ABSTRACT
This study determined the potential antidiarrhoeal potential of Pericopsis angolensis and Swartzia madagascariensis extracts against Escherichia coli O157, Shigella species and Salmonella Typhi. Extracts were obtained using the following methods: (i) hot water extraction (90°C) (LHWE), (ii) cold water extraction (CWED) and (iii) ethanolic extraction (EED). Antimicrobial effects of the extracts were determined using the well diffusion assay. Phytochemical analysis was performed using standard biochemical methods. The LHWE extracts exhibited significantly greater inhibition than CWED and EED extracts as follows: (i) P. angolensis bark extract at 0.8 mg/ml against Shigella spp. and (ii) P. angolensis bark extract at 1.6 mg/ml and S. madagascariensis bark extract at 1.6 mg/ml against S. Typhi. The aqueous methods largely resulted in P. angolensis and S. madagascariensis extracts rich in flavonoids, saponins and tannins. The aqueous extraction methods (CWED and LHWE) are therefore suitable to obtain extracts with high antimicrobial effects against E. coli O157, Shigella species and S. Typhi.
Key words: Antidiarrhoeal, phytochemicals, extraction, Pericopsis angolensis, Swartzia madagascariensis.
The use of plants or their products in traditional medicines has, since historic times, remained significant in the treatment of various medical ailments such as diarrhoea (Maroyi, 2016). Notably, there is a renewed public interest in the use of traditional medicines owing to the high costs of orthodox medicines and the associated side effects, especially antimicrobial resistance (Patwardhan et al., 2005). In African countries, approximately 80% of the population reportedly rely on traditional medicines owing to their low cost and ease of access, as well as for cultural reasons (Maroyi, 2016).
Diarrhoeal diseases have remained a global burden and a leading public health threat characterised by high morbidity and mortality especially among children under the age of 5 years (Maroyi, 2016). Diarrhoea is thought to account for between 1 and 9 million deaths among children aged 5 years or younger globally, with the highest rates occurring in low income countries (LIC) especially those in sub Saharan Africa and Asia (Njume et al., 2011).
Plants have maintained their place in traditional medicine owing to their rich composition of healthful bioactive chemical compounds/metabolites (Smith, 2007). Notably, most communities in LIC tend to rely of phytomedicines to manage various forms of diarrhoea including cholera, typhoid and various forms of gastroenteritis. Bioactive compounds are accumulated plant tissues as secondary metabolites (Smith, 2007). These metabolites are often accessed through the use of extraction techniques, with some plant materials being consumed whole to achieve the desired therapeutic or prophylactic effect (Semenya and Maroyi, 2013). The composition and bioactivity of an extract depends on its inherent chemical composition, the solvent used as well as the protocol followed during the extraction process (Muhamad et al., 2017). Traditional medicine has often relied on organic solvents including the use of cold and hot water (steeping) as extractants (Ngarivhume et al., 2015; Palombo, 2011; Wachtel-Galor, 2004). However, the utilisation of organic solvents is often limited by a low extraction yield (Wachtel-Galor, 2004). To enhance the extraction processes, scientists have adopted protocols that use a combination of organic solvents (Pilon et al., 2016). The traditional extraction methods, relying primarily on hot or cold water, utilise fresh or dried plant materials, with the obtained extracts being administered within hours from the time of plant material collection (De Wet and Ngubane, 2014; Odunmbaku et al., 2018). Different extractive strategies reportedly yield products with different clinical efficacies (Odunmbaku et al., 2018). With the availability of more modern extraction methods including maceration, percolation, reflux extraction, super critical fluid extraction (SFC), pressurised liquid extraction (PLE) and microwave assisted extraction (MAE)-with advantages that include enhanced extraction efficiency and improved extract bioactivities (Zhang et al., 2018), their utility compared to that of the traditional hot and cold water extraction methods have remained unappraised.
The current study compared the antidiarrhoeal potential of hot water extraction and cold water extraction methods (LHWE and CWED, respectively) to that of ethanolic extraction method (EED). Specifically, the current study sought to assess the validity and utility of the aqueous extraction methods, which are considered methods of choice in the preparation of traditional antidiarrhoeal medicines. Two plant species with a history of use in the traditional management of diarrhoea in Southern Africa, namely Pericopsis angolensis (Baker) Meeuwen and Swartzia madagascariensis (Desv.) J.H. Kirkbr. & Wiersama (Table 1), were used for the study. The extracts were tested for antimicrobial activities against selected diarrhoeagenic Escherichia coli O157, Shigella species and presumptive Salmonella Typhi. Additionally, the phytochemical composition of the extracts was determined. This study was considered important as it informs both the traditional and orthodox medicinal practices on the relative utilities of these extraction methods.
Collection and processing of plant materials
Fresh samples of P. angolensis bark, S. madagascariensis bark and S. madagascariensis leaves were collected in Chiraswa Village in Murehwa, Mashonaland East Province of Zimbabwe (-17°.69’71.55ËS, 31°.96’48.90ËE) during the months of October-December 2018. Species identification was done by qualified botanists at the National Herbarium and Botanic Garden in Harare (Zimbabwe). Voucher specimens were deposited in the Biological Sciences laboratory for future reference.
The collected plant materials were washed to remove debri and then separated into two batches. Half of each fresh sample was frozen in airtight plastic bags for future use. The other half of each plant sample was air-dried for 72 h, then ground into fine powders with an electric grinder and stored in air tight containers in the dark at room temperature.
Extraction
Cold water (CWED) and ethanolic extraction (EED)
The powdered samples were extracted into cold distilled water (150 ml) and 70% ethanol (150 ml). The plant-solvent mixtures were continuously swirled at 150 rpm on a rotary shaker for 72 h. The extracts were filtered through Whatman No. 41 filter paper (pore size 20-25 μm) and the collected filtrates were evaporated at room temperature. Each dried extract was resuspended into between 1 and 2 ml of sterile Ringers solution. Concentrations of each stock solution were stored at -20°C until further analysis.
Hot water extraction (THWE)
Fresh plant samples (10 g) were added to 100 ml of hot boiled water (90°C) and steeped for 30 min. The samples were filtered through Whatman No. 41 filter paper (pore size 20-25 μm) and were stored at -20°C for further tests.
Phytochemical analyses
Qualitative chemical analyses of the extracts were conducted using the following biochemical tests. Ringers solution was used as a negative control for all phytochemical tests.
Test for tannins (Ferric chloride test)
A few drops of 0.1 ferric chloride were added to 2 ml of aqueous extracts (CWED, EED and THWE). A blue coloration indicated the presence of tannins [16].
Test for flavonoids
Dilute ammonia (5 ml) solution was added to 1 ml of each plant extract. Concentrated sulphuric acid (5 ml) was added and a yellow coloration in each plant extract indicated the presence of flavonoids (Zohra et al., 2012).
Test for alkaloids
Aqueous 1% hydrochloric acid (0.2 ml) was added to 2 ml each extract. Each solution was heated in a steam bath for 10 min. The aqueous extract solution was treated with 6 to 10 drops of Dragendoff’s reagent. A creamish precipitate indicated the presence of alkaloids (Zohra et al., 2012).
Test for saponins
Aqueous extracts (2 ml) were mixed with distilled water (5 ml) and shaken vigorously for stable persistence froth. The froth was mixed with 3 drops of olive oil and was shaken vigorously. Emulsion indicated the presence of saponins (Zohra et al., 2012).
Test for reducing sugars (Benedict’s test)
To 1 ml of the plant extract, a few drops of Benedict’s reagent (alkaline solution containing cupric citrate solution) were added and boiled in a water bath. A reddish brown precipitate indicated the presence of reducing sugars (Avinash and Waman, 2014).
Bacterial strains
The microorganisms used in determination of the antibacterial activity of the plant extracts’ were as follows: presumptive E. coli O157, Shigella spp. and S. Typhi. All bacterial strains were obtained from our in-laboratory stock of environmental isolates. The isolated strains were maintained on Nutrient agar. The bacterial cultures were prepared by transferring a colony of the bacteria into a universal bottle containing 10 ml of nutrient broth and incubated overnight at 37°C. The concentration of the bacterial cultures was standardised to a concentration of 1 × 108 colony forming units per millilitre (CFU/ml) (internal protocol), which is equivalent to an optical density of 0.2 using a Biobase EL 10B Microplate Reader (Jinan, China) at optical density 620 nm. Ringer’s solution was used as a negative control for all antimicrobial tests.
Antibacterial screening
Antibacterial tests were performed using standard agar well diffusion assay as described by Soman and Ray (2016). Briefly, agar plates were prepared using sterile HiCrome O157: H7 agar (Sigma-Aldrich, Saint Quentin Fallavier, France) and XLD agar (Sigma-Aldrich, Saint Quentin Fallavier, France) for E. coli O157, Shigella spp. and S. Typhi, respectively. The standardised cultures were evenly spread onto the surface of the agar plates using sterile swabs. Wells were made in each plate with a sterile auger (10 mm diameter). 40 µl of ethanol and aqueous extracts (100 mg/ml) were added in each well, with streptomycin (300 µg, Mast Diagnostics, UK) being used as positive control. The plates were incubated at 37°C for 24 h. Each extract was tested in triplicate.
Antibacterial activity was tested by observing bacterial growth and was indicated as the presence of clear zones around the well (zones of inhibition). The absence of the zone of inhibition around the wells was interpreted as the absence of activity. The zones of inhibition were measured in millimetres. Only extracts that showed antimicrobial activities were used to determine the minimum inhibition and minimum bactericidal concentration of each preparation.
Determination of minimal inhibitory concentration (MIC)
The minimal inhibitory concentration of the plant extracts/control was determined using the well diffusion assay (Soman and Ray, 2016). Agar plates were prepared using sterile HiCrome 0157: H7 media for E. coli 0157 and XLD media for Salmonella and Shigella spp. The standardised cultures were evenly spread on the surface of the agar plates using sterile swabs under sterile conditions. Wells were made in each plate with a sterile auger (10 mm diameter). 40 µl of plants extracts (two fold concentrations ranging from 0.781 to 100 mg/ml) were added in each triplicate wells. Streptomycin (300 µg, Mast Diagnostics, UK) was used as the positive control. The diffusion of the extracts was allowed at room temperature for 1 h in a sterile laminar flow cabinet and the plates were incubated at 37°C for 24 h. The plates were observed for antimicrobial activity and the zones of inhibition (mm) indicated the minimum concentration at which the extracts inhibited the growth of the test microorganisms. The concentration at which there was no zones of inhibition were recorded as the minimum inhibition concentration.
Determination of minimal bactericidal concentration (MBC)
A modified assay to that described by as modified from the Soman and Ray (2016) method was used to determine the MBC of each extract. Briefly, using the agar plates from the MIC assay, a sterile inoculating loop was used to touch the zone of inhibition of different concentrations of extracts where there was invisible growth. The loops were used to streak labelled and prepared agar plates. The plates were incubated for 24 h and observed for growth at different concentration.
Extracts from plant materials listed in Table 1 were exposed to different solvents and conditions. Briefly, plant materials were exposed to the following: (i) hot water (steeping) for 1 h (LHWE), a method simulating the traditional extraction method; (ii) cold water for 72 h (CWED) followed by evaporation at room temperature and (iii) ethanol for 72 h followed by evaporation at room temperature (EED). Yields per extract (mg) were obtained by weighing the dried samples and subtracting the weight of the containers (Petri dishes). Table 2 provides details of the amount of material used and the yield of extract (mg).
Standard phytochemical analyses were conducted on extracts described in Table 3. Briefly, extracts were exposed to various chemicals in accordance with standard biochemical protocols. Colour and other changes in the extracts were used to show the presence of the target compounds. Relative phytochemical concentration was determined relying on intensities of the extracts. Table 3 provides information on chemical composition of each extract used.
The traditional hot water extraction method (LHWE) yielded extracts that contained the following phytochemicals [flavonoids (P. angolensis bark extract and S. madagascariensis bark and leaf extracts, saponins (P. angolensis bark extract and S. madagascariensis bark and leaf extracts) and reducing sugars (P. angolensis bark extract and S. madagascariensis bark and leaf extracts)] (Table 3). The cold water extraction method (with evaporation) (CWED) yielded extracts that contained the following phytochemicals [flavonoids (P. angolensis bark extract and S. madagascariensis bark and leaf extracts, saponins (P. angolensis bark extract and S. madagascariensis bark and leaf extracts), tannins (P. angolensis bark extract and S. madagascariensis bark and leaf extracts) and reducing sugars (P. angolensis bark extract and S. madagascariensis bark and leaf extracts)] (Table 3). The ethanolic extraction method (EED) yielded extracts with greater concentrations of reducing sugars (P. angolensis bark extract)] (Table 3). All extracts showed no presence of alkaloids.
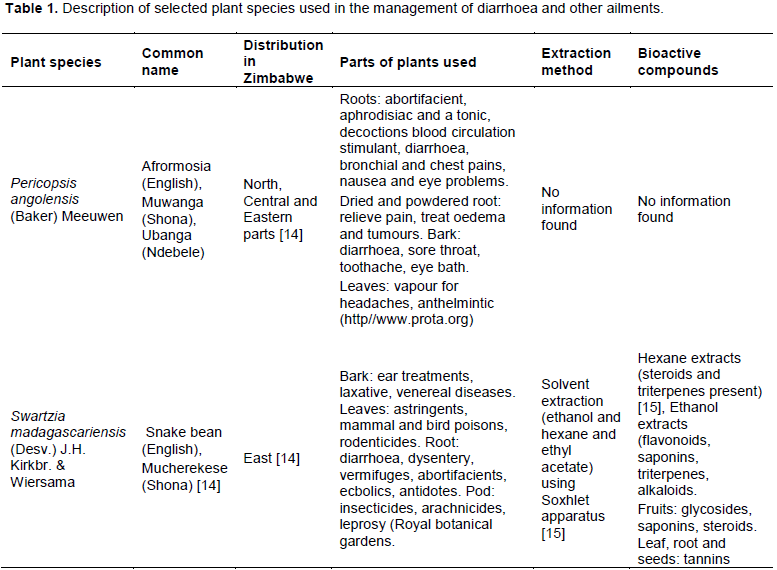
The traditional hot water extraction method (LHWE) yielded the highest antimicrobial activities in terms of the attainment of the lowest minimum inhibition concentration (MIC)/highest zone of inhibition (ZOI) against Shigella spp. in comparison with the other two extraction methods (CWED and EED) as follows: (i) P. angolensis bark extract (ZOI = 21 mm) (Figure 3) and (ii) S. madagascariensis bark extract (1.56 mg/ml) (Figure 1). The LHWE extract was shown to yield significantly greater ZOI at 100 mg/ml than CWED and EED against Shigella spp. (p = 0.001 and p = 0.0003, respectively) (Table A1). Additionally, LHWE extracts of the following extracts attained significantly greater antimicrobial activity (ZOI) against the strain of Shigella spp. (at 100 mg/ml) than Streptomycin (300 µg/ml) (ZOI = 20 mm) with the following: (i) S. madagascariensis leaf extract (ZOI = 23 mm) (Figure 2) and (ii) P. angolensis bark extract (ZOI = 21 mm) (Figure 1). Additionally, the LHWE extract retained the highest activity against Shigella spp. compared to the other two (CWED and EED) across all concentrations tested (Figures 1, 2 and 3).
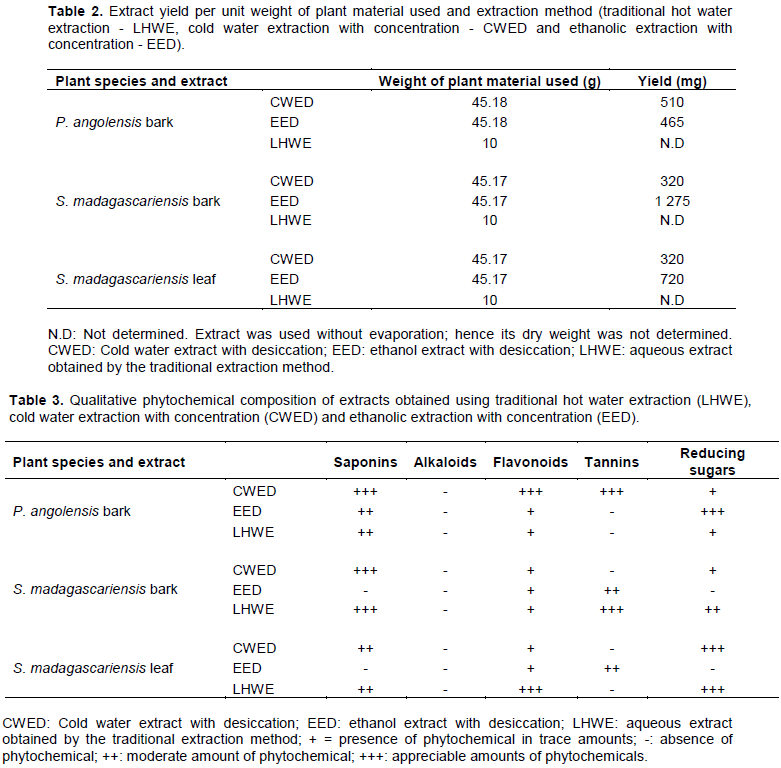
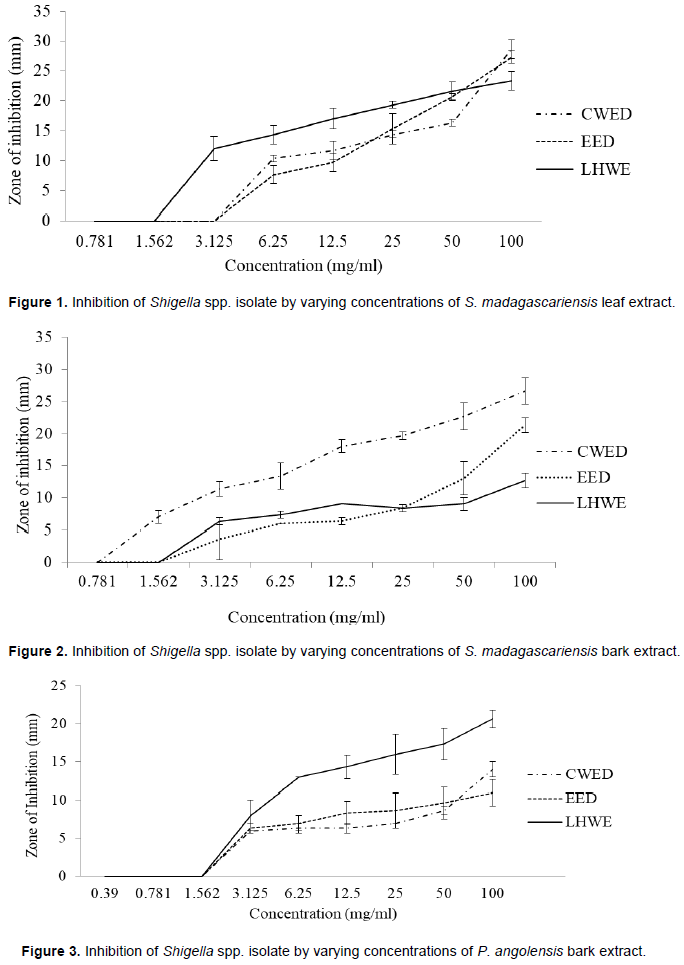
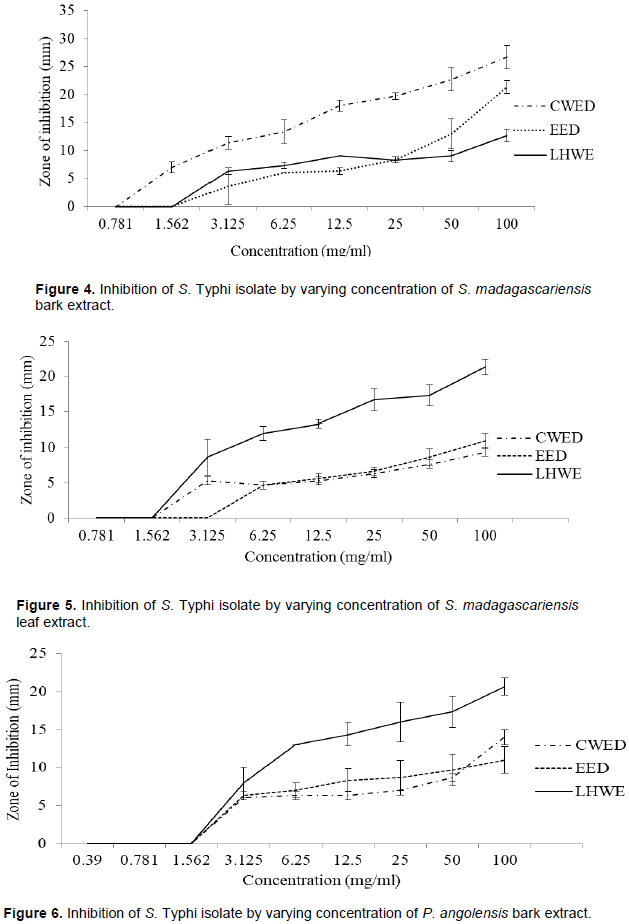
The LHWE extraction method was shown to yield extracts with significantly greater ZOI against Shigella spp. than those from the other methods with the following: (i) P. angolensis bark extract (LHWE > CWED - p = 0.001) and (ii) P. angolensis bark extract (LHWE > EED – p = 0.0003) (Table A1). Additionally, LHWE extracts were shown to have significantly greater ZOI at 100 mg/ml than other against the S. Typhi as follows: (i) S. madagascariensis bark extract (LHWE > CWED – p < 0.0001); (ii) S. madagascariensis bark extract (EED > LHWE – p = 0.002); (iii) S. madagascariensis leaf extract (LHWE > CWED – p = 0.0001); (iv) S. madagascariensis leaf extract (LHWE > EED – p = 0.0003); (v) P. angolensis bark extract (LHWE > CWED – p = 0.002) and (vi) P. angolensis bark extract (LHWE > EED – p = 0.0004) (Table A1, Figure 4 and 5).
The cold water extraction method with desiccation (CWED) yielded the highest antimicrobial activities in terms of the attainment of the lowest minimum inhibition concentration (MIC) / highest zone of inhibition (ZOI) against S. Typhi in comparison with the other two extraction methods (LHWE and EED) as follows: (i) S. madagascariensis bark extract (MIC = 0.78mg/ml) (Figure 1d) and S. madagascariensis leaf extract (MIC = 1.56mg/ml) (Figure 1e). Additionally, the CWED extract retained the highest activity against S. typhi compared to the other two (LHWE and EED) across all concentrations of S. madagascariensis bark extract (Figure 1d). Additionally, the CWED extract from P. angolensis bark was shown to have significantly greater ZOI at 100mg/ml than that of EED extract against the S. typhi (CWED > EED – p = 0.02) (Figure 6).
The hot water extraction method (LHWE) yielded extracts that had no antimicrobial activity against E. coli. The cold water extraction method with desiccation (CWED) of S. madagascariensis leaf extract (ZOI = 21 mm) yielded the higher antimicrobial activities in terms of the attainment of the highest zone of inhibition (ZOI) against E. coli than that of extracts from the other two extraction methods (LHWE and EED) (Figure 8). Additionally, CWED extracts attained greater antimicrobial activity against the strain of E. coli (at 100 mg/ml) than streptomycin (300 µg/ml) (ZOI = 23 mm) as follows:
(i) S. madagascariensis bark extract (ZOI = 26 mm) (Figure 7), (ii) S. madagascariensis leaf extract (ZOI = 34 mm) (Figure 8) and (iii) P. angolensis bark extract (ZOI = 30 mm) (Figure 9). Additionally, the CWED extract retained the highest activity against S. typhi compared to the other two (LHWE and EED) across all concentrations of S. madagascariensis leaf (Figure 8).
The following extracts were shown to have significantly greater ZOI at 100 mg/ml than the other against the E. coli: (i) S. madagascariensis bark EED extract > CWED extract: p = 0.02 and S. madagascariensis leaf CWED extract > EED extract: p < 0.0001 (Table A1).
The ethanolic extraction with desiccation method
yielded extracts with greater antimicrobial activities against E. coli O157 as follows: (i) S. madagascariensis bark extract (MIC = 0.39 mg/ml / ZOI = 29 mm) (Figure 7) and (ii) P. angolensis bark extract (ZOI = 31 mm) (Figure 9). The following extracts achieved greater ZOI than streptomycin against E. coli (300 µg/ml) (ZOI = 23 mm): (i) CWED and EED of S. madagascariensis bark extracts (26 and 29 mm) (Figure 7), (ii) CWED of S. madagascariensis leaf extract (34 mm) (Figure 8) and (iii) CWED and EED of P. angolensis bark extracts (30 and 31 mm) (Figure 9).
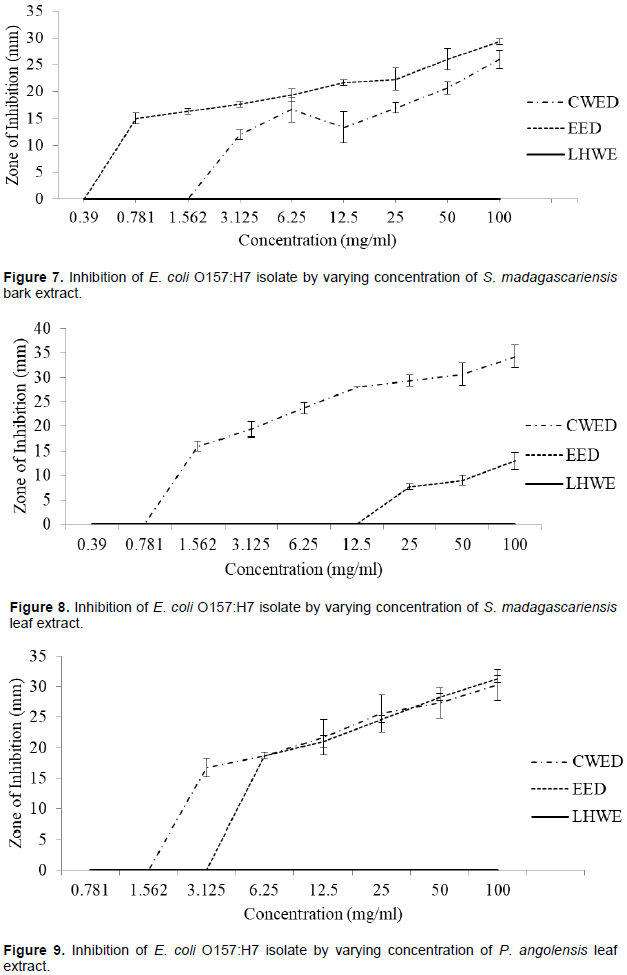
Overall, the aqueous extraction methods (CWED and LHWE) yielded extracts with greater antimicrobial activities than the other two methods in terms of the attainment of the lowest MIC values and higher ZOI per concentration used depending on plant species or plant part used. Generally, the LHWE method was largely better than the other two methods in (lowest MIC values and higher ZOI against Shigella spp. and presumptive S. Typhi per concentration used) with different plants. However, the LHWE technique yielded extracts with no antimicrobial activities against E. coli. The CWED and EED methods yielded greater antimicrobial activities against the bacteria as follows: (i) CWED with S. madagascariensis leaf extract (Figure 8) and (ii) EED with P. angolensis bark (Figure 9).
Table 4 shows ZOI of each extract (at the highest concentrations) against Shigella spp. that were greater than that for streptomycin (300 µg, Mast Diagnostics, UK) (20 mm). The extract from the traditional hot water extraction method (LHWE) had greater inhibitory activities than that for streptomycin (300 µg) against the following microorganisms with the following extracts (Shigella spp.: S. madagascariensis bark and leaf extracts and P. angolensis bark extract) (Table 4). The cold water extract of S. madagascariensis bark showed greater ZOI compared to that for streptomycin against E. coli and S. Typhi. The cold water extract of S. madagascariensis leaf showed greater ZOI compared to that of streptomycin against E. coli. Whereas the ethanolic extraction method yielded an extract of S. madagascariensis bark and P. angolensis bark with greater ZOI than streptomycin.
The traditional hot water extraction method (LHWE), cold water extraction with desiccation (CWED) and the ethanol with desiccation extraction (EED) methods yielded equal or similar MIC values, as seen with P. angolensis bark extract (0.78 mg/ml) (Figure 1). The CWED method yielded equal but higher MIC values than LHWE and EED method when used to obtain S. madagascariensis bark extract (CWED - 0.2 mg/ml vs. LHWE and EED - 0.78 mg/ml) (Table 5). The LHWE method yielded greater antimicrobial activities (ZOI) at concentrations higher that the respective MIC against presumptive S. typhi for P. angolensis bark extract and S. madagascariensis bark extract (Table 5). Overall, the LHWE method was largely better than the other two methods in (lowest MIC values and higher ZOI per concentration used) for P. angolensis bark extract and S. madagascariensis bark extract, whereas EED proved inferior (Figure 1).
The traditional hot water extraction method (LHWE) yielded extracts had no antimicrobial effects against the strain of E. coli O157 used (results not shown). The CWED method yielded higher MIC values than EED method and LHWE when used to obtain the following: P. angolensis (MIC = 0.78 mg/ml, greater activity between 0.78 and 1.56 mg/ml and S. madagascariensis (MIC = 0.196 mg/ml, greater activity between 0.196 and 6.25 mg/ml (Figure 1). The EED method yielded higher MIC values than CWED method and LHWE when used to obtain S. madagascariensis bark extract (MIC: EED = 0.098 mg/ml, CWED = 0.781 mg/ml).
Minimum inhibition concentration (MIC) of extracts obtained using traditional hot water extraction (LHWE), cold water extraction with concentration (CWED) and ethanolic extraction with concentration (EED) were obtained following the well diffusion protocol described earlier. Table 5 shows MIC values for each extract against each of the microorganisms tested.
The ethanolic extraction (with concentration) method (EED) yielded extracts that had dually greater antimicrobial effect (MIC) against the strains and extracts (than the other two methods): S. Typhi and E. coli with P. angolensis bark extract. The cold water aqueous extraction (with concentration) method (CWED) yielded extracts that had dually greater antimicrobial effect (MIC) against the strains and extracts (than the other two methods): S. Typhi and E. coli with S. madagascariensis bark.
In this study, extracts obtained from the extraction LHWE, CWED and EED were analysed for antimicrobial activity against presumptive E. coli O157, Shigella spp. and S. Typhi and were characterised for phytochemical composition. The extraction methods gave yields (dry mass of desiccated extracts) that lied between 711 and 2833 mg (0.07 and 0.25% respectively) per 100 g of plant material used. This means for one to obtain 1 kg of desiccated product, between 35 and 140 kg of dried plant material. Should the plants not be domesticated, harvest for widespread use in the management of diseases would not be sustainable. We recommend the domestication or replanting of such medicinal plants.
Qualitative phytochemical analysis revealed the presence of saponins, flavonoids, tannins and reducing sugars in plant extracts obtained from different extraction methods.
The traditional hot water extraction method (LHWE) yielded greater antimicrobial activities (significantly greater ZOI than the other extracts against S. Typhi: (i) S. madagascariensis bark extract (LHWE > CWED – p < 0.0001), (ii) S. madagascariensis bark extract (EED > LHWE – p = 0.002), (iii) S. madagascariensis leaf extract (LHWE > CWED – p = 0.0001), S. madagascariensis leaf extract (LHWE > EED – p = 0.0003), (v) P. angolensis bark extract (LHWE > CWED – p = 0.002) and (vi) P. angolensis bark extract (LHWE > EED – p = 0.0004). Similar dominance of LHWE was shown against Shigella spp. as follows: (i) P. angolensis bark extract (LHWE > CWED - p = 0.001) and (ii) P. angolensis bark extract (LHWE > EED – p = 0.0003). Swartzia madagascariensis has a history of being used in concoctions (mixed with Isoberlinia doka, Annona senegalensis, Gardenia ternifolia, Terminalia glaucescens and Erythrina senegalensis) that have shown significant antibacterial activities against Bacillus cereus, Mycobacterium fortuitum, Staphylococcus aureus, or Candida albicans (Magassouba et al., 2007). No evidence of use of S. madagascariensis or P. angolensis as sole antimicrobials in the traditional management of diseases was found.
The barks of P. angolensis and S. madagascariensis were shown to contain a number of phenolic compounds (pterocarpins) (Harper et al., 1969) which could account for the high antimicrobial activities of LHWE against S. Typhi and Shigella spp. The observed antimicrobial activities in the selected plants may be attributed to high composition of flavonoids and tannins in S. madagascariensis leaf extract or pterocarpins in P. angolensis (Harper et al., 1969). Flavonoids have been shown to harbour antimicrobial activities against Salmonella spp. (Dzoyem et al., 2017), for example quercetin (Wang et al., 2017), rutin (Arima et al., 2002) and others. Generally, no other studies reporting chemical composition of P. angolensis were found. Interestingly, all LHWE extracts did not yield antimicrobial effects against E. coli.
Ahmed et al. (2014), in a study on the effect of hot versus cold water extraction of Hibiscus sabdariffa calyxes revealed greater accumulation of total phenolics, total flavonoids and tannins with short time high temperature extraction process, as well as high antioxidant activity (DPPH assay) than with the cold water extraction method. Yung et al. (2010) demonstrated an increase in phenolics content and antioxidant activities of Pegaga (Centella asiatica) extracts with boiling temperature (90°C). The observed high accumulation of the phenolic substances (flavonoids and tannins) as well as saponins may be due to the increased dissolution of these substances with the hot water extraction method in the present study. Saponins are glycosidic secondary metabolites that exert a wide range of pharmacological properties (Podolak et al., 2010).
The LHWE could have attained greater antimicrobial activities due to the short processing time (30 min) that could have prevented antioxidative deterioration of phytochemicals within. Whereas extraction with the EED and CWED methods was done over a period of 72h, plus a desiccation step that took at least 48 h. The length of exposure to agents of the atmosphere and the time taken could have had deleterious effects on the chemicals.
The aqueous extraction method (with desiccation) (CWED) generally yielded extracts with higher antimicrobial activities against E. coli than against S. Typhi and Shigella spp. where the zones of inhibition were as follows (respectively): P. angolensis bark extract (31 mm for E. coli). CWED extracts were also shown to have greater concentrations of the following: flavonoids (P. angolensis bark extract), tannins (P. angolensis bark extract) and saponins (P. angolensis bark extract). The cold water extract of S. madagascariensis leaves (CWED) was shown to have significantly greater antimicrobial activity against E. coli than the ethanolic counterpart (EED) (p < 0.0001). The cold water extraction method (with evaporation) (CWED) yielded extracts of S. madagascariensis were shown to be rich in the following phytochemicals: flavonoids, saponins and tannins. These components are thought to account for the high antimicrobial activities of the S. madagascariensis extracts.
The aqueous extraction methods (CWED and LHWE) were shown to yield extracts with greater antimicrobial activities than the ethanolic extraction method (EED) (significantly lower MIC values or significantly higher ZOI against Shigella spp. and S. Typhi per concentration used) with the three selected plants. However, the LHWE technique yielded extracts with no antimicrobial activities against E. coli. The high antimicrobial activities of CWED and LHWE could be because of the presence of bioactive compounds that exert antimicrobial properties such as flavonoids, saponins, alkaloids and tannins. The hot water extraction method was shown to be an extraction method of choice as it resulted in significantly greater antimicrobial activities against the three diarrhoeagenic microorganisms with the three plant species. The novelty of the hot water extracted preparations is thought to lie with the freshness of such extracts (used within hours from extraction) – meaning reduced oxidative degradation of their phytochemistry. The current study therefore validates the widespread use of aqueous extraction methods in traditional medicinal practices.
The authors thank Mrs. A. Chingwaru for her sterling work in sourcing and identifying the plants used.
The authors have not declared any conflict of interests.
REFERENCES
|
Ahmed ZS, Abozed SS, Abd El-Kader AE (2014). Extraction Optimization and Quality Characterization of Traditionally Prepared Hibiscus sabdariffa Beverage Using Response Surface Methodology. World Journal of Dairy and Food Sciences 9:154-165.
|
|
|
|
Arima H, Ashida H, Danno GI (2002). Rutin-enhanced antibacterial activities of flavonoids against Bacillus cereus and Salmonella enteritidis. Bioscience, Biotechnology, and Biochemistry 66(5):1009-1014.
Crossref
|
|
|
|
|
Avinash DK, Waman SN (2014). Phytochemical Constituents of Leaves of Celastrus paniculatus Wild: Endangered Medicinal Plant. International Journal of Pharmacognosy and Phytochemical Research 6:792-794.
|
|
|
|
|
De Wet H, Ngubane SC (2014). Traditional herbal remedies used by women in a rural community in northern Maputaland (South Africa) for the treatment of gynaecology and obstetric complaints. S. South African Journal of Botany 94:29-139.
Crossref
|
|
|
|
|
Dzoyem JP, Melong R, Tsamo AT, Tchinda AT, Kapche DG, Ngadjui BT, McGaw LJ, Eloff JN (2017). Cytotoxicity, antimicrobial and antioxidant activity of eight compounds isolated from Entada abyssinica (Fabaceae). BMC Research Notes 10(1):118.
Crossref
|
|
|
|
|
Harper SH, Kemp AD, Underwood WGE, Campbell RVM (1969). Pterocarpanoid constituents of the heartwoods of Pericopsis angolensis and Swartzia madagascariensis. Journal of the Chemical Society 8:1109-1116.
Crossref
|
|
|
|
|
Magassouba FB, Diallo A, Kouyate M, Mara F, Mara O, Bangoura O, Camara A, Traore S, Diallo AK, Zaoro M, Lamah K (2007). Ethnobotanical survey and antibacterial activity of some plants used in Guinean traditional medicine. Journal of Ethnopharmacology 114(1):44-53.
Crossref
|
|
|
|
|
Maroyi A (2016). Ximenia caffra Sond. (Ximeniaceae) in sub-Saharan Africa: A synthesis and review of its medicinal potential. Journal of Ethnopharmacology 184:81-100.
Crossref
|
|
|
|
|
Muhamad II, Hassan ND, Mamat SN, Nawi NM, Rashid WA, Tan NA (2017). Extraction technologies and solvents of phytocompounds from plant materials: Physicochemical characterization and identification of ingredients and bioactive compounds from plant extract using various instrumentations. In Ingredients Extraction by Physicochemical Methods in Food pp. 523-560.
Crossref
|
|
|
|
|
Ngarivhume T, van Klooster CI, de Jong JT, Van der Westhuizen JH (2015). Medicinal plants used by traditional healers for the treatment of malaria in the Chipinge district in Zimbabwe. Journal of Ethnopharmacology 159:224-237
Crossref
|
|
|
|
|
Njume C, Jide AA, Ndip RN (2011). Aqueous and organic solvent-extracts of selected South African medicinal plants possess antimicrobial activity against drug-resistant strains of Helicobacter pylori: inhibitory and bactericidal potential. International Journal of Molecular Sciences 12(9):5652-5665.
Crossref
|
|
|
|
|
Odunmbaku LA, Sobowale SS, Adenekan MK, Oloyede T, Adebiyi JA, Adebo OA (2018). Influence of steeping duration, drying temperature, and duration on the chemical composition of sorghum starch. Food Science and Nutrition 6(2):348-355.
Crossref
|
|
|
|
|
Palombo EA (2011).Traditional medicinal plant extracts and natural products with activity against oral bacteria: potential application in the prevention and treatment of oral diseases. ECAM.
Crossref
|
|
|
|
|
Patwardhan B, Warude D, Pushpangadan P, Bhatt N (2005). Ayurveda and traditional Chinese medicine: a comparative overview. ECAM. 2(4):465-473.
Crossref
|
|
|
|
|
Pilon AC, Carnevale Neto F, Freire RT, Cardoso P, Carneiro RL, Da Silva Bolzani V, Castro-Gamboa I (2016). Partial least squares model and design of experiments toward the analysis of the metabolome of Jatropha gossypifolia leaves: extraction and chromatographic fingerprint optimization. Journal of separation science 39(6):1023-1030.
Crossref
|
|
|
|
|
Podolak I, Galanty A, Sobolewska D (2010). Saponins as cytotoxic agents: a review. Phytochemistry Reviews 9(3):425-74.
Crossref
|
|
|
|
|
Semenya SS, Maroyi A (2013). Medicinal plants used for the treatment of tuberculosis by Bapedi traditional healers in three districts of the Limpopo Province, South Africa. African Journal of Traditional, Complementary and Alternative Medicines 10(2):316-323.
Crossref
|
|
|
|
|
Smith E (2007). Plant secondary metabolites: occurrence, structure and role in the human diet. Phytotherapy Research 21(9):904-904.
Crossref
|
|
|
|
|
Soman S, Ray JG (2016). Silver nanoparticles synthesized using aqueous leaf extract of Ziziphus oenoplia (L.) Mill: characterization and assessment of antibacterial activity. Journal of Photochemistry and Photobiology 163:391-402.
Crossref
|
|
|
|
|
Wachtel-Galor S (2004). Herbal medicine. In Herbal and Traditional Medicine. CRC Press pp. 78-89.
|
|
|
|
|
Wang S, Yao J, Zhou B, Yang J, Chaudry MT, Wang M, Xiao F, Li Y, Yin W (2017). Bacteriostatic effect of Quercetin as an antibiotic alternative in vivo and its antibacterial mechanism in vitro. Journal of Food Protection 81(1):68-78.
Crossref
|
|
|
|
|
Yung OH, Maskat MY, Mustapha WAW (2010). Effect of Extraction on Polyphenol Content, Antioxidant Activity and pH in Pegaga (Centella asiatica) Extract. Sains Malaysiana 39(5):747-752.
|
|
|
|
|
Zhang QW, Lin LG, Ye WC (2018). Techniques for extraction and isolation of natural products: A comprehensive review. Chinese Medicine 13(1):20.
Crossref
|
|
|
|
|
Zohra SF, Meriem B, Samira S, Muneer MA (2012). Phytochemical screening and identification of some compounds from mallow. Journal of Natural Product and Plant Resources 2(4):512-516.
|
|