ABSTRACT
Otochilus albus Lindl is one of the important orchids especially used for the horticultural purpose and commercial use. It is listed as a rare and critically endangered species in Nepal. An attempt to preserve the important orchids was done by establishing an efficient in vitro regeneration protocol using seed. This study was carried out by using Murashige and Skoog (MS) medium and the MS medium supplemented with growth regulators namely 6-benzylaminopurine (BAP) and α-naphthalene acetic acid (NAA) in different concentration. The seed germination and protocorm formation started after 8 weeks of culture, and complete seedling was developed within 26 weeks of culture in the basal medium and medium supplemented with 0.5 mg/L BAP and 0.5 mg/L BAP + 0.5 mg/L NAA, respectively. Regardless of the NAA supplement, the protocorm formation and seedling development were delayed in the medium containing higher concentration of BAP.
Key words: Otochilus albus Lindl, in vitro, Murashige and Skoog (MS), 6-benzylaminopurine (BAP), α-naphthalene acetic acid (NAA).
Orchids are one of the unique plant groups and are important aesthetically, medicinally, and also regarded as an ecological indicator (Joshi et al., 2009). Orchid constitutes an order of royalty in the world of ornamental plants. Despite the ornamental application of orchids, they are equally used as herbal medicines. Hence, orchids have ornamental, medicinal as well as edible values. Typical medicinal values are identified as anti rheumatic, anti-inflammatory, anti-carcinogenic, antiviral, diuretic, anti-tumour, anti-aging, anti-microbial, wound healing, and many others. Orchids are of immense horticultural importance and play a significant role to balance the forest ecosystem (Kaushik, 1983).
Overexploitation and habitat loss extremes might cause them to be extinct in the near future (Pant et al., 2018). Orchids are highly sensitive to a slight disturbance to their natural habitat. With the rapid urbanization and pressure on utilizing forest resources, orchids and their habitats are being constantly under threat in the recent years.
On the other hand, the germination rate of orchid seeds in nature is only 2 to 5% (Rao, 1977). Orchid seed lacks functional endosperm, so the germination of seed requires a suitable fungus. The seedling takes 12 years to grow to maturity (Basker and Narmatha Bai, 2006). These difficulties in natural germination may cause these species to get extinct. Therefore, scientists need to preserve these valuable orchid species at least by utilizing an efficient artificial means to facilitate the seed germination. Micropropagation of orchids through seeds can produce a large number of orchid plantlets in a reasonable time. Thus, the present study was undertaken to develop an efficient protocol for in vitro propagation of Otochilus albus Lindl. through immature seed to seedling formation. A well-known MS medium and the medium supplemented with the varied concentration of BAP and NAA were used to study their effects on in vitro propagation of O. albus Lindl species.
The material used in the present investigation was the immature capsule of O. albus Lindl obtained from the natural habitat of the plant species, Dolakha District, Lamabagar VDC, Nepal. Nepal is situated in the lap of Himalaya, harbors 451 species of orchids from 107 genera (Rajbhandari, 2015). Nepal is rich in an incredible variety of orchid flora due to altitudinal variation, diverse habitats and varied climate conditions.
The capsule was sterilized by washing under running tap water after dipping in the detergent for 15-20 min for 1 h until the water became totally clear and transparent. The capsule was then rinsed in 70% ethyl alcohol for 2 min and dipped in 1% sodium hypochlorite solution for 10 min. Finally, the capsule was rinsed with sterile water for 5 min.
Murashige and Skoog (MS) medium (Murashige and Skoog, 1962) was used alone and in different concentrations of 6-benzylaminopurine (BAP) and α-naphthalene acetic acid (NAA) for the inoculation of seeds. The medium was adjusted to pH 5.8 before autoclaving and solidified with 0.8% w/v Difco Bacto Agar. About 20 ml medium per culture tube was poured into culture tubes, and each tube was tightly covered with aluminum foil. The culture tubes containing medium were autoclaved at 121°C and pressure of 15 lb/sq. inch for 20 min. After cooling down, the culture tubes were taken out and kept in culture room in slanting position. The sterilized capsule was transferred to pre-sterilized laminar airflow cabinet and dissected longitudinally into two halves using a sterile surgical blade. The seeds were then inoculated on the surface of MS medium alone and in different concentrations of BAP and BAP + NAA using sterile forceps. The cultures were incubated at 25±2°C under the photoperiod of 12-15 h and observed regularly.
The immature capsules of O. albus Lindl was selected for the research as they show better germination response in a short period (Pant, 2006). The nature and quantity of growth hormones have significant effects on the germination of orchid seeds (Arditti, 1979). Immature seeds of orchid species from northern temperate regions are more difficult to germinate than mature seeds (Arditti et al., 1982). In this study, the MS basal medium is nevertheless found to be effective for the in vitro seed germination. Similar results were obtained by Karki et al., (2005) in Vanilla planifolia. The Orchid seeds were successfully germinated in MS basal medium. Likewise, Pant and Gurung (2005) germinated seeds of Arides odorata Lour in the MS basal medium. On the other hand, the most effective germination response of O. albus Lindl. with the development of roots and shoots was obtained on MS medium supplemented with BAP (0.5 mg/L) + NAA (0.5 mg/L).
In the experiment, there was the selection of the most suitable medium on the basis of time taken for germination of seeds and their growth and development. Initiation of germination was observed after 8 weeks of culture in MS basal medium and 13 to 18 weeks of culture in MS basal medium supplemented in different combination BAP and NAA hormonal concentration (Table 1). Among the hormonal supplemented MS media, BAP (0.5 mg/L) supplement has demonstrated similar performance to that of MS basal medium for spherulation, protocorm formation, as well as shoot formation. However, it is interesting to note that the first root formation was significantly earlier in 0.5 mg/L BAP supplement than that of the MS basal medium without any supplement. This was supported by finding of Reddy et al. (1992), who studied the seed germination, and seedling growth of different orchids (Cymbidium aloifolium, Dendrobium crepidatum, Epidendrum radicans, Spathoglottis plicata) and found seed germination in 5 weeks.
Protocorms were obtained after 11 weeks of culture in MS basal medium and medium supplemented with 0.5 mg/L BAP respectively (Table 1). Basker and Narmatha Bai (2010) obtained similar findings in seed germination of Eria bambusifolia in which it took 7 weeks for protocorm formation in the MS basal media and on Phaius tancarvillea it took around 9 weeks for the protocorm formation (Pant et al., 2011). According to the recent studies, it is reasonable to expect that seed germination could be affected by phylogeny, life history attributes such as seed size, seed dispersal, life forms, etc (Xu et al., 2014).
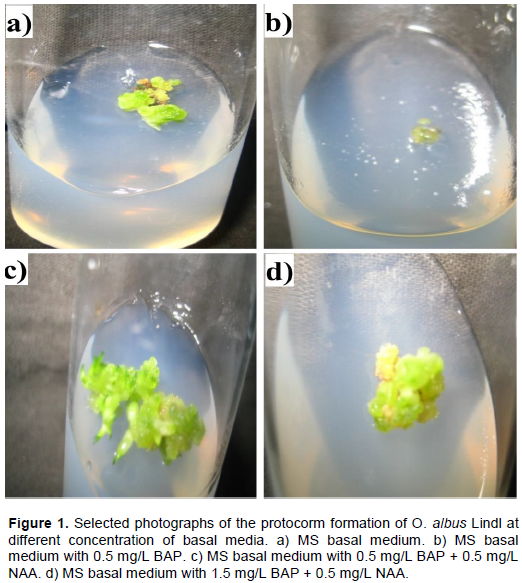
Auxins and cytokinins are most frequently used in nutrient media to increase the percentage of germination or to stimulate protocorm and seedling development (Gegi et al., 2018; Diengdoh et al., 2017). According to De Pauw et al. (1995) their importance in germination is limited but increased during the development of protocorms. As reported by Mitra (1986), cytokinins or their combination with auxin stimulates seed germination. The combination of auxins and cytokinins play an important role in seed germination. The signaling pathways of the hormone can stimulate seed germination through the release of coat dormancy, weakening of endosperm and expansion of embryo cell. MS basal medium does not contain hormonal effect whereas MS modified medium stimulates the germination through the release of coat dormancy. In other media such as 0.5 mg/L BAP + 0.5 mg/L NAA, and 1.5 mg/L BAP + 0.5 mg/L NAA, it took only 17 weeks for protocorm formation. The protocorms observed after inoculation of seeds in MS medium (8 weeks), and the medium supplemented with 0.5 mg/L BAP (8 weeks), 0.5 mg/L BAP + 0.5 mg/L NAA (18 weeks), and 1.5 mg/L BAP + 0.5 mg/L NAA (18 weeks) are shown in Figure 1. The protocorm obtained from the MS basal medium is more distinct and bigger as compared to the protocorm obtained from BAP supplement in early appearance. On the other hand, addition of 0.5 mg/L NAA along with the 0.5 mg/L BAP enhanced to formation of protocorm, which is larger, distinct greenish color, and an apparent branched structure. Further increase of the concentration of NAA to 1.5 mg/L, the protocorm appeared as oval shape with yellowish green in color. For other combinations of BAP and NAA supplements, the protocorm formation was delayed for 3 to 4 weeks.
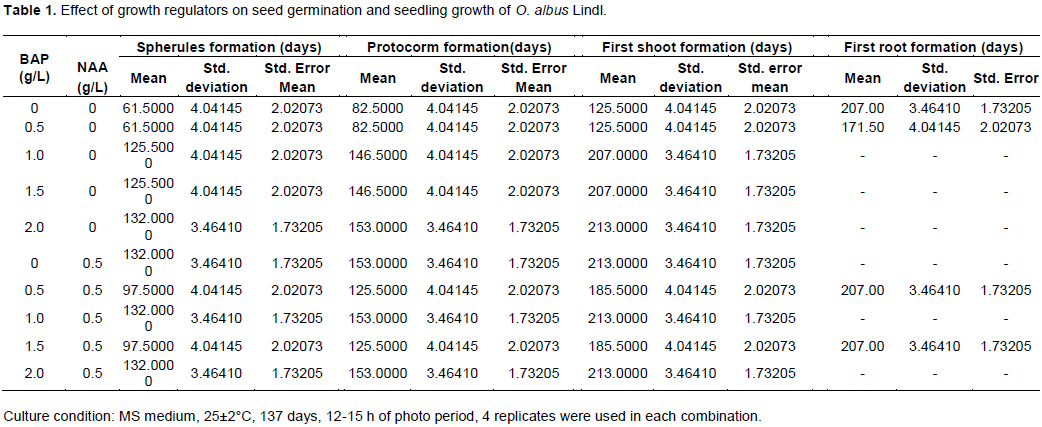
The protocorm obtained was differentiated into the seedling after 26 weeks of seed culture for the seed sample from the MS basal medium containing 0.5 mg/L BAP + 0.5 mg/L NAA. Explants of O. albus Lindl on the MS medium (30 weeks) and the medium supplemented with 0.5 mg/L BAP (26 weeks), 0.5 mg/L BAP + 0.5 mg/L NAA (30 weeks), and 1.5 mg/L BAP + 0.5 mg/L NAA (30 weeks) are shown in Figure 2. Other combinations of BAP and NAA in the MS media took nearly 30 weeks for the appearance of first shoot. From the Figure 2, it is clearly observed that the explant from MS basal medium containing 0.5 mg/L BAP + 0.5 mg/L NAA hormonal supplement has shown a healthy plantlet over other combinations of hormones. The dimension of the shoot is nearly 2 cm long. Moreover, the MS basal medium with 0.5 mg/L BAP supplement has revealed the early shoot/root appearance among all the variants. The root initiation was observed in the MS media with hormonal concentration 0.5 mg/L NAA + 0.5 mg/L BAP within 29 weeks of seed culture. The roots were observed as fine hairy structures. Hence, it is evident from (Figure 2d) that the higher concentration ratio of BAP to NAA is not as effective as the MS basal medium without supplement (Figure 2a). Accordingly, only BAP supplement is not adequate for the proper growth of the O. albus Lindl plantlet (Figure 2b) via in vitro seed culture. BAP acts as in vitro shoot proliferation hormone and NAA act as root proliferation hormone. The synergetic effect of BAP+NAA together induce root and shoot proliferation and seedling formation. Similar findings were also reported in previous studies on seed germination of Aerides odorata (Pant and Gurung, 2005), seed germination of Cymbidium spp., Dendrobium nobile and Dendrobium primulinum (Luan et al., 2006). However, this result is different from the result of Phaius trankervilliae (Luan et al., 2006).

The complete plantlet of O. albus Lindl was obtained within 29 weeks of culture. This was supported by the findings of Pant et al. (2011) on Phaius tancarvilleae which took 24 weeks to develop into complete plantlets and Paudel et al. (2012) on Esmeralda clarki which took 25 weeks. It is to be noted that the nutritional requirement to germinate orchid seeds vary with their physiological state and this may be species-specific (Yam et al., 1989). Hence, in the present investigation different strength of MS media were found to be effective for the germination of immature O. albus Lindl seeds.
This study is focused on the in vitro mass propagation of endangered orchid species O. albus Lindl for the conservation by tissue culture method. In O. albus Lindl, a combination of 0.5 mg/L BAP + 0.5 mg/L NAA hormonal supplements in the MS basal medium was found to be an effective concentration of BAP and NAA hormones for both shoot and root proliferation. Similarly, 0.5 mg/L BAP in the MS basal medium was also observed to be an effective concentration for early shoot/root initiation. However, an appropriate concentration of both the hormones, BAP and NAA, are recommended for in vitro seed germination and seedlings growth of O. albus Lindl using the modified MS basal media.
The authors have not declared any conflict of interests.
The authors gratefully appreciate the Central Department of Botany, Tribhuvan University, Kirtipur, Kathmandu, Nepal for providing the laboratory facilities and Mr. Mukti Paudel for providing the orchid capsule.
The authors gratefully appreciate the Central Department of Botany, Tribhuvan University, Kirtipur, Kathmandu, Nepal for providing the laboratory facilities and Mr. Mukti Paudel for providing the orchid capsule.
REFERENCES
|
Arditti J (1979). Aspects of the physiology of orchids. Advances in Botanical Research 7:421-655.
Crossref
|
|
|
|
Arditti J, Clements MA, Fast G, Hadley G, Nishimura G, Ernst R (1982). Orchid seed germination and seedling culture. Orchid Biology: Reviews and perspectives, vol II, Cornell University Press 243-370.
|
|
|
|
Basker S, Narmatha Bai V (2006). Micropropagation of Coelogyne stricta (D. Don) Schltr. via pseudobulb segment cultures. Tropical and Subtropical Agroecosystems 6:31-35.
|
|
|
|
Basker S, Narmatha Bai V (2010). In vitro propagation of an epiphytic and rare orchid Eria bambusifolia Lindl. Reasearch in Biotechnology 1:15-20.
|
|
|
|
De Pauw MA, Remphrey WR, Palmer CE (1995). The cytokinin preference for in vitro germination and protocorm growth of Cypripedium candidum. Annals of Botany 75:267-275.
Crossref
|
|
|
|
Diengdoh RV, Kumaria S, Tandon P, Das MC (2017). Asymbiotic germination and seed storage of Paphiopedilum insigne, an endangered lady's slipper orchid. South African Journal of Botany 112:215-224.
Crossref
|
|
|
|
Gegi GV, Williams BC, Suja RM (2018). Micropropagation of an endangered terrestrial orchid Geodorum Densiflorum (LAM.) Schltr. of Kanyakumari district, India. World Journal of Pharmaceutical Research 7(7):816-823.
|
|
|
|
Joshi GC, Tewari LM, Lohani N, Upreti K, Jalal JS, Tewari G (2009). Diversity of orchids in Uttarakhand and their conservation strategy with special reference to their medicinal importance. Report and Opinion 1(3):47-52.
|
|
|
|
Karki A, Rajbahak S, Saiju HK (2005). Micropropagation of Vanilla planifolia from seeds. Nepal Journal of Plant Sciences 1:42-44.
|
|
|
|
Kaushik P (1983). Ecological and Anatomical Marvels of the Himalayan Orchid. Today and Tomorrow's Printers and Publishers, New Delhi, India.
|
|
|
|
Luan VQ, Thien NQ, Khiem DV, Nhut DT (2006). In vitro germination capacity and plant recovery of some native and rare orchids. Preceding of International Workshop on Biotechnology in Agriculture, Nong Lam University, Ho Chi Minh City, October 20-21:175-177.
|
|
|
|
Mitra GC (1986). In vitro culture of orchid seeds for obtaining seedlings.
|
|
|
|
In: Biology, conservation and culture of orchids, Vij SP (Ed.), East-West Press Pvt. Ltd., New Delhi, pp. 401-409.
|
|
|
|
Murashige T, Skoog F (1962). A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiologia Plantarum,- 15(3):473-497.
Crossref
|
|
|
|
Pant B (2006). Biotechnology in orchid conservation. Preceding of the National Seminar on Natural Resource Management, Nepal Biological Society (NBS), pp. 221-225.
|
|
|
|
Pant B, Gurung R (2005). In vitro seed germination and seedling development in Aerides odorata Lour. The Journal of the orchid Society of India 19(1-2):51-55.
|
|
|
|
Pant B, Paudel M, Chand M, Pradhan S, Malla B, Raskoti B (2018). Orchid diversity in two community forests of Makawanpur District, central Nepal. Journal of Threatened Taxa 10(11):12523-12530.
|
|
|
|
Pant B, Shrestha S, Pradhan S (2011). In vitro seed germination and seedling development of Phaius tancarvilleae Blume. Scientific World 9(9):50-52.
Crossref
|
|
|
|
Paudel M, Pradhan S, Pant B (2012). In vitro seed germination and seedling development of Esmeralda clarkei Rchb. f. (Orchidaceae). Plant Tissue Culture and Biotechnology 22(2):107-111.
Crossref
|
|
|
|
Rajbhandari KR (2015). A Handbook of Orchids of Nepal. Department of Plant Resources, Nepal, pp. 1-168.
|
|
|
|
Rao AN (1977). Tissue culture in orchid industry. In: Applied and Fundamental Aspects of Plant, Cell, Tissue, and Organ Culture. Springer Verlag. Berlin, pp. 44-69.
|
|
|
|
Reddy PV, Nanjan K, Shanmugavelu KG (1992). In vitro studies in tropical orchids: seed germination and seedling growth. The Journal of the Orchid Society of India 6(1-2):75-78.
|
|
|
|
Xu J, Li W, Zhang C, Liu W, Du G (2014). Variation in seed germination of 134 common species on the Eastern Tibetan Plateau: Phylogenetic, Life History and Environmental Correlates. PLOS ONE 9 (6):
Crossref
|
|
|
|
Yam TW, Arditti J, Weatherhead MA (1989). The use of darkening agents in seed germination and tissue culture media for orchids: a review. The Journal of the orchid Society of India 3:35-39.
|