Explants were then treated with 0.1% HgCl2 for 5 min and were subsequently washed with distilled water three to four times.
Culture conditions
The modified MS media was used for shoot initiation of D. stocksii and B. tulda. The MS media (consisting of salt, vitamins and 3% sucrose) was prepared using 100 mg/l myoinositol. Different plant growth regulators (PGRs)-BAP, TDZ and NAA were added at various concentrations to the MS media, before adjustment of pH at 6.0. Then the media was autoclaved at 121°C at 6.80 kg (15 lb) for 20 min. The cultures of all growth stages were incubated under artificial physical conditions 22±2°C, 65 to 70% humidity and 16 h of photoperiod (using white fluorescent tube).
Axillary bud initiation
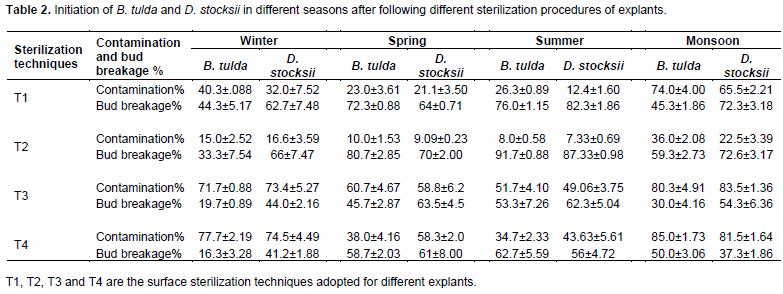
Following different sterilization treatments (T1, T2, T3 & T4), per cent bud breakage and contamination level in both genus (B. tulda and D. stocksii) were monitored in different seasons (winter, spring, summer and monsoon) (Table 2). Experiments were conducted thrice for each sterilization procedure for each season. 10 explants were used for one experiment and in each culture tube 15 ml nutrient media (MS+ NAA 0.1 mg/l+ BAP 1.0 mg/l) was employed. Contamination level and bud breakage per cent were calculated after 10 days of inoculation.
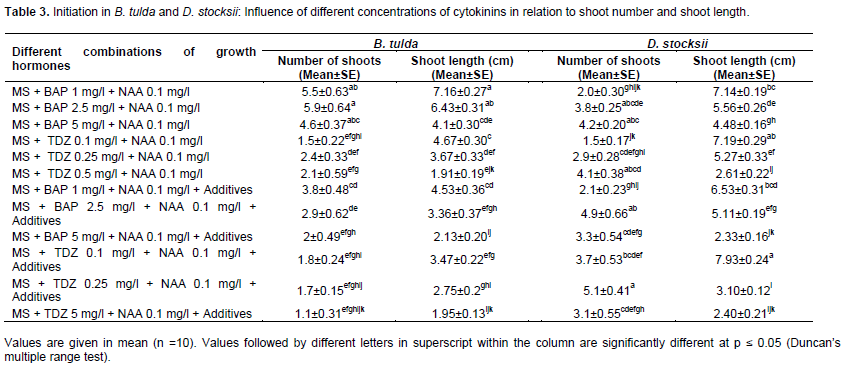
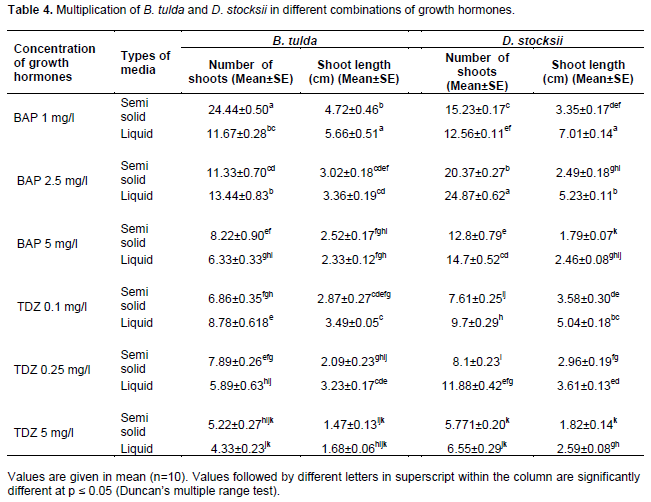
For monitoring the influence of various phytohormones on auxiliary bud initiation, explants were inoculated in test tubes containing 15 ml of initiation media supplemented with different concentrations of growth hormones (BAP, TDZ and NAA) alone or with additive. Three concentrations of BAP (1, 2.5 and 5 mg/l) and TDZ (0.1, 0.25 and 0.5 mg/l) were tested. Since TDZ being strong cytokinines and thereby, comparatively less concentration of this cytokinine was taken in our experiments. For observation, 10 tubes were taken for each treatment having one explant (observation unit) in each tube. The experiments were done in triplicate. For each genus a total of 360 experiment units, each time with 120 units were employed. Treatments were made in completely randomized design. After inoculation being made in nutrient media, explants were kept in culture room and observations were made after 21 days (3 weeks) onward till when the auxiliary bud broken and a number of shoots proliferated. Number of shoots was calculated by counting the shoot number in each explant. Shoot length was measured in cm by scale (Table 3).
In vitro shoot multiplication
Initiated axillary buds were separated and subsequently cultured for multiple shoot proliferations. Established aseptic cultures of proliferated clumps of shoots were propagated in semi-solid (clerigel used as solidifying agent) and liquid MS media under different cytokinins concentrations BAP (1, 2.5, and 5 mg/l) and TDZ (0.1, 0.25 and 0.5 mg/l). The clumps of 5 to 10 shoots were used for in vitro shoots multiplication. The sub-culturing of in vitro raised shoots was carried out by transferring in fresh MS media at regular intervals. For liquid media, sub-culturing time intervals were kept 8 to 10 days, however, for solid media it was in between 15 and 21 days. During sub-culturing, dead parts of the plantlets were removed carefully. Also, periodical transfers were made in order to avoid browning and leaching and to maintain healthy cultures. The performance of multiplication in different concentrations of growth hormones was calculated by observing the number of shoots and shoot length.
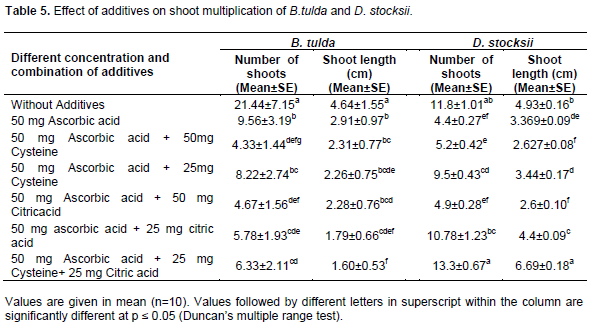
The shoot length and shoot number were also observed when media were supplemented with different concentrations of additives, Ascorbic acid (AA), Cysteine (Cys) and Citric acid (CA) applied alone or in combinations (50 mg/l AA, 50 mg/l AA + 50 mg/l Cys, 50 mg/l AA + 25 mg/lCys. AA + 50 mg/l CA, 50 mg/l AA + 25 mg/l CA, 50 mg/l AA + 25 mg/l Cys + 25 mg/l CA).
In vitro rooting
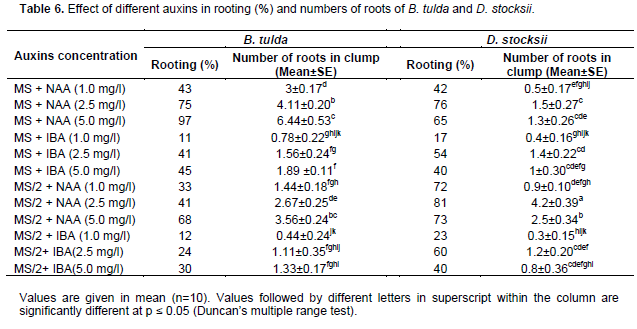
After 5 to 6 successful passages of cycles, the well-developed clump with 3 to 4 shoots was inoculated into the MS rooting media. The in vitro raised shoots of adequate heights were transferred to full strength and half strength of MS media containing different concentrations of auxins such as IBA (1.0, 2.5 and 5.0 mg/l), NAA (1.0, 2.5 and 5.0 mg/l) in order to access their response on rooting. The performance of in vitro rooting was calculated after 4 weeks (30 days) of incubation by observing root per cent and the mean number of roots in each clump.
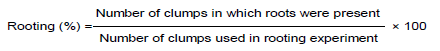
Acclimatization
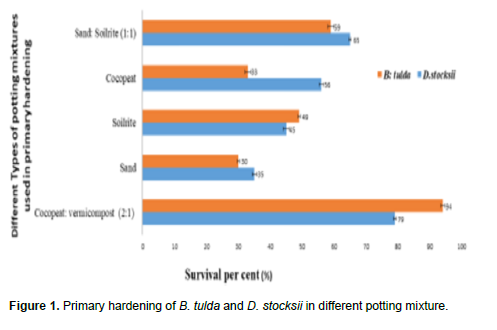
For acclimatization, in vitro raised bamboo plantlets seedlings were transferred into portrays containing different potting mixtures (Figure 1) and then they were subsequently allowed to grow inside the closed tunnel of green house for three weeks. Then the partially acclimatized plantlets were kept in green house (outside of closed tunnel) for 3 weeks. Primary hardened plantlets were subsequently transferred to the propagation bed of the Net/Shade house for a period of 45 days. The survival rate during hardening processes was observed in different seasons (winter, spring, summer and monsoons).
Experimental design and statistical analysis
The experiment was conducted thrice using a completely randomized design with 10 replicates per treatment. The effects of PGRs and additives on initiation and multiplication of both the species were calculated and the level of significance was determined through analysis of variance (ANOVA). Mean calculations were performed using Duncan’s Multiple Range Test (DMRT) at p ≤ 0.05. Evaluation of efficient sterilization techniques, axillary bud initiation and rooting per cent was made through the experimental designs as previously discussed.
RESULTS AND DISCUSSION
Initiation and establishment of in vitro aseptic culture
Initiation in different seasons
Seasons had marked impact on the establishment of aseptic cultures and on multiple bud breaks. In T2 treatment, there was maximum bud proliferation with least microbial contamination in all the seasons followed by treatment T1 (with the application of antibiotic streptomycin) (Tables 1 and 2). Comparatively less bud break was observed in explant treated with antibiotic as surface sterilants. Microbial contamination was the maximum during the monsoons in treatment T4 (85%) and T3 (80.3%). Earlier workers have also reported the retarded growth of explants with the applications of antibiotics (Yasodha et al., 2008; Negi and Saxena, 2011). Moreover, the treatment with antibiotics is not only cost effective but also time consuming. Negative effects of antibiotics at higher concentrations have been attributed to their toxic effects on morphogenesis (Mathias and Mukasa, 1987; Venkatasalam et al., 2013; Buckseth et al., 2017).
The rate of contamination and bud initiation percentage of both the species varied in different seasons. Summer climate (April-June) was the most congenial season for initiation and establishment of aseptic cultures. In case of D. stocksii the per cent bud break response was maximum (87.33%) during summer season followed by monsoons (72.6%), when they were treated as T2 sterilization technique. During the monsoons, the microbial contamination percent was comparatively high (22.5%). Microbial load was also maximum during rainy seasons (65.5, 83.5 and 81.5%) compared to other seasons. This might be due to prolonged high humidity coupled with high temperature prevailing in this locality during monsoons. In case of B. tulda also, the summer was the most suitable season for bud initiation due to high percent (91.7%) of bud breakage with minimal contamination level (8.0%), followed by spring season with 80.7% bud breakage and 10.0% microbial contamination (Table 2). Previous workers have also reported the influence of climatological factors on the levels of microbe contamination and establishment of aseptic cultures (Saxena and Dhawan, 1999; Kiran and Ansari, 2000; Choudhary et al., 2017; Sandhu et al., 2018). In an investigation, working on Dendrocalamus asper, Nadha et al. (2013) observed that the rainfall had the direct influence on contamination rate and survival per cent of explants. In D. stocksii, the contamination level was moderately low during winters and spring, 16.6 and 9.09%, respectively, when treated with T2 procedure. However, in the same sterilization procedure (T2), the bud proliferation percentage (66.1%) was comparatively lesser in winters compared to other seasons. It might be due to high phenol production during this season (Banerjee et al., 2011). In B. tulda also bud breakage percentage was less (33.3%) during winter, even after following the same sterilization technique. Browning and death are the common problems associated with the culture of woody plants which might be attributed to the oxidation of phenolic compounds in explants tissues (Choudhary et al., 2004; Arya et al., 2008; Suwal et al., 2020).
Influence of PGR
The influence of PGRs combinations varied in both the species of bamboos. In case of D. stocksii, TDZ when applied along with additives resulted in high number of shoots and also high shoot length (Table 3). The maximum number (5.1±0.41) of shoots was observed in the combinations of MS + TDZ 0.25 mg/l+ NAA .1 mg/l+ additive, followed by the combinations of MS+ BAP 2.5 mg/l + NAA 0.1 mg/l + additives, with 4.9 ± 0.66 shoots. The shoot length was high (7.93±0.24) in TDZ concentration of 0.1 mg/l in combination with additives. However, B. tulda, TDZ as cytokinine was not very effective for bud proliferation. In this case, BAP alone at the concentration of 2.5 mg/l and 1 mg/l was found as the most suitable phytohormones for bud breakage (Table 3). The number of shoots was 5.9±0.64 at concentration of 2.5 mg/l, followed by 5.5 ± 0.63 at the concentration of 1.0 mg/l. The shoot length was adversely affected with the increase in the concentrations of the PGR. At low concentration of PGR, there was high shoot length; however, at considerably higher concentrations there was minimum length. Similar findings were also observed in case of D. stocksii. The high level of BAP or cytokinine has been reported to induce programmed cell death in cultures (Singh et al., 2012). Moreover, cytokinine at higher level is also known to promote ethylene biosynthesis and thereby, adversely affecting the growth in culture (Suwal et al., 2020).
Different cytokinines (BAP and TDZ) had varied responses towards both the species. The BAP without additive resulted in significant numbers of bud breakage in B. tulda. Contrary to this, TDZ with additives had better response in D. stocksii. This might be due to the endogenous level of growth hormones in the tissues of the culture as well as the extent of phenolic which might vary in different strains of the same species (Sandhu et al., 2017; Jiménez, 2005).
Previous researchers working on different bamboo species have also reported high percentage of bud breakage, when BAP was applied without additives as in B. tulda (Choudhary et al., 2020), Dendrocalamus hamiltonii (Agnihotri and Nandi, 2009; Sood et al., 2002), Bambusa bambos (Arya and Sharma, 1998) and Guadua angustifolia (Jiménez et al., 2006). However, Kabade (2009) and Beena et al. (2012) observed multiple shoot induction when TDZ was applied in combination with NAA. Somesekhar et al. (2008) working on D. stocksii reported the synergistic effect of BAP with NAA for high shoot induction with adequate shoot length. The endogenous auxins and the added auxins in the media might have resulted in increased total auxin levels of seedlings in cultures resulting thereby in high apical dominance and subsequent suppression of shoot proliferation. However, interaction with cytokinine might promote shoot proliferation (Rasool et al., 2009; Bin Azizan, 2017).
Shoot multiplication
Influence of PGR concentrations
After 45 days of inoculation, shoots developed well in a group of 5-20, then the clumps of 5-10 micro-shoots were transferred to different types of nutrient media (MS semi solid and liquid media) containing different concentrations of cytokinins (BAP and TDZ). The number of shoots as well as shoot lengths of D. stocksii and B. tulda cultures was evaluated subsequently after 15 to 21 days of sub culturing (Table 4). In case of D. stocksii, the number of shoots was maximum (24.87±0.62) in liquid media supplemented with BAP 2.5 mg/l, followed by 20.37±0.27 in the semisolid media; however, with same concentration (2.5 mg/l) of BAP. Shoot length was maximum (7.0±0.14) at the BAP concentration of 1.0 mg/l followed by 5.23±0.11 at the concentration of 2.5 mg/l of BAP. Moreover, for D. stocksii the liquid media supplemented with 2.5 mg/l BAP was most congenial as it resulted in maximum shoot number with adequate length (5.23±0.11). Another cytokinine (TDZ) was not very effective due to slow rate of multiplication along with dwarf shoots.
In B. tulda, the multiplication rate and shoot length were high in semi-solid media. The number of shoots was observed maximum (24.44±0.50) in semi-solid media supplemented with BAP at 1.0 mg/l, followed by 13.44±83 shoots at BAP concentration of 2.5 mg/l.
Physical state of media (liquid/semisolid) requirements depends upon the nature of the explant used for the micropropagation. In case of D. stocksii, MS liquid media resulted in better response than semi solid media. Browning and leaching were comparatively lower in this media. This might be due to easier uptake of nutrients and regulators without any restrictions compared to gelling agents (Negi and Saxena, 2011). Earlier, Saxena (1990) reported multiplication of B. tulda in liquid media. However, in case of B. tulda we found semisolid media (with clerigel) as most suited for mass propagation. In liquid media, there was slower rate of multiplication after 2 to 3 cycles, which might be due to hyperhydricity (Saxena and Bhojwani, 1993; Arshad et al., 2005). Among the different cytokinines, BAP at the concentrations of 1 to 2.5 mg/l was found most suitable phytohormones for in vitro multiplication of bamboo shoots (Choudhary et al., 2016; Mane et al., 2020). However, concentrations above this range influenced multiplication but resulted in stunted shoot growth. Previous workers have also confirmed it while working on different species of bamboos (Arya and Arya, 1997; Bag et al., 2000; Bhadrawale et al., 2018).
Influence of additives
Influence of different types of additives and their combined effects was also evaluated. After 1 to 2 sub-culturing, cultures of D. stocksii became brown and died due to secretion of phenolic compounds. In order to overcome the browning and leaching of those cultured plants, the effect of various combinations of additives in different concentrations was monitored. Additives in combination of 50 mg AA + 25 mg CA + 25 Cys. mg/l resulted in maximum (13.3±0.67) shoot multiplication with high (6.69±0.18 cm) shoot length (Table 5). Our findings are in conformity with the observations of previous workers (Rathore and Ravishankar Rai, 2005; Somashekar et al., 2008; Rajput et al., 2020). However, in case of B. tulda, MS basal media without the addition of any additives resulted in maximum number of shoots (21.44±7.15) and shoot length (4.64±1.55 cm) (Table 5). Earlier, Thorpe et al. (1991) and Prutpongse and Gavinlertvatana (1992) have also reported micropropagation of some of the bamboo species in MS media supplemented either with cytokinine or BAP.
Rooting
In vitro rooting was observed in both the species of bamboos by adding different concentrations of auxin either in full or half strength of MS basal media. In D. stocksii, the half strength of MS media was suffice for in vitro rooting, however, in case of B. tulda full strength resulted comparatively in better rooting percentage (Table 6). In D. stocksii, rooting percentage was maximum (81%) with 4.2±0.39 roots when half strength of MS media was supplemented with NAA 2.5 mg/l (Figure 4f and g). However, in B. tulda, full strength of MS media containing NAA 5 mg/l resulted in maximum percent (97%) of rooting with 6.44±2.15 roots in average (Figure 3f and g). Among different auxins, NAA was the most effective hormone for root induction and growth for both species. However, previous workers reported high efficiency of rooting in IBA alone in case of Bomarea glaucescens (Shirin and Rana, 2007) or by supplementing combinations of auxins in Dendrocalamus strictus (Chaturvedi et al., 1993). Responses of phytohormones on cultures might be influenced with the nature of strains of different species. There are several reports, suggesting half MS strength as most appropriate for rooting, however, in case of B. tulda we found MS full strength as most suitable for in vitro rooting. In half strength of MS, there was profuse rooting but the mortality rate was comparatively high due to dryness and browning. This might be due to nutrient deficiency. Root induction is the high energy demanding process and thereby, the endogenous requirements of metabolic substrates might vary in different species (Yasodha et al., 2008; Sandhu et al., 2018).
In conformity with the previous reports on different species of bamboos, we observed half strength of MS media as more effective in root induction than MS full strength for D. stocksii. Singh et al. (2012) on D. asper and D. hamiltonii, Ramanayake and Yakandwall (1997) on Dendrocalamu giganteus, Rathore and Ravishankar Rai (2005) on D. stocksii. Somasheker et al. (2008) found rooting in one fourth strength of MS media. The low strength of MS media create partial nutrient stress (Singh et al., 2012) and also provide low osmotic potential resulting thereby, plantlets to produce more roots, early adaptation during acclimatization and to induce them to become autotrophic (Arab et al., 2018).
Acclimatization and field transfer
Primary hardening
Well developed in vitro rooted plantlets were transferred to greenhouse under closed tunnel. The development and growth of plants were monitored in five different potting mixtures (cocopeat + vermicompost; sand; soilrite; cocopeat; sand + soilrite) for primary hardening. The use of 2:1 ratio of cocopeat and vermicompost was found most suitable potting mixture with high survival rate (79% in D. stocksii and 94% in B. tulda) for both species (Figure 1). The vermicompost being used in potting mixture was effective in providing high porosity and better aeration for root growth (Singh et al., 2012).
Secondary hardening and field transfer
Influence of seasons on survival of seedlings in hardening was also monitored. After 30 to 45 days of primary hardening, plants were transferred to the Net or Shade house on mother bed constituting of sand and cow dung (1:1). For both species of bamboos, survival rate was maximum during monsoons (93% in B. tulda and 85 % D. stocksii) (Figure 2). High survival rate in hardening during July and August has also been reported earlier on different bamboos (Mishra et al., 2008; Singh et al., 2012, 2021).
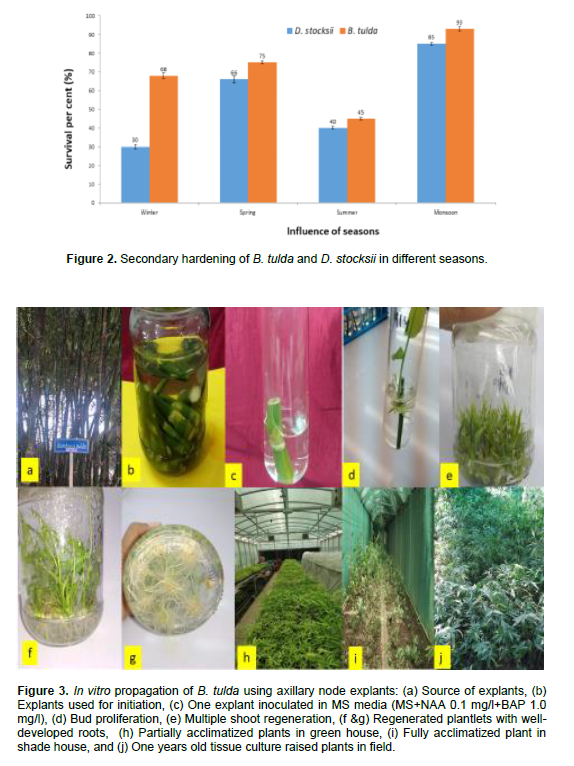
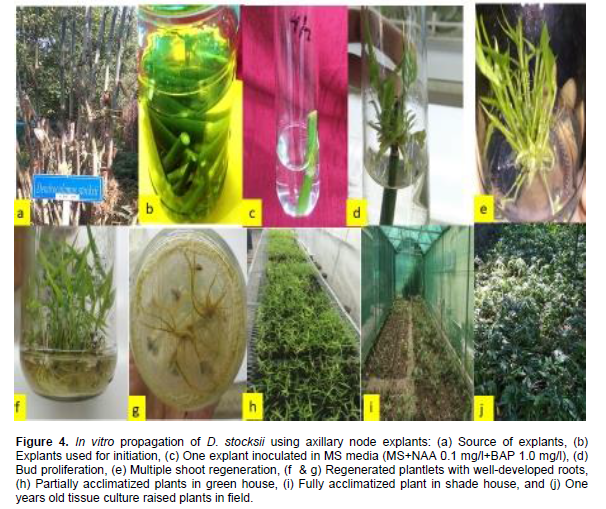
With the important variables taken into account, the study reveals about the large scale mass clonal propagation of two important bamboo species (B. tulda and D. stocksii). These findings can be helpful for industrial adoption of in vitro propagation technology for large scale commercial production.
However, in tissue culture raised plants, heterogeneity to some extent limit the purpose of in vitro propagation system. Therefore, it is advisable to test clonal fidelity after 10 sub culturing cycles. In our investigations, we followed up to 9-10 cycles of sub-culturing during multiplication. Axillary branching is, however, least susceptible to soma clonal variations (Negi and Sexena, 2011).
CONCLUSION
Because of the long flowering cycles of B. tulda and D. stocksii and the limitations of vegetative propagation, the difficulties are confronted with regards to the supply and the growing demand of these two commercially important bamboo species. In the present investigation, an efficient refined protocol for large scale mass clonal propagation, multiplication with high rooting and acclimatization in the soil with abundant growth performance has been developed. These findings can be helpful for industrial adoption of in vitro propagation technology for large scale production of high quality planting materials (true to the type). These two bamboo species have tremendous potential to develop agro industrialization in rural areas and to bring marginal lands into use.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
ACKNOWLEDGEMENT
The authors are thankful to the Department of Environment, Forest and Climate change (DEFCC), Government of Bihar for financial support (Sanction no.:Yo.Wa.13/14-2227/2014).
REFERENCES
|
Agnihotri RK, Nandi SK (2009).In vitro shoot cut: a high frequency multiplication and rooting method in the bamboo Dendrocalamus hamiltonii. Biotechnology 8(2):259-263. |
|
|
Arab MM, Yadollahi A, Eftekhari M, Ahmadi H, Akbari M, Khorami SS (2018). Modeling and optimizing a new culture medium for in vitro rooting of GXN15 Prunus root stock using Artificial Neural Network Genetics Algorithm. Scientific Reports 8(1):1-18. |
|
|
Arshad SM, Kumar A, Bhatnagar SK (2005). Micropropagation of Bambusa wamin through proliferation of mature nodal explants. Journal of Biological Research 3:59-66. |
|
|
Arya ID, Arya S (1997). In vitro culture establishment of exotic bamboo Dendrocalamus asper. Indian Journal of Experimental Biology 35(11):1252-1255. |
|
|
Arya S, Sharma S (1998). Micropropagation technology of Bambusa bambos through shoot proliferation. Indian Forest 124:725-731. |
|
|
Bag N, Chandra S, Palni LMS, Nandi SK (2000). Micropropagation of Dev-ringal [Thamnocalamuss pathiflorus (Trin.) Munro]-a temperate bamboo, and comparison between in vitro propagated plants and seedlings. Plant Science 156(2):125-135. |
|
|
Banerjee M, Gantait S, Pramanik BR (2011). A twostep method for accelerated mass propagation of Dendrocalamus asper and their evaluation in field. Physiology and Molecular Biology of Plants 17(4):387-393. |
|
|
Beena DB, Rathore TS, Rao PS (2012). Effects of carbohydrates on in vitro axillary shoot initiation and multiplication of Bambusa pallida Munro. Journal of Phytology 4(5):55-58. |
|
|
Bhadrawale D, Mishra JP, Mishra Y (2018). An improvised in vitro vegetative propagation technique for Bambusa tulda: influence of season, sterilization and hormones. Journal of Forestry Research 29(4):1069-1074. |
|
|
Bin Azizan MNA (2017). The effect of BAP and NAA treatment on micropropagation of Cucumis sativas L. International Journal of Science and Research (IJSR) 6(11):170-176. |
|
|
Buckseth T, Singh RK, Sharma AK, Sharma S, Modgil V, Saraswati A (2017). Effect of streptomycin and gentamycin on in vitro and cultural contaminants of potato cultivars. International Journal of Current Microbiology and Applied Sciences 6(12):4038-4043. |
|
|
Chaturvedi HC, Sharma M, Sharma AK (1993). In vitro regeneration of Dendrocalamus strictus Nees through nodal segments taken from field-grown culms. Plant Science 91(1):97-101. |
|
|
Choudhary P, Das M, Sikdar SR, Pal A (2004). Influence of the physiological age and position of the nodal explants on micropropagation of field-grown Dendrocalamus strictus Nees. Plant Cell Biotechnology and Molecular Biology 5(1-2):45. |
|
|
Choudhary AK, Kumari P, Ranjan A (2017). Refinement of protocol for rapid clonal regeneration of economical bamboo, Bambusa balcooa in the agroclimatic conditions of Bihar, India. African Journal of Biotechnology 16(10):450-462. |
|
|
Choudhary AK, Ranjan A, Kumari P (2016). In vitro shoot proliferation for rapid and mass production of quality planting materials of Bambusa nutans in the climatic conditions of Bihar, India. Indian Journal of Energy 5(2):1-11. |
|
|
Choudhary AK, Ranjan A, Kumari S, Tudu S (2020). In vitro culture and adventitious rhizogenesis of Bambusa tulda Roxb. In agro- climatic conditions of Bihar, India. South Asian Journal of Experimental Biology 10(1):26-36. |
|
|
Goyal AK, Sen A (2016). In vitro regeneration of bamboos, the "Green Gold": An overview. Indian Journal of Biotechnology 15:9-16. |
|
|
Hossain MF, Islam MA, Numan SM (2015). Multipurpose uses of bamboo plants: A review. International Research Journal of Biological Sciences 4(12):57-60. |
|
|
Hoogendoorn JC, Benton A (2014). Bamboo and rattan production and the implications of globalization. In Forest and Globalization. Routledge pp. 178-179. |
|
|
India (2021). A reference Annual publication Division, Ministry of Information and Broadcasting, Govt. of India. P 799. |
|
|
Jiménez VM (2005). Involvement of plant hormones and plant growth regulators on in vitro somatic embryogenesis. Plant Growth Regulation 47(2):91-110. |
|
|
Jiménez VM, Castillo J, Tavares E, Guevara E, Montiel M (2006). In vitro propagation of the neo-tropical giant bamboo, Guadua angustifolia Kunth, through axillary shoot proliferation. Plant Cell, Tissue and Organ Culture 86(3):389-395. |
|
|
Kabade AU (2009). Studies on refinement of protocols for rapid and mass In vitro clonal propagation, evaluation of genetic fidelity and growth performance of bamboo species-Bambusa bambos (L.) Voss and Dendrocalamus strictus (Roxb.) Nees. Forest Research Institute Dehradun. |
|
|
Kiran A, Ansari SA (2000). Adventitious rhizogenesis in relation to seasonal variation, size of culm branch cuttings and IAA treatment in bamboos. Indian forester 126(9):971-984. |
|
|
Liese W, Kohl M (2015). Bamboo: The plant and its uses. Tropical Forestry Springer International Publishing. |
|
|
Mane C, Meshram MP, Patil SR, Deshmukh V, Kamadi SR, Baviskar SB (2020). In vitro micropropagation of Dendrocalamus stocksii Munro through nodal explants. Journal of Soils and Crops 30(2):310-314. |
|
|
Mathias RJ, Mukasa C (1987). The effect of cefotaxime on the growth and regeneration of callus from varieties of barley (Hordeum vulgare L). Plant Cell Reports 6(6):454-457. |
|
|
Mishra Y, Patel PK, Yadav S, Shirin F, Ansari SA (2008). A micropropagation system for cloning of Bambusa tulda Roxb. Scientia Horticulturae 115(3):315-318. |
|
|
Mustafa AA, Derise MR, Yong WTL, Rodrigues KFA (2021). Concise Review of Dendrocalamus asper and related bamboos: Germplasm Conservation, Propagation and Molecular Biology Plant 10:1897. |
|
|
Nadha HK, Salwan R, Kasana RC, Anand M, Sood A (2012). Identification and elimination of bacterial contamination during in vitro propagation of Guadua angustifolia Kunth. Pharmacognosy Magazine 8(30):93. |
|
|
Negi D, Saxena S (2011b). Micropropagation of Bambusa balcooa Roxb. through axillary shoot proliferation. In Vitro Cellular and Developmental Biology-Plant 47(5):604-610. |
|
|
Prutpongse P, Gavinlertvatana P (1992). In vitro micropropagation of 54 species from 15 genera of bamboo. HortScience 27(5):453-454. |
|
|
Rasool R, Kamili AN, Ganai BA, Akbar S (2009). Effect of BAP and NAA on shoot regeneration in Prunella Vulgaris. J Journal of Natural Sciences and Mathematics 3(1):1-26. |
|
|
Rajput BS, Jani MD, Sasikumar K, Manokari M, Sekhawat MS (2019). An improved micropropagation protocol for Manga bamboo- Pseudoxytenanthera stocksii (Munro) T. Q. Nguyen. World News of Natural Sciences 25:141-154. |
|
|
Rajput BS, Jani MD, Ramesh K, Manokari M, Jogam P, Allini VR, Kher MM, Shekhawat MS (2020). Large- scale clonal propagation of Bambusa balcooa Roxb. An industrially important bamboo species. Indust. Industrial Crops and Products 157:112905. |
|
|
Ramanayake SMSD, Yakandawala K (1997). Micropropagation of the giant bamboo (Dendrocalamus giganteus Munro) from nodal explants of field grown culms. Plant Sciences 129(2):213-223. |
|
|
Rathore TS, Ravishankar Rai V (2005). Micropropagation of Pseudoxytenanthera stocksii Munro. In Vitro Cellular and Developmental Biology-Plant 41(3):333-337. |
|
|
Sawarkar AD, Shrimankar DD, Kumar A, Kumar A, Singh L, Kumar S, Kumar R (2020). Commercial clustering of sustainable bamboo species in India. Industrial Crops and Products 154:112693. |
|
|
Saxena S (1990). In vitro propagation of the bamboo (Bambusa tulda Roxb.) through shoot proliferation. Plant Cell Reports 9(8):431-434. |
|
|
Saxena S, Bhojwani SS (1993). In vitro clonal multiplication of 4-year old plants of the bamboo, Dendrocalamus longispathus Kurz. In Vitro Cellular and Developmental Biology-Plant 29(3):135-142. |
|
|
Saxena S, Dhawan V (1999). Regeneration and large-scale propagation of bamboo (Dendrocalamus strictus Nees) through somatic embryogenesis. Plant Cell Reports 18(5):438-443. |
|
|
Sandhu KV, Sherwin E, Schellekens H, Stanton C, Dinan TG, Cryan JF. (2017). Feeding the microbiota-gut-brain axis: diet, microbiome, and neuropsychiatry. Translational Research 179:223-244. |
|
|
Sandhu M, Wani SH, Jimenez VM (2018).In vitro propagation of bamboo through axillary shoot proliferation: a review Plant Cell Tiss. Plant Cell, Tissue and Organ Culture (PCTOC) 132(1):27-53. |
|
|
Sharma P, Sharma KP (2013). In vitro propagation of Bambusa tulda: An important plant for better environment. Journal of Environmental Research and Development 7:1216-1223. |
|
|
Shirin F, Rana PK (2007). In vitro plantlet regeneration from nodal explants of field-grown culms in Bambusa glaucescensWilld. Plant Biotechnology Reports 1(3):141-147. |
|
|
Singh SR, Dalal S, Singh R, Dhawan AK, Kalia RK (2012). Seasonal influences on In vitro bud break in Dendrocalamus hamiltonii rn. ex Munro nodal explants and effect of culture microenvironment on large scale shoot multiplication and plantlet regeneration. Indian Journal of Plant Physiology 17(1):9-21. |
|
|
Singh L, SridharanS, Thul ST, Kokate P, Kumar P, Kumar P, Kumar S, Kumar R (2020). "Eco-rejuvenation of degraded land by microbe assisted bamboo plantation. Industrial Crops and Products 155:112795. |
|
|
Singh S, Singh H, Sharma SK, Nautiyal R (2021).Seasonal variation in biochemical responses of bamboo clones in the sub-tropical climate of Indian Himalayan foothill. Heliyon 7(4):e06859. |
|
|
Somashekar PV, Rathore TS, Shashidhar KS (2008). Rapid and simplified method of micropropagation of Pseudoxytenanthera stocksii. In: Ansari SA, Narayanan C, Mandal AK (eds) Forest biotechnology in India. Satish Serial Publishing House, Delhi, pp. 165-182. |
|
|
Somashekar PV, Rathore TS, Fatima T (2018). In vitro plant regeneration of Dendrocalamus stocksii (Munro) M. Kumar, Ramesh & Unnikrisnan, through somatic embryogenesis. American Journal of Plant Sciences 9(12):2429-2445. |
|
|
Sood A, Ahuja PS, Sharma M, Sharma OP, Godbole S (2002). In vitro protocols and field performance of elites of an important bamboo Dendrocalamus hamiltonii Nees et Arn. Ex Munro. Plant Cell, Tissue and Organ Culture 71(1):55-63. |
|
|
Suwal MM, Lamichhane J, Gauchan DP (2020). Regeneration Technique of bamboo species through nodal segments: A Review. Nepal Journal of Biotechnology 8(1):54-68. |
|
|
Thorpe TA, Harry IS, Kumar PP (1991). Application of micropropagation to forestry. In: Debergh PC, Zimmerman RH (eds) Micropropagation. Kluwer, Dordrecht pp. 311-335. |
|
|
Venkatasalam EP, Pandey KK, Singh BP, Thakur V, Sharma S, Sood R, Sharma AK (2013). Efficacy antimicrobial agent on In vitro Micropropagation potential of potato. Potato Journal 40(1):45-54. |
|
|
Waghmare VG, Raut VK, Kale AN, Awachare PK (2021). Rapid in vitro propagation of BambusabalcooaRoxb. (Bamboo). International Journal of Current Microbiology and Applied Sciences 10(03):651-657. |
|
|
Waikhom SD, Louis B (2014). An effective protocol for micropropagation of edible bamboo species (Bambusa tulda and Melocanna baccifera) through nodal culture. The Scientific World Journal P 14. |
|
|
Yasodha R, Kamala S, Kumar SPA, Kumar PD, Kalaiarasi K (2008). Effect of glucose on In vitro rooting of mature plants of Bambusanutans. Scientia Horticulturae 116:113-116. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0