ABSTRACT
Bluetongue virus (BTV) is the ‘type’ species of the genus Orbivirus within the family Reoviridae. The BTV genome is composed of ten linear segments of double-stranded RNA (dsRNA), each of which codes for at least one of ten distinct viral proteins. Phylogenetic analysis based on VP2, VP3, VP5, VP7 and NS3 gene has been advocated by different researchers around the world. However, very little information about the phylogenetic analysis based on the NS1 gene of BTV-16 isolates is available. In this study, a partial sequence of segment 5 (Seg 5) from the BTV-16 Hisar isolate was sequenced. Sequence analysis of the Seg 5 of the BTV-16 Hisar isolate revealed highest nucleotide sequence identity of 98.9% (BTV-21), -96.4% (BTV-9) with sequences of NS1 genes of Indian isolates and 97.8% (BTV-3),-93.4%(BTV-1) isolates from other countries. The lowest sequence identity detected was 91.2% between sequences of NS1 gene of JQ924824 of BTV 16 Indian isolate and JX861502 BTV-1 of France. The study also assessed the comparative identity from the deduced amino acid sequences and found maximum homology (100% identity) with Indian isolates and 95.2 to 98.8% identity with Western isolates with only three amino acid difference. Phylogenetic analysis of the NS1 gene of the BTV-16 Hisar isolate with others around the world has revealed two major clusters which includes the Indian (Eastern) linage and Western linage separately. The BTV-16 Hisar isolate was found to be closely related with the BTV-9 and 21 Indian isolates based on its NS1 gene. Phylogenetic analysis of segment 5 is highly conserved among the different serotypes however, NS1 of BTV-3 of USA clustered with the Eastern lineage indicating introduction of Western BTV strains and a possible reassortment between Eastern and Western field strains in India.
Key words: Bluetongue virus, genome, NS1, Orbivirus, phylogeneticanalysis, segment-5, serotype.
Bluetongue is an arthropod-transmitted hemorrhagic disease of wild and domestic ruminants. It is enzootic between approximately 45 to 53°N and 35°S, in many tropical, subtropical and some temperate regions, including much of the Americas, Africa, southern Asia and northern Australia, and coincides with the geographic
distribution and seasonal activity of competent Culicoides vector insects (Purse et al., 2005, 2009).
Bluetongue virus (BTV) is the prototype of the genus Orbivirus in the family Reoviridae (Mertens et al., 2004; Bitew et al., 2017; Abera et al., 2018). Currently, there are 27 recognized serotypes with recent additions of the 25th serotype (“Toggenburg orbivirus”) from Switzerland in goat and 26th serotype from Kuwait in sheep and goat (Hofmann et al., 2008; Maan et al., 2011, 2012c; Batten et al., 2013) and 27th from Corsica, France (Schulz et al., 2016). Bluetongue virus is a non-enveloped virus, approximately 90 nm in diameter, with a triple-layered icosahedral protein capsid. Its genome consists of 10 double-stranded (ds) RNA segments coding for 7 structural proteins (VP1–VP7) and 4 non-structural proteins (NS1, NS2, NS3/NS3a, NS4) of approximately 19.2kb pairs in total (Singh et al., 2005; Sperlova and Zendulkova, 2011; Ratinier et al., 2011; Bitew et al., 2017; Abera et al., 2018).
The outer capsid of the virion is composed of 60 trimers of VP2 and 120 trimers of VP5 (Zhang et al., 2011) and differences within this outer capsid define the 26 BTV serotypes which have been described so far (Hofmann et al., 2008; Maan et al., 2007). The outer capsid proteins, VP2in particular, stimulate virus neutralizing antibodies which in general protect only against the homologous serotype (DeMaula et al., 2000). The internal core is formed by two layers, constituted by VP3 (subcore) and the immunodominant VP7 (intermediate layer) (Grimes et al., 1998). Three minor enzymatic proteins, VP1 (RNA dependent RNApolymerase), VP4 (capping enzyme and transmethylase) and VP6(RNA dependent ATPase and helicase) are contained within the core that is transcriptionally active in infected cells (Mertens and Diprose, 2004; Sutton et al., 2007; Roy, 2008; Noad and Roy, 2009; Boyce et al., 2004; Wehrfritz et al., 2007; Singh et al., 2005). The BTV genome encodes also 4 non-structural proteins: NS1, NS2, NS3/NS3a and NS4 (Ratinier et al., 2011). NS1 and NS2 are highly expressed viralproteins and their multimers are morphological features of BTV infected cells. Multimers of the NS1 protein form tubules (approximately 50 nm in diameter and up to 1000 nm in length) that appear to be linked to cellular cytopathogenicity (Owens et al., 2004; Ratinier et al., 2011), while NS2 is the major component of the viral inclusion bodies. NS2 plays a key role in viral replication and assembly as it has a high affinity for single stranded RNA (ssRNA) and possesses phosphohydrolase activity (Horscroft and Roy, 2000; Ratinier et al., 2011; Belhouchet et al., 2011). NS3/NS3a is glycosylated proteins involved in BTV exit. There are two isoforms of NS3: NS3 and NS3a with the latter lacking the N-terminal 13 amino acid residues (Celma and Roy, 2009; Han and Harty, 2004; Boyce et al., 2008). It has been recently identified that BTV expresses a fourth non-structural protein (NS4) encoded by an open reading frame in segment 9 overlapping the open reading frame encoding VP6. NS4 is 77 to 79 amino acid residues in length and highly conserved among several BTV serotypes/strains (Ratinier et al., 2011; Belhouchet et al., 2011).
BTV-16 was first isolated in 1960 in Pakistan (Maan et al., 2012a). It is the most prevalent virus in India. It has been isolated from sheep in Madhya Pradesh, Maharashtra, Tamil Nadu, Uttar Pradesh and Jammu and Kashmir. BTV-16 field strains from China and Australia have been fully sequenced (Boyle et al., 2012; Yang et al., 2012), as well as reference strain RSArrrr/16 and BTV-16 strain Goat/10/Ind/ABT/Hisar (Maan et al., 2012a; Minakshi et al., 2012). Phylogenetic analysis of VP2, VP3 and VP7 genes have been recommended to determine the relationship between the orbiviruses. Phylogenetic analysis based on VP2, VP3, VP5, VP7 and NS3 gene has been advocated by different researchers around the world (Dash et al., 2005; Singh et al., 2005; Maan et al., 2007; Maan et al., 2012b, d). However, little information about the phylogenetic analysis based on NS1 gene of BTV-16 isolates is available. This study was carried out with the objectives to study the extent of nucleotide and the amino acid sequence variation in the BTV-16 Hisar isolate in comparison with 23 other BTV serotypes isolated from different geographical locations of the world and to study the phylogenetic analysis of the BTV-16 Hisar isolate based on partial sequence of segment 5 (NS1) genes.
Virus
The BTV-16 Hisar isolate used in this study was acquired from the India Network Programme on Bluetongue (AINP-BT) project repository, Virus Laboratory, Center for Animal Disease Research and Diagnosis (CADRAD), Indian Veterinary Research Institute (IVRI), Izatnagar, U.P. (243122), India. The BTV 16 prototype virus was propagated in baby hamster kidney (BHK)-21 cell cultures. Two 75 cm2 cultures of BHK-21cells at 70 to 80% cell confluency were infected with 0.1 multiplicity of infection (MOI) per cell, and infected cells were harvested and pelleted by centrifugation (2000 g for 10 min at 4°C). RNA extraction was performed when approximately 80% of the infected cells showed cytopathology (approx. 72 hpi).
Viral dsRNA extraction
Total RNA was extracted from 250 µl infected BHK-21 cell suspension with 750 µl Trizol-LS reagent (Life Technology, USA), according to the manufacturer’s recommended method. RNA was precipitated with isopropanol and washed with 70% ethanol. Double-stranded RNA (dsRNA) was purified by 2M LiCl differential precipitation of ssRNA as described previously (Wilson et al., 1990). The dsRNA was dried in a dry bath and resuspended in 30 µl DEPC treated water. The quality of the dsRNA was assessed by 1% agarose gel electrophoresis (7 V/ cm-1 for 1 h) in Tris acetate EDTA (TAE) buffer containing 0.5 mg ethidium bromide ml-1. The concentration of dsRNA was determined using a nanophotometer by measuring absorbance at 260 nm. Extracted RNA was stored at -80°C until complimentary DNA (cDNA) synthesis was performed.
cDNA synthesis and amplification bypolymerase chain reaction (PCR)
Reverse transcriptase polymerase chain reaction (RT-PCR) was done with two steps as described by Wade-Evans et al. (1990). Reverse transcription was performed in a final volume of 20 µl from BTV dsRNA using segment 5 (NS1) gene specific primers [Forward primer (FR) 5’- GTTCTCTAGTTGGCAACCACC-3’(nt.=10-30) and reverse primer (RP) 5’- AAGCCAGACTGTTTCCCGAT-3’(nt. 264-283)] and MMLV-RT enzyme (Promega, USA) according to manufacturer’s protocols. The primers were custom synthesized by M/s Eurofins, Bangalore. The cDNA mix containing 4 µl of BTV dsRNA (500 ng), 1 µl of forward primer (20 pmol), 1 µl of reverse primer (20 pmol) and 3 µl of DPEC treated water was incubated at 94°C for 4 min for denaturation of the dsRNA and to remove secondary structures, and then chilled on ice for the primer annealing step. A master mix was prepared containing 4 µl of 5× reaction buffer (50 mM Tris/HCl, pH 7.5, 3 mM MgCl2 and 75 mM KCl) (Thermoscientific, USA ), 1 µl of dNTP mix (10 mM each dATP, dCTP, dGTP, dTTP), 2µl DTT (0.1 M), 1.5 μl of DEPC water, 0.5µl of RibolockTM (Ribonuclease-inhibitor, 40 U/µl)(Fermentas, USA) and 1 µl of reverse transcriptase (Revert Aid H minus, 200 units/μl)(Thermoscientific, USA). The master mix was added to each reaction mixture tube in 10 µl aliquots and mixed. Reverse transcription was carried out by incubating the reaction mixture at 42°C for 60 min and the MMLV-RT inactivated by incubating the tube at 70°C for 10 min.The cDNA was stored at -20°C until PCR was performed. The PCR master mix consisted of 2.5 µl of 10Xmagnesium free PCR buffer (10mM Tris-HCl, 50mM KCl, 0.1% Triton X), 1.5 µl of 25 mM MgCl2, 1 µl of 10 mM each of four dNTP, 1 µl each of (20pmol) forward and reverse primers, 0.5 µl of 1 U/µl Taq DNA polymerase (Promega, USA), 1 µl of cDNA and nuclease free water (NFW) was added. The PCR was carried out by the GeneAmp® PCR system 9700 thermal cycler machine (Applied biosystems®, USA). The cycling conditions were initial denaturation at 95°C for 5 min, 35 cycles of 94°C/30 s denaturation, 58°C/30 sprimer annealing and 72°C/30 s extension, followed by a final extension of 72°C for 10 min. The RT-PCR product was resolved on a 1.5% agarose gel in 1X TAE buffer. The size of RT-PCR products was determined by a 100 bp DNA ladder (Fermentas, USA) run simultaneously. Gel was stained with ethidium bromide (Life technology, USA) and visualized by UV illumination using the Gel Documentation System (Gel Doc TM XR+, imaging system, BIORAD, USA).
Cloning of the amplified NS1 partial gene in pJET1.2/blunt vector
RT-PCR product was resolved on a 1.2% low melting point agarose gel and 274 bp fragments were cut for elution. The 274 bp PCR product was purified by using GeneJET gel extraction kit (Fermentas, Lithuania) and then cloned into the pJET1.2/blunt vector using Clone- JET PCR cloning kit (Fermentas, Lithuania). The purified PCR product was ligated in pJET1.2/blunt vector by using Clone- JET PCR cloning kit (Fermentas, Lithuania) in 1:1 ratio. Escherichia coli strain DH5α cells were prepared employing the CaCl2 method prior to ligation and stored at -70°C. Ligation mixtures were transformed in chemically competent E. coli strain DH5α cells by heat shock method according to the manual protocol (Sambrook and Russell, 2001). After transformation, 50 µl of transformed material was inoculated onto a Petri dish containing LB agar with 100 μg/ml ampicillin. The colonies were screened for the recombinant plasmid DNA. Re-circularized pJET1.2/blunt vector (vector without the insert) expresses a lethal restriction enzyme after transformation and hence bacteria cells are not propagated. As a result, only recombinant clones containing the insert grow on culture plates. Boiling followed by RT-PCR from supernatant was used as a screening tool. Plasmid DNA was isolated from selected colonies using GeneJet Miniprep plasmid isolation kit (Fermentas, Lithuania).
Sequencing of partial NS1 gene
The recombinant plasmids containing the 274 bp insert were sequenced by M/s Eurofins Genomics India Private Limited, Bangalore. Automated DNA sequencing system (ABI 3700) from applied Biosystems was used along with ABI PRISM genetic analyzer (Applied Biosystems, Foster City, California, USA) using BigDyeTM terminator cycle sequencing kit according to manufacturer's instructions. The BTV-16 NS1 partial gene was sequenced by using T7 promoter primer. The chromatograms received after sequencing were analyzed using Chromas Lite v2.01 software (http://www.technelysium.com.au) and sequence reading errors if any were edited and assembled using the Bioedit, Edit Seq and mega Align module of lasergene-5software package (DNASTAR Inc, USA) and compared with NS1 gene sequences reported in GenBank public database.
Nucleotide sequence data assembly and phylogenetic analysis
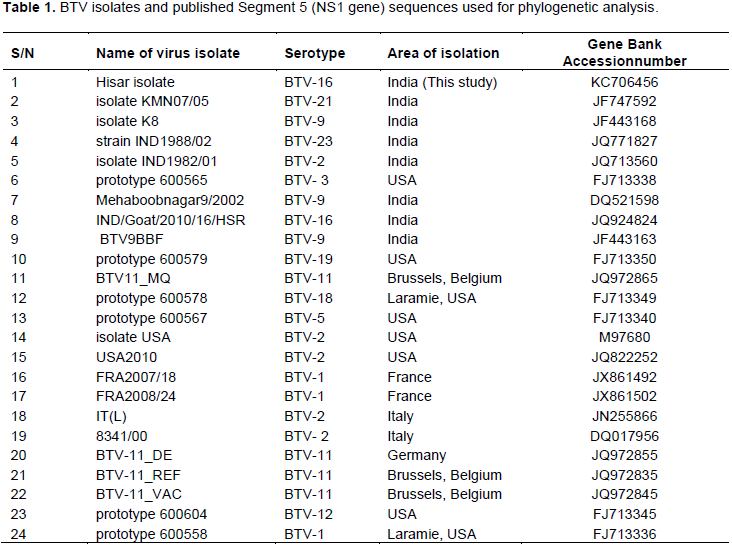
Homology studies were done through standard nucleotide and protein BLAST software available at NCBI web site (http://www.ncbi.nlm.nih.gov/Blast).The consensus sequence of the 274 bp fragment was obtained out of 7 clones used to obtain the genetic sequence for phylogenetic and sequence analysis. The sequences were analyzed along with the sequences of 23 selected other Indian and International BTV isolates belonging to various serotypes (Table 1). The multiple sequence alignment was done using the CLUSTALW program integrated in MEGA v5.1 software and Open Reading Frame (ORF) Finder (http://www.ncbi.nlm.nih.gov/gorf/gorf.html). Phylogenetic analysis was done by MEGA v5.1 software (Tamura et al., 2011). The percentage of nucleotide identity and diversity was done by MegAlign module of lasergene-5software package (DNASTAR Inc, USA).The evolutionary history was inferred using the Neighbor-Joining (N-J) method (Saitou and Nei, 1987). The optimal tree with the sum of branch length = 0.23054784 is shown. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown next to the branches (Felsenstein, 1985). The tree was drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the Kimura 2-parameter method (Kimura, 1980) and are in the units of the number of base substitutions per site. The analysis involved 24 nucleotide sequences. Codon positions included were 1st+2nd+3rd+Noncoding. All positions containing gaps and missing data were eliminated. There were a total of 274 positions in the final dataset for nucleotide analysis. For deduced protein phylogenetic analysis, 249 positions were included, because ORF starts from position 26 with total of 83 amino acids (Tamura et al., 2011).

The prototype BTV-16 virusHisar isolate having titer of 107.2 ml-1 was propagated in baby hamster kidney (BHK)-21 cells in 75 cm2 cell culture flask. Cells were infected by using 0.1 multiplicity of infection (MOI), and revealed distinct cytopathic effects (CPE) characterized by appearance of foci of rounded, refractile and increasing the number of granular cells at 24 hpi. This was followed by aggregation of enlarged and vacuolated cells, foamy degeneration and death of infected cells resulting in detachment of cell monolayer from the surface after 72 hpi. Control cells did not reveal any cytopathic effect. RNA extraction was performed when approximately 80% of the infected cells showed cytopathology (approx. 72 h).
Synthesized cDNA was used as a template in PCR amplification, using gene specific primers, designed to amplify 274 bp fragment of segment 5 (NS1) genes. Annealing temperature was optimized at 58°C via gradient PCR and Pfu polymerase was used to amplify the product to reduce the error rate in the amplification process. Total volumes of RT-PCR products were resolved on 1.5% agarose gel along with 100 bp molecular weight marker.
Sequence analysis
In this study, the nucleotide and amino acid sequences of the NS1 gene of BTV-16 strains were compared with several other BTV serotypes isolated from different geographical locations of the world. The nucleotide sequence of the insert obtained by sequencing (274bp) and the deduced amino acid (83aa) have been submittedtoNCBI GenBank and assigned Accession number KC706456. The sequence was analyzed along with the sequences of 23selectedother Indian and global BTV isolates belonging to various serotypes (Table 1). Multiple sequence alignment of BTV-16 for this study revealed highest nucleotide sequence identity [98.9% (BTV-21, JF747592) -96.4% (BTV-9, JF443163)] with sequences of NS1 gene of Indian isolates, and isolates from other countries [97.8% (BTV-3, FJ713338)-93.4%(BTV-1,FJ713336)]. However, there were 3 nucleotide changes between BTV-16 for this study and Indian isolates and many nucleotide changes have been detected in Western isolates (Figure 1). The lowest sequence identity detected between other sequences was 91.2% between sequences of NS1 gene of JQ924824 of BTV 16 Indian isolate and JX861502 BTV-1 of France. The study also assessed the comparative sequence identity from the deduced amino acid level and found maximum homology (100% identity) with Indian isolates and 95.2-98.8% identity with Western isolates with only three amino acid difference. The conservation of critical amino acids was seen in this region (Figure 2).
Phylogenetic analyses
Phylogenetic analysis of NS1 gene of BTV-16 Hisar isolate with others around the world has revealed two major clusters consisting of the Indian (Eastern) linage and Western linage, with the exception of BTV-3 FJ713338 of the USA which clustered within the Indian isolate group (Figure 3). BTV-16 NS1 gene was found to be closely related with BTV-9 and 21of Indian isolates. Similarities apparent at both NS1 gene and amino acid level demonstrate that some related serotypes have sequences in common, indicating a relatively closer common ancestry. These relationships appear to depend on the geographical origin of individual isolates and serotypes (Figure 3).
Bluetongue is endemic in most parts of India (Dash et al., 2005; Singh et al., 2005; Maan et al., 2007). Twenty one out of the possible 27 serotypes of BTV are found circulating in different parts of India (Singh et al., 2005; Bitew et al., 2013). Phylogenetic analysis of VP2, VP3 and VP7 genes have been recommended to determine the relationship between the orbiviruses (Singh et al., 2005). However, molecular phylogenetic analysis including VP5 and NS3 genes have also been promoted by different researchers around the world (Dash et al., 2005; Singh et al., 2005; Maan et al., 2007, 2012a). Segment 5 (Seg-5) encodes the NS1 protein (553 amino acids), which accumulates in the cytoplasm of infected cells as tubules (Ratinier et al., 2011); however, the function of NS1 is unknown. Previous phylogenetic analysis of Seg-5 identified distinct Western and Eastern topotypes (Boyle et al., 2012). However, little information about the phylogenetic analysis of NS1 gene of BTV-16 isolates is available. In this study nucleotide and the amino acid sequence of the NS1 gene of BTV-16 isolates were compared with several other BTV serotypes isolated from different geographical locations of the world. A bluetongue virus isolated from Hisar, India was used in this study. The BTV-16 propagated in BHK21 cells revealed characteristic cytopathic effect. The extraction of dsRNA by Trizol method following differential Lithium Chloride precipitation resulted in pure BTV dsRNA. RT-PCR amplification resulted in 274 bp amplicons and this product was cloned and sequenced. This result was in line with different authors (Maan et al., 2012a; Minakshi et al., 2012; Boyle et al., 2012; Yang et al., 2012).
The comparison of nucleotide sequence of different portion of the NS1 gene revealed a few number of nucleotide substitutions among various BTV serotypes. The percent nucleotide and amino acid sequences homology among various BTV serotypes, varies from 96.4 to 98.9% and 95.2 to 100% respectively. This confirms the fact that the NS1 gene is highly conserved among the different serotypes. The result is in agreement with Maan et al. (2012a) who sequenced all genome segments of the BTV-16 reference strain (RSArrrr/16) and showed >99% sequence identity to the BTV-16 vaccine strain (RSAvvvv/16) that was derived from it and to the Chinese BTV-16 (strain BN96/16) isolated from a sheep in Yunnan province during 1996. In contrast, the genome segments of RSArrrr/16 show lower levels of identity (90 to 95%) with the BTV-16 from Australia (strain DPP96), indicating that it represents a distinct virus
strain/lineage, although still within the major Eastern topotype. In contrast to this, Dash et al. (2005) found that 39 to 73% and 25 to 82% nucleotide and amino acid sequences homology respective among various BTV serotypes based on L2 gene which is the variable segment of BTV.
Phylogenetic analysis of NS1 gene of BTV-16 Hisar isolate with others around the world has revealed two major clusters which includes the Indian (Eastern) linage and Western linage separately. However, BTV-3, FJ713338 of USA clustered within the Indian isolate group. This suggests the introduction of Western BTV strains and re-assortment between Eastern and Western field strains in India. BTV-16 has shown to be closely related with BTV-9 and 21of Indian isolate with its NS1 gene. This is in line with the works of Boyle et al. (2012) and Maan et al. (2011) who identified distinct Western and Eastern topotypes with previous work on phylogenetic analysis of Seg-5 on (BTV 1, 7, 9, 20, and 21) and BTV-26 respectively. Phylogenetic analyses of BTV-3 (IND2003/08 isolate) by Maan et al. (2012c) show that nine genome segments belong to an Eastern lineage/topotype, with Seg-2 and Seg-6 (encoding outer capsid proteins VP2 and VP5) sharing the highest identity (90 and 91%, respectively) with earlier Eastern isolates of BTV-3 (Japanese strain ON-6/B/98). However, Seg-5/NS1 of IND2003/08 showed up to 99% identity with the Western topotype viruses (prototype 600565 strain), providing further evidence for the introduction of Western BTV strains and reassortment between Eastern and Western field strains in India (Maan et al., 2012d, b; Wilson et al., 2009). Western topotype or reassortant BTV strains may be at least partly responsible for the increased virulence of bluetongue outbreaks seen in India, in indigenous sheep breeds. Seg-5 of IND2003/08 showed only 89% identity with a Western BTV-10 vaccine strain detected in India (Maan et al., 2012b) and is therefore unlikely derived from this source. Singh et al. (2005) also revealed the level of genetic diversity in genome segment 5, between different BTV serotypes, as well as between isolates of the same serotype. The BTV-3 isolate from USA is genetically closely related in genome segment 5 (NS1 encoding region), to strains from India, but is quite distinct from those from other isolates from USA and Europe. Singh et al. (2005) also reported the same result in his work on phylogenetic analysis based on segment 6 of BTV-3.
The authors have not declared any conflict of interests.
This work was supported by the all India network program on bluetongue disease, Indian Council of Agricultural Research (ICAR), New Delhi, India. The authors would like to appreciate the Director, Indian Veterinary Research Institute for providing necessary facilities to carry out this study.
REFERENCES
|
Abera T, Bitew M, Gebre D, Mamo Y, Deneke Y, Nandi S (2018). Bluetongue Disease in small ruminants in south western Ethiopia: Cross-sectional Sero-epidemiological study. BMC Research Notes 11(1):112.
Crossref
|
|
|
|
Batten CA, Henstock MR, Steedman HM, Waddington S, Edwards L, Oura AL (2013). Bluetongue virus serotype 26: Infection kinetics, pathogenesis and possible contact transmission in goats. Veterinary Microbiology 162:62-67.
Crossref
|
|
|
|
|
Belhouchet M, Mohd Jaafar F, Firth AE, Grimes JM, Mertens PPC, Attoui H (2011). Detection of a Fourth Orbivirus Non-Structural Protein. PLoS ONE 6(10):e25697.
Crossref
|
|
|
|
|
Bitew M, Nandi S, Ravishankar C, Sharma A, Chander V. (2017). Humoral immune response and protective efficacy of Binary ethylenimine (BEI) inactivated pentavalent bluetongue vaccine after challenge with homologous virus in sheep. International Journal of Virology 13(1):43-52.
Crossref
|
|
|
|
|
Bitew M, Nandi S, Ravishankar C., Somvanshi R. (2013). Serological and molecular evidence of bluetongue in sheep and goats in Uttar Pradesh, India. African Journal of Biotechnology 12(19):2699-2705.
|
|
|
|
|
Boyce M, Celma CC,Roy P (2008). Development of reverse genetics systems for bluetongue virus: recovery of infectious virus from synthetic RNA transcripts. Journal of Virology 82:8339-8348.
Crossref
|
|
|
|
|
Boyce M, Wehrfritz J, Noad R, Roy P (2004). Purified recombinant bluetongue virus VP1 exhibits RNA replicase activity. Journal of Virology 78:3994-4002.
Crossref
|
|
|
|
|
Boyle DB, Bulach DM, Amos-Ritchie R, Adams M M, Walker PJ, Weirb R (2012). Genomic sequences of Australian bluetongue virus prototype serotypes reveal global relationships and possible routes of entry into Australia. Journal of Virology 86(12):6724.
Crossref
|
|
|
|
|
Celma CC, Roy P (2009). A viral nonstructural protein regulates bluetongue virus trafficking and release. Journal of Virology 83:6806-6816.
Crossref
|
|
|
|
|
Dash PK, Nandi S, Pande A, Mondal B, Bandyopadhyay SK (2005). Comparative phylogenetic analysis of bluetongue virus Based on sequencing of two different regions of L2 gene. Indian Journal of Comparative Microbiology Immunology and Infectious Diseases 26(2):79-85.
|
|
|
|
|
DeMaula CD, Bonneau KR, MacLachlan NJ (2000). Changes in the outer capsid proteins of bluetongue virus serotype ten that abrogate neutralization by monoclonal antibodies. Virus Research 67:59-66.
Crossref
|
|
|
|
|
Felsenstein J (1985). Confidence limits on phylogenies: An approach using the bootstrap. Evolution 39:783-791.
Crossref
|
|
|
|
|
Grimes JM, Burroughs JN, Gouet P, Diprose JM, Malby R, Ziéntara S, Mertens PP, Stuart DI (1998).The atomic structure of the bluetongue virus core. Nature 395:470-478.
Crossref
|
|
|
|
|
Han Z, Harty RN (2004). The NS3 protein of bluetongue virus exhibits viroporin-like properties. Journal of Biological Chemistry 279:43092-43097.
Crossref
|
|
|
|
|
Hofmann MA, Renzullo S, Mader M, Chaignat V, Worwa G (2008). Genetic characterization of toggenberg orbivirus, a new bluetongue virus, from goats, Switzerland. Emerging and Infectious Disease 14:1855-1861.
Crossref
|
|
|
|
|
Horscroft NJ, Roy P (2000). NTP binding and phosphohydrolase activity associated with purified bluetongue virus non-structural protein NS2. Journal of General Virology 81:1961-1965.
Crossref
|
|
|
|
|
Kimura M (1980). A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. Journal of Molecular Evolution 16:111-120.
Crossref
|
|
|
|
|
Maan NS, Maan S, Guimera M, Nomikou K, Morecroft E, Pullinger G, Belaganahalli MN, Mertens PPC (2012b). The genome sequence of a reassortant bluetongue virus serotype 3 from India. Journal of Virology 86(2):6375-6376.
Crossref
|
|
|
|
|
Maan NS, Maan S, Nomikou K, Guimera M, Pullinger G, Singh KP, Belaganahalli MN, Mertens PPC (2012a). The genome sequence of bluetongue virus type 2 from India: evidence for reassortment between Eastern and Westerntopotype field strains. Journal of Virology 86:5967-5968.
Crossref
|
|
|
|
|
Maan S, Maan NS, Nomikou K, Batten C, Antony F, Belaganahalli MN, Samy AM, Reda AA, Al-Rashid SA, Batel M El, Oura CAL, MertensPPC (2011). Novel bluetongue virus serotype from Kuwait. Emerging and Infectious Disease 17:886-889.
Crossref
|
|
|
|
|
Maan S, Maan NS, Pullinger G, Nomikou K, Morecroft E, Guimera M, Belaganahalli MN, Mertens PPC (2012d). The genome sequence of bluetongue virus type 10 from India: evidence for circulation of a Western topotype vaccine strain. Journal of Virology 86: 5971-5972.
Crossref
|
|
|
|
|
Maan S, Maan NS, Samuel AR, Rao S, Attoui H, Mertens PPC (2007). Analysis and phylogenetic comparisons of full-length VP2 genes of the 24 bluetongue virus serotypes. Journal of General Virology 88:621-630.
Crossref
|
|
|
|
|
Maan S, Maan NS, Singh KP, Belaganahalli MN, Guimera M, Pullinger G, Nomikou K, Mertens PPC (2012c).Complete Genome Sequence Analysis of a Reference Strain of Bluetongue Virus Serotype 16. Journal of Virology 86(18):10255.
Crossref
|
|
|
|
|
Mertens PP, Diprose J (2004). The bluetongue virus core: a nano-scale transcription machine. Virus Research 101:29-43.
Crossref
|
|
|
|
|
Mertens PP, Diprose J, Mann S, Singh KP, Attoui H (2004). Bluetongue virus replication, molecular and structural biology. VeterinariaItaliana 40:426-437.
|
|
|
|
|
Minakshi P, Singh R, Ranjan K, Kumar P, Joshi CG, Reddy YKM, PrasadG (2012). Complete genome sequence of bluetongue virus serotype 16 of goat origin from India. Journal of Virology 86(15):8337.
Crossref
|
|
|
|
|
Noad R, Roy P (2009). Bluetongue virus replication and assembly. In: Mellor P, Baylis M, Mertens P, eds. Bluetongue. Amsterdam: Academic Press pp. 53-76.
Crossref
|
|
|
|
|
Owens RJ, Limn C, Roy P (2004). Role of an arbovirus nonstructural protein in cellular pathogenesis and virus release. Journal of Virology 78: 6649-6656.
Crossref
|
|
|
|
|
Purse BV, Brown HE, Harrup L, Mertens PP, Rogers DJ (2008). Invasion of bluetongue and other orbivirus infections into Europe: the role of biological and climatic processes. Revue scientifique et technique 27:427-442.
Crossref
|
|
|
|
|
Purse BV, Mellor PS, Rogers DJ, Samuel AR, Mertens PP, Baylis M (2005). Climate change and the recent emergence of bluetongue in Europe. Nature Review Microbiology 3:171-181.
Crossref
|
|
|
|
|
Ratinier M, Caporale M, Golder M, Franzoni G, Allan K, Nunes FS, Armezzani A, Bayoumy A, Rixon F, Shaw A, Palmarini M (2011). Identification and characterization of a novel non-structural protein of bluetongue virus. PLoS Pathogens 7(12):e1002477.
Crossref
|
|
|
|
|
Roy P (2008). Bluetongue virus: dissection of the polymerase complex. Journal of General Virology 89:1789-1804.
Crossref
|
|
|
|
|
Saitou N, Nei M (1987). The neighbor-joining method: A new method for reconstructing phylogenetic trees. Molecular Biology and Evolution 4:406-425.
|
|
|
|
|
Schulz C, Bréard E, Sailleau C, Jenckel M,Viarouge C, Vitour D, Palmarini M, Gallois M, Höper D, Hoffmann B, Beer M, Zientara S (2016). Bluetongue virus serotype 27: detection and characterization
|
|
|
|
|
of two novel variants in Corsica, France. Journal of General Virology 97(9):2073-2083.
|
|
|
|
|
Singh KP, Maan S, Samuel AR, Rao S, Meyer A, Mertens PP (2005). Phylogenetic analysis of bluetongue virus genome segment 5 (encoding NS1) from different serotypes. VeterinariaItalaliana 40:479-483.
|
|
|
|
|
Sperlova A, Zendulkova D (2011). Bluetongue: a review.Veterinary Medicine 56:430-452.
Crossref
|
|
|
|
|
Sutton G, Grimes JM, Stuart DI, Roy P (2007). Bluetongue virus VP4 is an RNA-capping assembly line. Nature Structural and Molecular Biology 14:449-451.
Crossref
|
|
|
|
|
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011). MEGA5: Molecular Evolutionary Genetics Analysis using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Molecular Biology and Evolution 28:2731-2739.
Crossref
|
|
|
|
|
Wade-Evans AM, Mertens PPC, Bostock CJ (1990). Development of the polymerase chain reaction for the detection of bluetongue virus in tissue samples. Journal of Virological Methods 30:15-24.
Crossref
|
|
|
|
|
Wehrfritz JM, Boyce M, Mirza S, Roy P (2007). Reconstitution of bluetongue virus polymerase activity from isolated domains based on a three-dimensional structural model. Biopolymers 86:83-94.
Crossref
|
|
|
|
|
Wilson WC, Hindson BJ, O'Hearn ES, Hall S, Tellgren-Roth C, Torres C, Naraghi-Arani P, Mecham JO, Lenhoff RJ (2009). A multiplex real-time reverse transcription polymerase chain reaction assay for detection and differentiation of Bluetongue virus and Epizootic hemorrhagic disease virus serogroups. Journal of Veterinary Diagnostic Investigation 21:760-770.
Crossref
|
|
|
|
|
Yang T, Liu N, Xu Q, Sun E, Qin Y, Zhao J, Feng Y, WuD (2012). Complete genomic sequence of bluetongue virus serotype 1 from China. Journal of Virology 86(2):1288.
Crossref
|
|
|
|
|
Zhang X, Boyce M, Bhattacharya B, Zhang XK, Schein S, Roy P, Zhou ZH (2010).Bluetongue virus coat protein VP2 contains sialic acid-binding domains, and VP5 resembles enveloped virus fusion proteins. Proceedings of the National Academy of Sciences of the United States of America 107:6292-6297.
Crossref
|
|