ABSTRACT
Drought induced oxidative stress triggers anti-oxidative system in cell to minimize oxidative damage where catalase plays vital role to neutralize H2O2. In this work, catalase activity was evaluated to examine the role of catalase in barley (Hordeum vulgare L.) seedlings under drought stress. As compared to control, catalase activity increased with decreasing soil moisture where 219% higher activity were recorded at 10% soil moisture of field capacity (FC) compared to control (75% FC), but was reduced below 10% FC. Four different catalase isozymes that specifically accumulated in barley leaves in response to drought (10% FC) which nominated catalase, particularly CAT4 and CAT2, as key players for H2O2 scavenging were identified. However, for future study, one catalase was purified from barley leaves with an apparent molecular weight of 54 kDA and specific activity of 871.32 µmol min-1 mg-1 protein. Therefore, in this study, it was found that four CAT isozymes in barley leaf under drought, and the purified catalase needs characterization at molecular level for further biotechnical use.
Key words: Catalase, isozymes, barley, drought, purification.
The fast growing population is facing difficulty to feed the people worldwide due to reducing situation of arable land. Moreover, increased stress due to climatic change has caused higher risk for agricultural production. Bangladesh is one of the most over populated countries in the world with ranking 8th for population and 92nd for area. Moreover, about 30% of cultivable land in the southern coastal belt is affected by salinity (Rohman et al., 2019a); salinity has affected crop production in 100 million ha. On the other hand, about 0.28 million ha of land is dry (locally, Charland) and often faces drought (Sattar and Islam, 2010). Such types of problematic soils need tolerant crop species. Barley (Hordeum vulgare ssp. vulgare) inherently exhibits a higher level of abiotic stress tolerance than other crops (Baik et al., 2011; Nevo et al., 2012; Powell et al., 2012), and it has potential role as human food in different salinity and drought affected areas in the world (Zhou, 2010). It ranks the fourth most important cereal crop on a global scale (FAOSTAT, 2018), which predicts its future prospects for food production in problem areas like salinity and drought affected ones. Moreover, because of its relatively simple diploid genetics along with tight relationship between other cereals, barley has gained importance as a resistance source (Wiegmann et al., 2019). Under abiotic stress, a plant, tolerant or susceptible, undergoes a series of morphological, biochemical, physiological and molecular changes (Gill and Tujeta, 2010). However, the tolerance mechanism to abiotic stress, particularly saline and drought stress is very complex and still not clear.
Drought is the most detrimental stress that inhibits the growth and yield of crops. In plant species, drought causes osmotic stress resulting in oxidative stress in plants through declining stomatal conductivity that limit CO2 entry into the leaves which reduces the leaf internal CO2 resulting in the formation of reactive oxygen species (ROS) (Foyer and Noctor, 2012; Choudhury et al., 2013). ROS are highly cytotoxic in different ways: firstly, through lipid peroxidation resulting in increasing membrane leakage and reducing membrane fluids that damages ion channels, membrane proteins, enzymes and receptors; secondly, through oxidizing proteins that hinder or change activities and make plants more susceptible to proteolytic attack; and thirdly, through DNA damage (base deletions and modifications, strand breaks, cross-links and pyrimidine dimers) which ultimately reduces or injures protein syntheses, damages cell membrane, unstabilizes DNA replication, genomic stability and transcription (Filiz et al., 2019). ROS constitute oxidant molecules like superoxide radical (O2•−), singlet oxygen (1O2), hydrogen peroxide (H2O2), hydroxyl radicals (OH•) and alkoxyl radical (RO•), and cause cellular damage by oxidizing organelles like enzymes, proteins, DNA and lipids (Gill and Tujeta, 2010).
Plants have very well-organized ROS scavenging system consisting of both enzymatic and non-enzymatic antioxidants. Among enzymatic antioxidants are superoxide dismutase (SOD; EC 1.15.1.1), catalase (CAT; EC 1.11.1.6), ascorbate peroxidase (APX; EC 1.11.1.11), glutathione peroxidase (GPX; EC 1.11.1.9), glutathione reductase (GR; EC 1.6.4.2), peroxidase (GPX; EC 1.11.1.7), monodehydroascorbate reductase (MDHAR; EC 1.6.5.4), dehydroascorbate reductase (DHAR; EC 1.8.5.1) and glutathione S-transferase (GST; EC 2.5.1.18). On the other hand, ascorbic acid (ASA), glutathione (GSH), alkaloids, tocopherol some amino phenolic compounds, carotenoids and flavonoids are non-enzymatic components (Gill and Tujeta, 2010). ROS generation or accumulation is an unavoidable consequence of normal metabolic processes in plants, and in normal growth condition, there is a balance between ROS production and its scavenging; whereas any stressful condition imbalances the equilibrium by increasing cellular ROS (Sharma et al., 2012).
Under stressful condition, SOD provides first line protection from O2•- mediated oxidative damage through its dismutation to H2O2 (Apel and Hirt, 2004). H2O2 is also produced in other enzymatic and non-enzymatic metabolic pathways. In peroxisomes, the generation of H2O2 is catalyzed by glycolate oxidase involving glycolate oxidation, the b-oxidation of fatty acids and catabolism of lipids (Halliwell, 2006). H2O2 that accumulates in cell can be then metabolized by CAT, POD, APX, GPX and GST (Gill and Tujeta, 2010; Sharma et al., 2012). It is important that metabolism of H2O2 by CATs is different from other enzymatic H2O2 metabolisms, as they do not require any substrate (Mhamdi et al., 2010). Therefore, CATs are the most important tools for mitigating oxidative stress in plants. Considering those, the experiments were designed to examine the role of catalase in barley under saline stress, and a CAT was purified from barley leaves.
Plant materials
Seedlings of BARI barley-6 were used as plant materials. The leaves of seedlings were used to examine the regulation of CAT. For purification, 10 days old leaves of seedlings were used.
Stress treatment
Seedlings were grown in soil media (Soil: organic matter =3:1) in 30 L plastic bucket in the green house of Plant Breeding Division of Bangladesh Agricultural Research Institute. Fifteen days old seedlings were subjected to water withdrawal after attaining soil moisture of 30% (field capacity, FC) of experimental soil. Seedlings were observed until soil moisture attained 5% of FC. Data were taken from fully expanded leaves at 75 (as control), 25, 10 and 5% FC. Soil moisture level was monitored with a digital soil moisture meter (Lutron PMS-714, Taiwan). The experiment was repeated three times each containing three replications.
Enzyme extraction for CAT assay and isozyme analysis
Using a pre-cooled mortar and pestle, 0.5 g of leaf tissue was homogenized in 1 ml of 50 mM ice-cold potassium-phosphate buffer (pH 7.0) containing 100 mM KCl, 1 mM ascorbate, 5 mM β-mercaptoethanol and 10% (w/v) glycerol. The homogenates were centrifuged at 11,500 × g for 10 min, and the supernatants were used for determination of enzyme activity. All procedures were performed at 0 to 4°C.
Determination of protein
The protein concentration in the leaf extracts was determined according to the method of Bradford (1976) using albumin from bovine serum (BSA) as a protein standard.
Assay of CAT activity
CAT (EC: 1.11.1.6) activity was measured as per description of Rohman et al. (2019b) by monitoring the decrease of absorbance at 240 nm for 1 min caused by the decomposition of H2O2. The reaction mixture contained 50 mM K-phosphate buffer (pH 7.0), 15 mM H2O2, and enzyme solution in a final volume of 0.7 ml. The reaction was initiated with enzyme extract, and the activity was calculated using the extinction coefficient of 39.4 M−1 cm−1. CAT isozymes were analysed using SDS-PAGE under non-reduced and non-denatured conditions at 4°C according to Laemmli (1970). CAT isoenzymes were determined by using 10% separating gel implementing the method of Woodbury et al. (1971) with modification. The gels were treated with 0.01% H2O2 for 10 min. Then the gels were rinsed with distilled water, and stained with 1% FeCl3 and 1% K3Fe(CN)6. Photograph was taken as the CAT bands appeared in the staining solution.
Protein extraction for CAT purification
Thirty grams of barley fresh leaves were extracted by homogenizing in an equal volume of 25 mM Tris–HCl buffer (pH 8.0) containing 1 mM EDTA, 1% (w/v) ascorbate and 10% (w/v) glycerol with mortar pestle. The homogenate was centrifuged at 11500 × g for 15 min, and the supernatant was used as a soluble protein solution for CAT purification.
DEAE-cellulose chromatography
Proteins were precipitated by ammonium sulfate at 65% saturation from the supernatant and centrifuged at 11,500 × g for 10 min. The proteins were dialyzed against 10 mM Tris-HCl buffer (pH 8) containing 0.01% (w/v) β-mercaptoethanol and 1 mM EDTA (buffer A) overnight to completely remove low molecular inhibitors. The dialyzate (crude enzyme solution) was applied to a column (1.77 × 20 cm) of DEAE-cellulose (DE-52, Whatman, UK) that had been equilibrated with buffer A and eluted with a linear gradient of 0 to 0.20 M KCl in 750 ml of buffer A.
Hydroxyapatite chromatography
The pooled sample of CAT, separated by DEAE-cellulose column chromatography, was applied on a hydroxyapatite column (1.5 × 5.5 cm) that had been equilibrated with buffer A. The column was eluted with a 300 ml linear gradient of potassium phosphate buffer (K-P buffer; 0-20 mM, pH 7.0) in buffer A. The high active fraction (5 ml) was found to elute which was collected and further purified on Phenyl Sepharose CL-4B chromatography.
Phenyl sepharose CL-4B chromatography
The pooled sample of CAT, purified by hydroxyapatite chromatography, was applied on phenyl sepharose CL-4B chromatography (1.5 × 5.0 cm) that had been equilibrated with buffer A. The column was eluted with a 200 ml linear gradient of 0-50 mM potassium phosphate buffer (pH 7.0) in buffer A. The high active fractions were collected and purity was tested in an SDS-PAGE.
SDS-PAGE and CBB dying
To check the purification, different fractions were run into a SDS-PAGE of 12.5% (w/v) gel containing 0.1% (w/v) SDS by the method of Laemmli (1970) followed by staining with Coomassie Brilliant Blue (G-250). Molecular weight was measured using Alpha Innotech Gel Imaging System.
Statistical analysis
Data obtained from drought stress were analysed by statistical software Statistix 10 following complete randomized design (CRD), and the mean differences were compared by least significant test (LSD), and p≤0.05 was considered to be significant.
The activity of CAT increased surprisingly with decreasing soil moisture (Figure 1). At control condition, the activity was 101 µmol min-1 mg-1 protein. The activity increased significantly and continuously with decreasing soil moisture (Figure 1). However, the activity decreased after 10% of soil moisture of FC. As compared to control, the activity was 99 and 219% higher at 25 and 10% FC, respectively.
CATs are heme-containing tetrameric enzymes with crucial role for de-toxification of H2O2 into H2O in different stresses (Garg and Manchanda, 2009). They are located in all major sites of H2O2 production in the cellular environment (such as peroxisomes, mitochondria, cytosol and chloroplast) of higher plants. CAT have the highest turnover rate of detoxification of H2O2 to H2O per one molecule per minute. Therefore, highly induced CAT activity played essential role in the removal of H2O2 (Figure 1). CAT activity has been reported to increase in drought stress in alfalfa (Rubio et al., 2002), Arabidopsis thaliana (Koussevitzky et al., 2008), pea (Türkan et al., 2005), citrus (Balfagón et al., 2018; Zandalinas et al., 2017), Coffea canephora (Lima et al., 2002), cotton (Ratnayaka et al., 2003; Zhang et al., 2016), (Safronov et al., 2017), maize (Jiang and Zhang, 2002; Rohman et al., 2016), Populus przewalskii (Lei et al., 2006), rice (Guo et al., 2006), tobacco (Badawi et al., 2004; Jung, 2004) and wheat (Cheng et al., 2016; Shan et al., 2018).
Recently it has been established that catalase is present as multiple isoforms encoded by multiple genes expressed in organelle, temporal and stress specific manners. For further confirmation, the enzymatic protein extracts were subjected to isozyme analysis (Figure 2). Surprising, increment in intensification of CAT activity bands up to 10% FC as compared to control. Four CAT isozymes were appeared at 10% FC, although CAT 2 and CAT4 were clearer than CAT1 and CAT3.
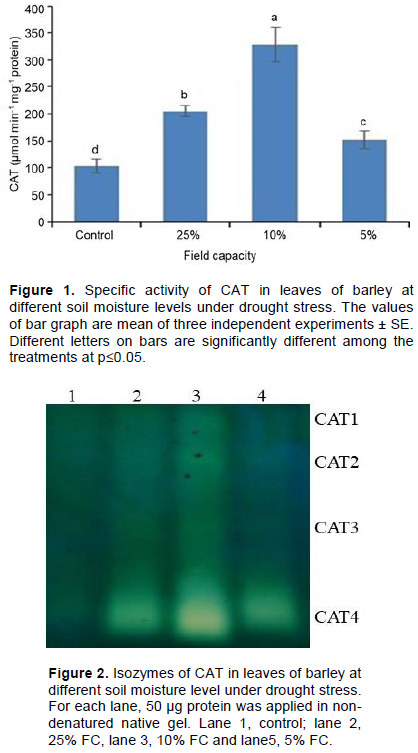
The modulation of H2O2 by the catalase isozymes within specific cells or organelles at specific time and developmental phases directly or indirectly interferes with signal transduction in plants, and the expression of CAT gene shows time, species and stress specificity (Sharma and Ahmad, 2014). Previously, Azevedo et al. (1998) reported two isozymes in barley root. In this study, four isozymes in barley leaves (Figure 2) particularly, under drought condition were found. Therefore, the isozymes that appeared under drought condition have important role in H2O2 metabolism. Previously, Mallik et al. (2011) investigated CAT activity in diverse groups of plants, such as a unicellular alga, Chlorella sp., an aquatic macrophyte, Najas graminea, and a mangrove plant, Suaeda maritima under saline stress, and reported highly induced CAT activity with formation of new isoforms under only severe saline stress. CAT isozymes have also been studied in many higher plants like 3 isoforms (CAT1, CAT2 and CAT3) in maize (Scandalios, 1990), in sweet potato (Sharma and Ahmad, 2014) and in Arabidopis (Filiz et al., 2019). On the other hand, four isozymes have been reported in Helianthus annuus cotyledons (Azpilicueta et al., 2007), 12 isozymes in Brassica (Frugoli et al., 1996) and only one prominent CAT in the leaves, stems and roots of Broussonetia papyrifera with different responses to salinity stress (Zhang et al., 2013). Polidoros and Scandalios (1999) showed positive relationship of two isozymes CAT1 and CAT2 with H2O2 metabolism in maize. Filiz et al. (2019) evaluated expression profile of two gene CAT2 and CAT3 coding for CAT under salt, cold, heat and light stress in natural Arabidopsis ecosystem through micro array system, where high light, salt and cold stresses substantially up-regulated the expression of the genes, but down regulated in cold stress. They also reported one CAT1 gene coded for SOD. On the other hand, Skadsen et al. (1995) characterized CAT1 and CAT2 gene in barley, but their expression was not reported under abiotic stress. Jeong and Kim (2004) reported two CAT isozymes in barley root with differential expression in aluminium stress. On the other hand, one isozyme was reported under salinity (Mohammad et al., 2015) and drought (Salekjalali et al., 2012), where highly expression of the activity was observed under drought. Therefore, CAT isozymes varied with different research group probably due to use different genotypes of barley. In this study, the variety used showed the presence of four isozymes under drought. Therefore, we believe that this study bears importance to study the four isozymes under different abiotic stress.
Purification of catalase from barley leaves
Catalase always draws the attention of researchers due to its efficient catalytic and regulatory properties among all antioxidant enzymes of the plant system. Thus, it was purified from barley leaves in this study for future characterization at the genetic, biochemical, and molecular level.
The soluble protein fraction was prepared from 30 g fresh leaves. The soluble protein was precipitated by (NH4)2SO4 at 65% saturation and dialyzed overnight and the dialyzate was applied on DEAE-cellulose column chromatography (i.d. 1.7 × 20 cm) and eluted with a linear gradient of KCl (0-0.2 M) (Figure 3). A total of 125 fractions, each containing 5 ml, were collected. The CAT activities and absorbance at 280 nm were measured. A high active peak was eluted at 67 mM of KCl.
The fractions showing high CAT activity were pooled and applied onto a hydroxylapatite column chromatography (Figure 4). A total of 300 ml gradient solution containing 0 to 20 mM potassium-phophate (K-P) buffer, pH 7 was passed. A total of 70 fractions were collected and CAT activity and absorbance at 280 nm was recorded for each fraction (Figure 4). An active CAT peak was found.
Finally, the active fractions were applied to phenyl sepharose CL-4B and eluted with 50 mM K-P buffer. The CAT activity and absorbance were taken and presented in Figure 5. Only one active peak of CAT activity was found. Therefore, all the fractions were applied on a SDS-PAGE and stained with coomassie brilliant blue (CBB) (Figure 6).
The CBB staining showed that the subsequent purification method eliminated the undesirable protein gradually. Finally, CAT protein was purified and moved with a single band showing the apparent molecular weight of 54 kDA. The summary of the purification is shown in Table 1.
It was found that the purified protein contained activity of 351.45 mmol min-1 (Table 1). The specific activity and amount of protein were 371.32 and 0.43 mg, respectively, with 0.52% recovery and 4.83 purification fold. In purification of CAT from barley, it was apparently observed in SDS-PAGE that the purified CAT protein is a polypeptide of 58 kDa (Figure 6). Beulah and Ramana (2013) reported a CAT of 51.3 kDa in Phyllanthus reticulates. The CAT purified from cotyledon of germinating pumpkin seed was 55 kDa (Yamaguchi and Nishimura, 1984) while CAT purified from Zantedeschia aethiopica had a molecular weight of 54 kDa (Trindade et al., 1988).
From the above data, it was found that CAT activity increased with increasing droughts, and after 10% FC, the activity decreased. Strong evidence was provided by analysing isozymes where four isozymes CAT1, CAT2, CAT3 and CAT4 were visualized in drought stress. Therefore, it is very clear that in barley, new CAT isozymes are synthesized under drought, and likely to have essential role in H2O2 scavenging. On the other hand, a CAT from barley leaf was purified, and the purified catalase had an apparent molecular weight of 54 kDA. Therefore, further study is required for its molecular and biochemical characterization.
The authors have not declared any conflict of interests.
REFERENCES
|
Apel K, Hirt H (2004). Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annual Review of Plant Biology 55:373-399.
Crossref
|
|
|
|
Azevedo RA, Alas RM, Smith RJ, Lea PA (1998). Response of antioxidant enzymes to transfer from elevated carbon dioxide to air and ozone fumigation, in leaves and roots of wild-type and catalase-deficient mutant of barley. Physiologia Plantarum 104(2):280-92.
Crossref
|
|
|
|
|
Azpilicueta CE, Benavides MP, Tomaro ML, Gallego SM (2007). Mechanism of CATA3 induction by cadmium in sunflower leaves. Plant Physiology and Biochemistry 45(8):589-595.
Crossref
|
|
|
|
|
Badawi GH, Kawano N, Yamauchi Y, Shimada E, Sasaki R, Kubo A, Tanaka K (2004). Overâ€expression of ascorbate peroxidase in tobacco chloroplasts enhances the tolerance to salt stress and water deficit. Physiologia Plantarum 121(2):231-238.
Crossref
|
|
|
|
|
Baik BK, Newman CW, Newman RK (2011). Barley: Production, Improvement, and Uses, edited by Ullrich SE. Wiley-Blackwell, Oxford, UK pp.3-14.
|
|
|
|
|
Balfagón D, Zandalinas SI, Baliño P, Muriach M, Gómez-Cadenas A (2018). Involvement of ascorbate peroxidase and heat shock proteins on citrus tolerance to combined conditions of drought and high temperatures. Plant Physiology and Biochemistry 127:194-199.
Crossref
|
|
|
|
|
Beulah K, Ramana T (2013). Purification, properties and kinetic studies of catalase from leaves of Phyllanthus reticulates. International Journal of Pharmacy and Biological Sciences 3(3):940-948.
|
|
|
|
|
Bradford MM (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry 72(1-2):248-54.
Crossref
|
|
|
|
|
Cheng L, Wang Y, He Q, Li H, Zhang X, Zhang F (2016). Comparative proteomics illustrates the complexity of drought resistance mechanisms in two wheat (Triticum aestivum L.) cultivars under dehydration and rehydration. BMC Plant Biology 16(1):88.
Crossref
|
|
|
|
|
Choudhury S, Panda P, Sahoo L, Panda SK (2013). Reactive oxygen species signaling in plants under abiotic stress. Plant Signaling and Behavior 8(4):e23681.
Crossref
|
|
|
|
|
FAOSTAT (2018). FAOSTAT. Available at, http://www.fao.org/faostat/en/#compare Date 27.05.2020
|
|
|
|
|
Filiz E, Ozyigit II, Saracoglu IA, Uras ME, Sen U, Yalcin B (2019). Abiotic stress-induced regulation of antioxidant genes in different Arabidopsis ecotypes: Microarray data evaluation. Biotechnology and Biotechnological Equipment 33(1):128-143.
Crossref
|
|
|
|
|
Foyer CH, Noctor G (2012). Ascorbate and glutathione: The heart of the redox hub. Plant Physiology 155(1):2-18.
Crossref
|
|
|
|
|
Frugoli JA, Zhong HH, Nuccio ML, McCourt P, McPeek MA, Thomas TL, McClung CR (1996). Catalase is encoded by a multigene family in Arabidopsis thaliana (L.). Plant Physiology 112(1):327-336.
Crossref
|
|
|
|
|
Garg N, Manchanda G (2009). ROS generation in plants: Boon or bane? Plant Biosystems 143(1):81-96.
Crossref
|
|
|
|
|
Gill SS, Tujeta N (2010). Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiology and Biochemistry 48(12):909-930.
Crossref
|
|
|
|
|
Guo Z, Ou W, Lu SY, Zhong Q (2006). Differential responses of antioxidative system to chilling and drought in four rice cultivars differing in sensitivity. Plant Physiology and Biochemistry 44(11-12):828-836.
Crossref
|
|
|
|
|
Halliwell B (2006). Reactive species and antioxidants, redox biology is fundamental theme of aerobic life. Plant Physiology 141(2):312-322.
Crossref
|
|
|
|
|
Jeong MS, Kim SH (2004). Aluminium stress in the roots of naked barley. Journal of Plant Biology 47(2):65.
Crossref
|
|
|
|
|
Jiang M, Zhang J (2002). Water stressâ€induced abscisic acid accumulation triggers the increased generation of reactive oxygen species and upâ€regulates the activities of antioxidant enzymes in maize leaves. Journal of Experimental Botany 53(379):2401-2410.
Crossref
|
|
|
|
|
Jung S (2004). Variation in antioxidant metabolism of young and mature leaves of Arabidopsis thaliana subjected to drought. Plant Science 166(2):459-466.
Crossref
|
|
|
|
|
Koussevitzky S, Suzuki N, Huntington S, Armijo L, Sha W, Cortes D, Shulaev V, Mittler R (2008). Ascorbate peroxidase 1 plays a key role in the response of Arabidopsis thaliana to stress combination. Journal of Biological Chemistry 283(49):34197-34203.
Crossref
|
|
|
|
|
Laemmli UK (1970). Cleavage of structural proteins during the assembly of head of bacteriophage T4. Nature 227:680-685.
Crossref
|
|
|
|
|
Lei Y, Yin C, Li C (2006). Differences in some morphological, physiological, and biochemical responses to drought stress in two contrasting populations of Populus przewalskii. Physiologia Plantarum 127(2):182-191.
Crossref
|
|
|
|
|
Lima ALS, DaMatta FM, Pinheiro HA, Totola MR, Loureiro ME (2002). Photochemical responses and oxidative stress in two clones of Coffea canephora under water deficit conditions. Environmental and Experimental Botany 47(3):239-247.
Crossref
|
|
|
|
|
Mallik S, Nayak M, Sahu BB, Panigrahi A.K, Shaw BP (2011). Response of antioxidant enzymes to high NaCl concentration in different salt-tolerant plants. Biologia Plantarum 55(1):191-195.
Crossref
|
|
|
|
|
Mhamdi A, Queval G, Chaouch S, Vanderauwera S, Van Breusegem F, Noctor G (2010). Catalase function in plants: a focus on Arabidopsis mutants as stress-mimic models. Journal of Experimental Botany 61(15):4197-4220.
Crossref
|
|
|
|
|
Mohammad B, Mostafa V, Mohammad MV (2015). Catalase and peroxidase antioxidant enzyme activities in barley cultivars seedling under salt stress. Bulletin of Environment, Pharmacology and Life Science 4:29-35.
|
|
|
|
|
Nevo E, Fu YB, Pavlicek T, Khalifa S, Tavasi M, Beiles A (2012). Evolution of wild cereals during 28 years of global warming in Israel. Proceedings of the National Academy of Sciences 109(9):3412-3415.
Crossref
|
|
|
|
|
Polidoros NA, Scandalios JG (1999). Role of hydrogen peroxide and different classes of antioxidants in the regulation of catalase and glutathione S-transferase gene expression in maize (Zea mays L.). Physiologia Plantarum 106(1):112-120.
Crossref
|
|
|
|
|
Powell N, Ji X, Ravash R, Edlington J, Dolferus R (2012). Yield stability for cereals in a changing climate. Functional Plant Biology 39(7):539-52.
Crossref
|
|
|
|
|
Ratnayaka HH, Molin WT, Sterling TM (2003). Physiological and antioxidant responses of cotton and spurred anoda under interference and mild drought. Journal of Experimental Botany 54(391):2293-2305.
Crossref
|
|
|
|
|
Rohman MM, Begum S, Talukder MZ, Akhi AH, Amiruzzaman M, Ahsan AF, Hossain Z (2016). Drought sensitive maize inbred shows more oxidative damage and higher ROS scavenging enzymes, but not glyoxalases than a tolerant one at seedling stage. Plant Omics 9(4):220.
Crossref
|
|
|
|
|
Rohman MM, Islam MR, Naznin T, Omy SH, Begum S, Alam SS, Amiruzzaman M, Hasanuzzaman M (2019a). Maize production under salinity and drought conditions: Oxidative stress regulation by antioxidant defense and glyoxalase systems. In Plant Abiotic Stress Tolerance. Springer, Cham, pp. 1-34.
Crossref
|
|
|
|
|
Rohman MM, Islam MR, Monsur MB, Amiruzzaman M, Fujita M, Hasanuzzaman M (2019b). Trehalose protects maize plants from salt stress and phosphorus deficiency. Plants 8(12):568.
Crossref
|
|
|
|
|
Rubio MC, González EM, Minchin FR, Webb KJ, Arreseâ€Igor C, Ramos J, Becana M (2002). Effects of water stress on antioxidant enzymes of leaves and nodules of transgenic alfalfa overexpressing superoxide dismutases. Physiologia Plantarum 115(4):531-40.
Crossref
|
|
|
|
|
Safronov O, Kreuzwieser J, Haberer G, Alyousif MS, Schulze W, Al-Harbi N, Arab L, Ache P, Stempfl T, Kruse J, Mayer KX (2017). Detecting early signs of heat and drought stress in Phoenix dactylifera (date palm). PloS ONE 12(6):1-17.
Crossref
|
|
|
|
|
Salekjalali M, Haddad R, Jafari B (2012). Effects of soil water shortages on the activity of antioxidant enzymes and the contents of chlorophylls and proteins in barley. American-Eurasian Journal of Agricultural and Environmental Science 12:57-63.
|
|
|
|
|
Sattar SA, Islam MN (2010). Char lands of Bangladesh: Their extent, management and future research needs. Presented in a workshop on Soil Fertility, Fertilizer Management and Future Strategy held at Bangladesh Agricultural Research Council, Farmgate, Dhaka 18:1-9.
|
|
|
|
|
Scandalios JG (1990). Response of plant antioxidant defense genes to environmental stress. Advances in Genetics. Academic Press 28:1-41.
Crossref
|
|
|
|
|
Shan C, Zhang S, Ou X (2018). The roles of H2S and H2O2 in regulating AsA-GSH cycle in the leaves of wheat seedlings under drought stress. Protoplasma 255(4):1257-1262.
Crossref
|
|
|
|
|
Sharma I, Ahmad P (2014). Catalase: a versatile antioxidant in plants. In Oxidative Damage to Plants. Academic Press pp. 131-148.
Crossref
|
|
|
|
|
Sharma P, Jha AB, Dubey RS (2012). Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. Journal of Botany 2012:1-26.
Crossref
|
|
|
|
|
Skadsen RW, Schulze-Lefert P, Herbst JM (1995). Molecular cloning, characterization and expression analysis of two catalase isozyme genes in barley. Plant Molecular Biology 29(5):1005-14.
Crossref
|
|
|
|
|
Trindade H, Karmali A, Pais MS (1988). One-step purification and properties of catalase from leaves of Zantedeschia aethiopica. Biochimie 70(12):1759-1764.
Crossref
|
|
|
|
|
Türkan I, Bor M, Özdemir F, Koca H (2005). Differential responses of lipid peroxidation and antioxidants in the leaves of drought-tolerant P. acutifolius Gray and drought-sensitive P. vulgaris L. subjected to polyethylene glycol mediated water stress. Plant Science 168(1):223-231.
Crossref
|
|
|
|
|
Wiegmann M, Maurer A, Pham A, March TJ, Al-Abdallat A, Thomas WT, Bull HJ, Shahid M, Eglinton J, Baum M, Flavell AJ (2019). Barley yield formation under abiotic stress depends on the interplay between flowering time genes and environmental cues. Scientific Reports 9(1):1-16.
Crossref
|
|
|
|
|
Woodbury W, Spencer AK, Stahmann MA (1971). An improved procedure using ferricyanide for detecting catalase isozymes. Analytical Biochemistry 44(1):301-305.
Crossref
|
|
|
|
|
Yamaguchi J, Nishimura M, Akazawa T (1984). Maturation of catalase precursor proceeds to a different extent in glyoxysomes and leaf peroxisomes of pumpkin cotyledons. Proceedings of the National Academy of Sciences 81(15):4809-13.
Crossref
|
|
|
|
|
Zandalinas SI, Balfagón D, Arbona V, Gómez-Cadenas A (2017). Modulation of antioxidant defense system is associated with combined drought and heat stress tolerance in citrus. Frontiers in Plant Science 8:953.
Crossref
|
|
|
|
|
Zhang H, Ni Z, Chen Q, Guo Z, Gao W, Su X, Qu Y (2016). Proteomic responses of drought-tolerant and drought-sensitive cotton varieties to drought stress. Molecular Genetics and Genomics 291(3):1293-1303.
Crossref
|
|
|
|
|
Zhang M, Fang Y, Ji Y, Jiang Z, Wang L (2013). Effects of salt stress on ion content, antioxidant enzymes and protein profile in different tissues of Broussonetia papyrifera. South African Journal of Botany 85:1-9.
Crossref
|
|
|
|
|
Zhou MX (2010). Genetics and improvement of barley malt quality, edited by Zhang G and Li C (Springer-Verlag Berlin Heidelberg, Berlin, Heidelberg), pp. 1-17.
|
|