Full Length Research Paper
ABSTRACT
Ten pepper cultivars (Capsicum annuum L), that is, Doux D’Alger, Sonar, Esterel, Doux, Marconi, Magister, Belconi, Italico II, Lipari, Arabal, and Doux d’Espagne commercially grown in Algeria were inoculated with six isolates of Phytophthora capsici Leon, and susceptibility of pepper was assessed by evaluation of disease severity in different plant organs. The cultivar Italico II was found to be more resistant to P. capsici isolates compared to other cultivars, for example Esterel which was the very susceptible one. In addition, the pepper cultivars differed significantly (P < 0.01) in their susceptibility to different P. capsici isolates, on the rate of stem necrosis. However, isolated virulent fungal isolates displays similar pattern when they were inoculated to the studied cultivars (P > 0.05). However, velocity of disease development varied between resistant pepper cultivars, that is, Italico II and susceptible ones, for example Esterel. In conclusion, data suggest that pepper cultivars differ in their susceptibility to P. capsici isolates.
Key words: Susceptibility, Capsicum annuum, Phytophthora capsici, greenhouse conditions.
INTRODUCTION
Disease caused by Phytophthora capsici strains, is one of the serious issues for peppers grown in Algeria and in other regions of the world (Silvar et al., 2006). It was first identified in the Mesilla Valley of southern New Mexico in 1922 and is considered the causal agent of pepper wilt and infects virtually any under-ground or upper-ground organs of pepper plants (Ristaino and Johnston, 1999; Hausbeck and Lamour, 2004; Tamietti and Valentino, 2001). The disease is manifested by the appearance of several symptoms with various alteration forms of all plant organs (Manohara, 2007). Pathogen translocation and consequently disease outbreak is largely due to latent infection in plantlets and the contaminated nursery medium (Aravind et al., 2011).
Due to the high susceptibility of pepper cultivars to P. capsici, pesticide treatments are used before and after crop settlement in the field to control this pathogen (Hwang and Kim, 1995). However, chemical control has various environmental and safety limitations and often sometimes is ineffective against Phytophthora strains.
Many studies have indicated that the biological control approach is not effective (Oelke et al., 2003). The effectiveness of chemical control of pepper root rot, on the other hand, is only minimal, hence the efforts to identify resistant/less susceptible pepper cultivars. Similarly, several fungicides against this disease are available, but their effectiveness varies with respect to experimental conditions. The fact that P. capsici is a soil pathogen makes natural and chemical control very difficult (Thabuis et al., 2003). The development of adapted Phytophthora-resistant pepper cultivars is considered to be an essential approach for the control of Phytophthora. Hence, it is necessary to screen for varieties/ cultivars resistant or tolerant to P. capsici that persist in soil (Divya and Sharada, 2014).
The aim of this investigation was to understand the susceptibility of commercially cultivated sweet pepper cultivars, in three regions of Algeria to P. capsici isolates.
MATERIALS AND METHODS
Plant material and growth conditions
Ten sweet pepper (Capsicum annuum L) cultivars were used in this study. They are recognized in Algeria as Doux D’Alger, Sonar, Esterel, Doux Marconi, Magister, Belconi, ItalicoII, Lipari, Arabal, and Doux d’Espagne. Most varieties were hybrids except Doux d’Espagne, Doux Marconi, and Doux d’Alger which are stable (ITCMI, 2001). They were sown under greenhouse conditions in plastic conical pots (42 × 6.5 cm) containing sterile black peat and sand (2/3 and 1/3 v/v). Each pot was seeded with one or two seeds. Cultures were irrigated until soil saturation and regularly every 3 days, and they were incubated at 20 to 25°C for 45 days (Biles et al., 1992). Seedlings were transplanted to plastic conical pots (122 × 21 cm) containing steam sterilized mixture of sand and loam soil. Seedlings were irrigated with a mineral solution for 45 days. Small seedlings were collected and washed well with tap water and sterile distilled water, and then they were placed in glass bottles containing 150 ml of NPK solution (15:15:14 w/v) and 50 ml of Richard medium. The pH of the mixture was 7.8.
Sampling, isolation and identification of P. capsici
Isolates of P. capsici were collected from symptomatic plant tissues, including roots, stems, and fruits of sweet pepper. Sweet pepper samples were obtained from three different areas in Algeria, that is, Jijel, Constantine and Biskra, known as important pepper producing regions. They were transported to the laboratory and stored overnight under refrigeration until analysis. Plant tissues were surface-disinfested with 95% ethanol, and a small piece from lesion margins was placed on V-8 juice agar. The dishes were incubated at 25°C in the dark for 7 to 10 days. Six strains of P. capsici were selected for further studies. Strain J1 from spotted leaves and strain J2 from rotten roots were obtained from Jijel region; strain C1 from rotten fruit and strain C2 from rotten roots were isolated from Constantine region; whereas, strain B1 from rotten roots and strain B2 from diseased stems were isolated from Biskra region. Thereafter, isolates were observed under a microscope, and were identified based on morphological characteristics (Tsao and Alizadeh, 1988; Gerrettson-Cornell, 1989; Stamps et al., 1990; Tsao, 1991). Cultures of P. capsici were regenerated monthly on V-8 juice agar and conserved at 22°C.
Artificial inoculation of pepper plants
Stems, leaves and roots of pepper plants (C. annuum L.) of different varieties were artificially contaminated with P. capsici Leon isolates. The experiments were conducted in a controlled room at temperature 22 ± 2°C, 12 h photoperiod and 100% relative humidity. Young fungal strains (less than 10 days of age) were used, and pepper plants were in the vegetative phase (Barksdale et al., 1984). Pathogenicity of the six isolated fungal strains and resistance limits of different organs of pepper cultivars were studied. The score of the virulence of P. capsici was evaluated by measuring the necrotic spots on stems, leaves and roots.
Inoculation of stems
Seedlings of the ten pepper cultivars were adapted to the new environment for 5 days before the start of the experiment. Then, stems were decapitated under the last leaf, and a disc (4 mm in diameter) was punched out from a young culture of each fungal isolate cultivated on a solid medium. Each disc was placed on injury created on the stem of each pepper cultivar. The applied inoculums were covered with an aluminum foil covering the entire cross section of each stem as a chamber to maintain high humidity at the top of the stem. Necrosis extension was measured every 3 days for 15 days. 180 plants (pots) are used, the 10 tested cultivars are inoculated by the 6 isolates of fungus, thus 3 replications were done, and this test was done 2 times. The measured daily necrosis rate provides useful information about the rate of mold development in the stem tissue (Pochard et al., 1976).
Inoculation of leaves
Mature pepper leaves of different cultivars (Doux d’Alger, ItalicoII, and Esterel) were used to score resistance levels, when inoculated by a highly virulent strain of P. capsici Leon (Isolate J2). Leaves were placed in Petri dishes on a thin film formed by sterile distilled water. They were inoculated in the middle veins with a mycelial disc (Ø =4 mm in diameter). The tested leaves were 18 inoculated with one isolate (J2) each cultivar is represented by 6 leaves, each two leaves are put in a Petri dish, that experience was repeated twice. The resistance of leaves was estimated by measuring the necrotic spots extension from the inoculation site after 24, 48, 72 and 96 h (Molot et al., 1984).
Inoculation of roots
Sweet pepper cultivars (Lipari, Esterel, ItalicoII and Belconi) were used for studying resistance levels of roots. Before transplanting plants into glass bottles containing mineral solution, 4 discs (Ø= 4 mm) of a newly growing virulent fungal strain on V-8 juice agar were thrown into this liquid to release motile zoospores. The tested plants were inoculated with one isolate (J2), each cultivar is represented by 10 plants, this test is repeated 3 times, and the total number of plants (pots) tested is 120. Plants death may have resulted from direct effects of root rot or crown rot. Mortality percent was used to estimate pepper resistance (Satour and Butler, 1967; Yildiz and Delen, 1979).
Statistical analysis
Data were analyzed by the one way analysis of variance (ANOVA) and the test with PË‚0.05 was considered as statistically significant. This was followed by Fisher’s test when the number of treatments was under than 5 and over 2 (leaf and root treatments), and Duncan’s Duncan’s when the number of treatments were over 5(stem treatments).
RESULTS
Fugal isolation and characterization
Colony and sporangium morphology were variable. The colony shapes observed were stellate, rosaceous, and radial. Sporangium shapes observed were ellipsoid, glo-bose, obovoid, ovoid, and distorted. The fungus did not form chlamydospores. The growth rate of all isolates was similar at 25°C. The overall optimum temperature for vegetative growth was between 25 and 30°C. Some isolates grew optimally at 25°C, while others grew best at 30°C.
Evaluation of the resistance in stems
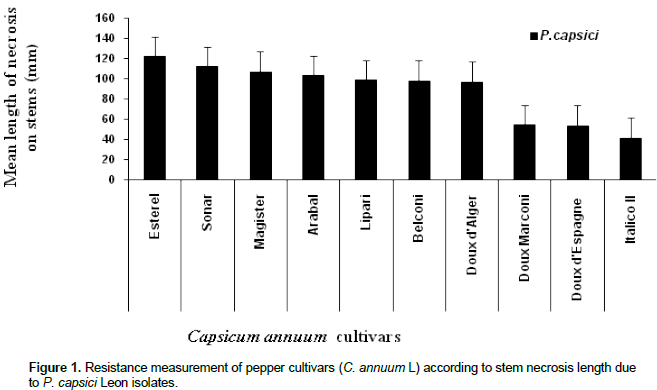
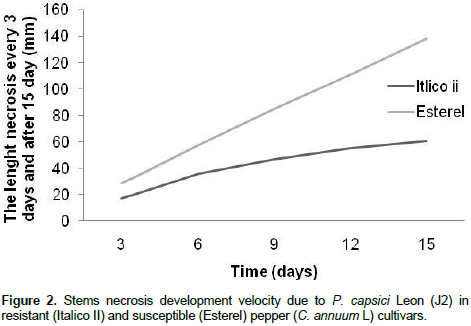
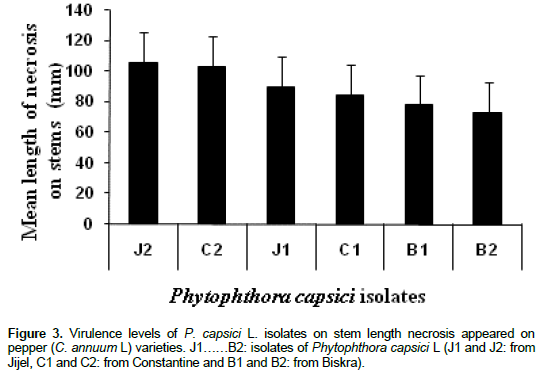
Results of incidence and severity of stem necrosis caused by different P. capsici Leon isolates against ten sweet pepper cultivars is shown in Table 1. In general, most of pepper plant cultivars were highly susceptible to the studied fungal isolates. Brown necrosis on inoculated stems was observed for all plants. Mean necrosis length ranged from 80 to 140 mm for most pepper plants analyzed (7 cultivars). The other three cultivars (Doux d’Espagne, Doux Marconi and Italico II) developed less length necrosis on their stems (< 70 mm). The results indicate that Italico II was highly resistant cultivar and Esterel, in contrast was the most sensitive to the highest virulence fungal isolates selected from roots J2 (Figure 1) and C2. In addition, statistical analysis (Two way ANOVA) indicates that, pepper cultivar have significant effects on the rate of stem necrosis (P < 0.01; ddf: 85/80; F = 5.124) and the necrotic lesion length (P < 0.01; ddf: 9/80, F = 482.899) caused by different P. capsici isolates (Figure 1). In contrast, fungal isolates displays similar pattern when they were inoculated to the studied cultivars (P > 0.05). Italico II cultivar was the most resistant. Comparing, the disease severity and velocity using the highly virulent fungal isolate (J2), significant differences were obtained between stem necrosis development velocities of the resistant pepper cultivar Italico II and the susceptible cultivar Esterel (Figure 2). Considering the most sensitive cultivar (Esterel), stem necrosis length increased linearly and a constant development velocity (9.26 mm/day) during the evolution of infection and suddenly stopped. In contrast, the most resistant pepper cultivar (Italico II), stem necrosis developed with irregular velocity. Necrosis increased in length from the third day to the sixth one post-treatment and became slower during the next experimental period. Fungal isolate, as a playing factor, affects very significantly pepper resistance pattern (P < 0.01; ddf: 5/80, F = 4148.054). Figure 3 presents virulence levels of selected six P. capsici Leon isolates. The results indicate clearly that the isolate J2 (from the Jijel region) was the highly virulent one, while isolate B2 (from Biskra region) has low virulence potential.
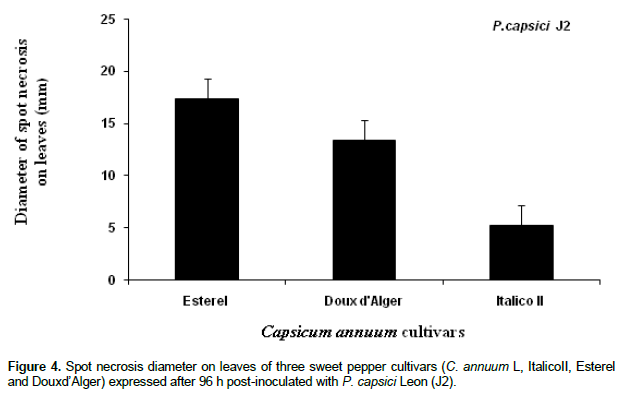
Disease development on detached leaves
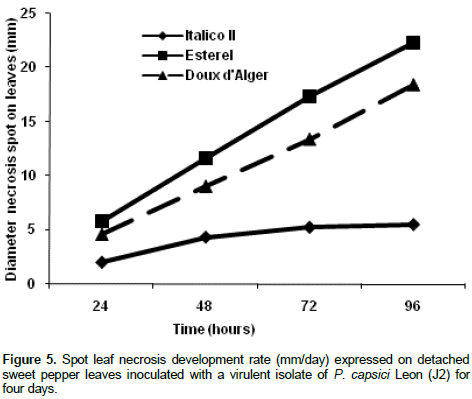
Inoculation of detached leaves, obtained from three different pepper cultivars, with the virulent fungal isolate (P. capsici J2) allowed the evaluation of the pepper plants pattern resistance (Figure 4). At the beginning of the experiment, wet dark green necrotic spots were developed, and then turned out to brown colour. Statistical analysis revealed clearly significant differences (P < 0.01; ddf: 2/15, F = 525,441) between disease symptoms in ItalicoII, Esterel and Doux d’Alger leaves pepper cultivars during 96 h post-inoculation. In the first cultivar, limited necrotic spots with average diameter 5.5 mm appear. In contrast, in the case of the two other susceptible cultivars, that is, A and B, larger necrotic spots with average diameter of 22.33 mm and18.41, respectively, were expressed (Figure 4). Leaf spot necrosis development in the three studied cultivars followed two different rates. Similar pattern, with constant development rate was observed in both Esterel and Douxd’Alger cultivars (5.6 and 4.6 mm/ day respectively) during the course of infection. However, diameter of leaf spot necrosis in Italico II cultivar increased with a slower rate (1.4 mm/day) during the four days post-inoculation. Therefore, it seems that Italico II was the most resistant cultivar, compared to the other ones, that is, Esterel and Douxd’Alger. Italico II cultivar expresses the resistant pattern in the third day (Figure 5).
Evaluation of resistance in roots
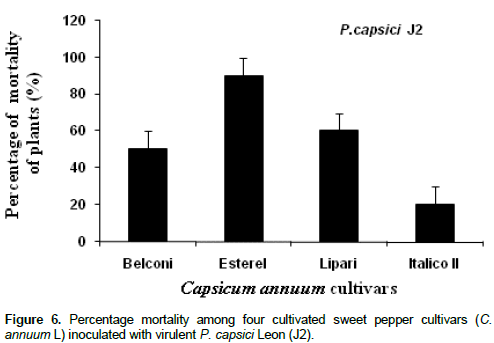
Inoculation of four cultivated pepper cultivars (Lipari, Esterel, Belconi and Italico II) with the virulent P. capsici Leon isolate “J2” allowed the evaluation of the pepper plants susceptibility to this pathogen by measuring the plant mortality. Infection and brown-grey necrosis of various plant parts were observed (root, root collars, plant tissues and plant crown). The infected plants wilt, dray and die consequently. Depending on cultivar type, significant differences between percentage mortality of cultivated plants were recorded (P < 0.01; ddf: 3/8, f = 21, 443). It seems that Italico II was the most resistant cultivar with only 16.66% mortality. In contrast Esterel, was the highly susceptible cultivar with 70% mortality. In addition, moderate resistance 43.33 and 40% mortality rate was recorded in the case of "Lipari" and "Belconi" cultivars, respectively (Figure 6).
DISCUSSION
Artificial inoculation of different organs (stems, leaves, and roots) of pepper (C. annuumL) with a range of strains of P. capsici Leon, led to the appearance of symptoms similar to those described previously (Clerjeau et al., 1976; Kim and Hwang, 1992; Walker and Bosland, 1999; Foster and Hausbeck, 2010; Koç and Üstün, 2012.). Considering necrosis on pepper stem, velocity of disease development varied between resistant pepper cultivars (Italico II) and susceptible ones (Esterel). Furthermore, highly significant cultivar/isolate effects were found, indicating a differential host-pathogen interaction. The ability of a virulent fungal strain to induce necrotic lesions on stems depends on several factors especially plant defence mechanisms. Foster and Hausbeck (2010) reported a difference between pepper cultivars to different P. capsici strains after an artificial inoculation conducted in greenhouse. The same results were also obtained by Walker and Bosland (1999), Andrés Ares et al. (2005), Byron et al. (2010) and Koç and Üstün (2012) studying the interaction of pepper/ P. capsici isolates.
According to study of Clerjeau et al. (1976), the application of 23 isolates of the fungus P. capsici Leon on two varieties resistant "Phyo" and susceptible "YoloWonder" of pepper (C. annuum L) varying significantly, have given answers between the two varieties. Both isolate 13 and isolate 101 of the fungus gave necrotic lesions significantly shorter on the resistant variety compared to the isolates 96 and 112, and the opposite was observed when they were applied to the susceptible variety. The heterogeneity of genetic material plants is the explanation of heterogeneity of resistance responses (Pochard et al., 1976). There were other examples of strain-range interaction, where more reference strains were used. More aggressive, strains 71, 73 and 107 are less discerning between the two varieties. In any case, the criterion is the total length of necrosis extended for a week or 10 days, most of this period the stems of the variety "Yolo Wonder" may have a whole necrosis in the presence of more aggressive strains (Clerjeau et al., 1976). Studying the total length of necrosis on the stem, they found that the speed of development has a relationship with the development of the mycelial hyphae (Molot et al., 1976). They found that after a period of rapid decline in the rate of necrosis, in partner resistant (R), it is stabilized by a constant value and varies depending on the strain used, that characterizes the behavior of our range "Italico II", but in partner susceptible (S), the speed begins to increase after stabilizes with a high value that will decrease rapidly, stability is less permanent, we take into consideration that the fungus is rapidly approaching the base of the stem, and the best representative of this behavior is the cultivar "Esterel" (Pochard and Daubèze, 1980). The expression of resistance to the various organs of pepper is quantitative, is different depending on the variety. The genes responsible for this natural resistance reduce the rate of penetration of the fungus P. capsici Leon in pepper tissues (Pochard et al., 1983). The resistance is expressed discontinuously between the organs in some varieties or continuously in others, such as the case of the varieties tested, the resistance remains at the root level associated with a high level of resistance in the stem. According to Pochard and Daubèze (1980), the sensitivity of the roots to the fungus P. capsici Leon may be linked to partial loses of resistance from the summit to the crown of the plant,
In addition, the fact that there was a great variation in virulence among fungal isolates would reflect the possible occurrence of pathogenic specialization of P. capsici on the various pepper cultivars grown in Algeria for a long period. Similar supported findings were reported by various investigations in the same line (Pochard and Daubèze, 1980; Yang et al., 1989; Kim and Hwang, 1992). In addition, the cultivar / isolate compatibility may be proved by the disease severity and symptoms development velocity. In resistant cultivars, disease symptoms appear with a slower rate as compared to the susceptible cultivars. Differences in genetic background of pepper cultivars is a direct factor for different resistance response expressed (Pochard et al., 1976).
CONCLUSION
The late blight disease caused by P. capsici Leon is characterized by its severity on sweet pepper (C. annuum L) cultivars. The artificial inoculation of various organs of some commercial pepper cultivars by Algerian P. capsici isolates in order to select the resistant ones provides that there was a relationship cultivar-isolate. Italico II cultivar showed high resistance to the studied fungal isolates. But under the same conditions, "Esterel" proved to be the most susceptible one. In conclusion, our data suggest that there are different interactions between P. capsici isolates and some pepper cultivars at normal plant growth stage. This study demonstrates that information from one geographic area may not accurately predict the response of a resistant cultivar used in another region, including diverse geographic regions within a single state.
CONFLICT OF INTERESTS
The author(s) did not declare any conflict of interest.
ACKNOWLEDGMENTS
This work was supported by the Algerian Ministry of Higher Education and Scientific Research. The authors would like to acknowledge all those who contributed directly or indirectly in the development of this work.
REFERENCES
|
Andrés Ares JL, Rivera Martìnez A, Fernández Paz J (2005). Resistance of pepper germplasm to Phytophthora capsici isolates collected in northwest Spain. Spanish J. Agric. Res. 3(4):429-436. |
|
|
Aravind R, Aundy K, Dinu A, Santhosh JE (2011). Single tube duplex PCR for simultaneous detection of Phytophthora capsici and Radopholus similis infecting black pepper (Piper nigrum). Indian Phytopathol. 64(4):353-357. |
|
|
Barksdale TH, Papavizas GS, Johnston SA (1984). Resistance to foliar blight and crown rot of pepper caused by Phytophthora capsici. Plant Dis. 68:506–509. |
|
|
Biles GL, Lindsey DL, Liddell CM (1992).Control of Phytophthora root rot of chile peppers by irrigation practices and fungicides. Crop Protect. 11:225-228. |
|
|
Byron LC, Patrick JC, Pingsheng J (2010). Screening Capsicum annuum Accessions for Resistance to Six Isolates of Phytophthora capsici. Hort. Sci. 45(2):254–259. |
|
|
Clerjeau M, Pitrat M, Nourrisseau JG (1976). La résistance du piment (Capsicum annuum L) à Phytophthora capsici Leon. IV. Etude de l'agressivité de divers isolats au niveau des feuilles, des tiges et du collet de plantes sensibles et résistantes. Ann. Phytopathol. 8(4):411-423. |
|
|
Divya CR, Sharada MS (2014).Screening of Piper nigrum L. Varieties/cultivars against quick wilt caused by Phytophthora capsici Leon. under greenhouse condition. Int. J. Recent Sci. Res. 5(11):2028-2030. |
|
|
Foster JM, Hausbeck MK (2010). Resistance of pepper to Phytophthora crown, root, and fruit rot is affected by isolate virulence. Plant Dis. 94:24-30. |
|
|
Gerrettson-Cornell L (1989). A compendium and classification of the species of the genus Phytophthora de Bary by the canons of the traditional taxonomy. Forestry Commission N. S.W. (Australia). Tech. 45:101-103. |
|
|
Hausbeck MK, Lamour KH (2004). Phytophthora capsici on vegetable crops: Research progress and management challenges. Plant Dis. 88:1292-1303. |
|
|
Hwang BK, Kim AH (1995). Phytophthora blight of pepper and its control in Korea. Plant Dis. 79:221-227. |
|
|
ITCMI (2001). Guide pratique du piment sous serre. Institut technique des cultures maraîchères et Industrielles, Staouéli, Algérie. p.13. |
|
|
Kim ES, Hwang BK (1992). Virulence to Korean pepper cultivars of isolates of Phytophthora capsici from different geographic areas. Plant Dis. 76:486-489. |
|
|
Koç E, Üstün AS (2012). Influence of Phytophthora capsici L. inoculation on disease severity, necrosis length, peroxidase and catalase activity, and phenolic content of resistant and susceptible pepper (Capsicum annuum L.) plants. Turk. J. Biol. 36:357-371. |
|
|
Manohara D (2007). Formation and pathogenesity variation of Phytophthora capsici infecting black pepper. Microbiol. Indones. 1(2):61-64. |
|
|
Molot PM, Clerjeau M, Nourrisseau J, Ricci P (1976). La résistance du piment (Capsicum annuum) à Phytophthora capsici. III. Etude, sur extraits de tiges sensibles et résistantes, du pouvoir antifongique induit par la contamination. Ann. Phytopathol. 8(4):399-407. |
|
|
Molot PM, Mas P, Lecoq H, Marchoux G (1984). Action, vis-à-vis de quelques agents parasitaires, de deux fractions élicitrices issues de Phytophthora capsici appliquées sur organes en survie et plantules de diverses espèces végétales. Agronomie 4(9):835-842. |
|
|
Oelke LM, Steiner R, Bosland PW (2003). Differentiation of race specific resistance to Phytophthora root rot and foliar blight in Capsicum annuum. J. Am. Soc. Hort. Sci. 128:213–218. |
|
|
Pochard E, Clerjeau M, Pitrat M(1976). La résistance du piment. Capsicumannuum L. à Phytophthora capsici Leon. Ann. Amélioration des Plantes. 26 (1) : 35-50. |
|
|
Pochard E, Daubeze AM (1980). Recherche et évaluation des composantes d'une résistance polygénique: La résistance du piment à Phytophthora capsici. Ann. Amélioration des Plantes. 30(4):377-398. |
|
|
Pochard E, Molot PM, Dominguez G (1983). Etude de deux nouvelles sources de résistance à Phytophthora capsiciLeon. chez le piment: confirmation de l'existence de trois composantes distinctes dans la résistance. Agronomie 3:333-342. |
|
|
Ristaino JB, Johnston SA (1999). Ecologically based approaches to management of Phytophthora blight on bell pepper. Plant Dis. 83:1080-1089. |
|
|
Satour MM, Butler EE (1967). A root and crown rot of tomato caused by Phytophthora capsici and Phytophthora parasitica. Phytopathology 57:510-515. |
|
|
Silvar C, Merino F, Díaz J (2006). Diversity of Phytophthora capsici in northwest Spain: Analysis of virulence, metalaxyl response, and molecular characterization. Plant Dis. 90:1135-1142. |
|
|
Stamps DJ, Waterhouse GM, Newhook FJ, Hall GS (1990). Revised tabular key to the species of Phytophthora. Mycological Papers 162. Commonwealth Mycological Institute, Kew, Surrey. P 22. |
|
|
Tamietti G, Valentino D (2001). Physiological characterization of a population of Phytophthora capsici Leon from northern Italy. J. Plant Pathol. 83:199-205. |
|
|
Thabuis A, Palloix A, Pflieger S, Daubèze AM, Caranta C, Lefebvre V (2003). Comparative mapping of Phytophthora resistance loci in pepper germplasm: evidence for conserved resistance loci across Solanaceae and for a large genetic diversity. Theor. Appl. Genet. 106(8):1473-1485. |
|
|
Tsao PH (1991). The identities, nomenclature, and taxonomy of Phytophthora isolates from black pepper. In: Sama YR, Premkumareds T, Disease black pepper proceedings of the international pepper comity of workshop on black pepper disease. Kerala, India. pp. 185-211. |
|
|
Tsao PH, Alizadeh A (1988). Recent advances in the taxonomy and nomenclature of the so-called 'Phytophthora palmivora' MF4 occurring on cocoa and other tropical crops. In Proceeding of the Tenth International Cocoa Research Conference, 17-23 May 1987, Santo Domingo, Dominican Republic. pp. 441-445. |
|
|
Walker SJ, Bosland PW (1999). Inheritance of Phytophthora Root Rot and Foliar Blight Resistance in Pepper. J. Am. Soc. Hort. Sci. 124(1):14–18. |
|
|
Yang SS, Sung NK, Choi DI, Kim CH (1989). Pathogenic variation of Phytophthora capsici on red-pepper in Korea. Korean J. Plant Pathol. 5:370-376. |
|
|
Yildiz M, Delen N (1979). Some results of fungicide tests on Phytophthora capsici Leon. of pepper. Turk. Phytopathol. 8(1):29-39. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0