Full Length Research Paper
ABSTRACT
This research work aimed at screening for different microorganisms associated with Irvingia gabonensis var. gabonesis Kernels, its nutritional value and detection of aflatoxins from some of the infested I. gabonensis Kernels sold in Oyo town. A total of 30 different I. gabonensis var. gabonesis Kernels were randomly purchased from six different points in the five major markets in Oyo town and isolation was done on Nutrient agar (NA), McConkey agar, Eosine Methylene Blue Agar (EMB) and Potato Dextrose Agar (PDA) using pour plate method. The isolates were culturally, morphologically and biochemically characterized. The mineral, proximate and aflatoxin detection of the I. gabonensis Kernels with high microbial load and growth of Aspergillus flavus was carried out using standard methods. A total of 25 bacteria and 18 fungal were isolated which include Bacillus spp., Staphylococcus spp., Aspergillus spp., Penicillium spp., and yeast. Irvingia gabonensis seeds with growth of A. flavus (OOW1) had the least mineral composition with 5.4% sodium, 20.0 mg/kg vitamin C, 29.4 mg/kg calcium, 0.9 mg/100g iron, 34.4 mg/100 g magnesium and 0.02 mg/100 g zinc. The I. gabonensis Kernels with the growth of A. flavus (OOW1) had the least mineral composition with 5.5% moisture content, 4.2% crude protein, 45.7% crude fat, 9.1% crude fibre and 1.5% total ash. The I. gabonensis Kernels with the growth of A. flavus (OOW4, OOJ6 and OOW1) had aflatoxin level of 3.47, 3.69 and 5.10 ppb, respectively. Irvingia gabonensis seed with high microbial load and growth of A. flavus had low nutritional value making them unsafe for consumption.
Key words: Nutritional value, kernels, mineral composition, microbial load, proximate analysis.
INTRODUCTION
Food is a vital part of the cultural identity of people all over the world. In African countries such as Nigeria, some foods are consumed during religious or cultural festivals, while condiments and soup thickeners such as melon and Irvingia gabonensis Kernels (Ogbono) are consumed as a normal culinary practice (Chibundu et al., 2016). I. gabonensis commonly known as Dikanut, Africa mango, bush mango or wild mango is an essential product that serve as a source of valuable income to both rural and urban settlers in Africa (Arowosoge, 2017). It is known in South Western part of Nigeria (Yoruba land) as “Apon” and South Eastern part of Nigeria (Igbo land) as “Ogbono”.
The tree of I. gabonensis grows well in tropical rainforests of Africa. The freshly harvested seeds are sun-dried, grinded and used as recipe for “ogbono” soup (Bamidele et al., 2015). The two known species of I. gabonensis Kernels that grow freely are the l. Gabonensis var. Gabonesis and I. gabonensis var. Excelsa. The pulp of the I. gabonesis var. Excelsa is classified as oil seed due to its high fatty matter (54 to 67%) (Bamidele et al., 2015; Akusuand and Kiin-Kabari, 2016). The kernels of I. gabonensis contain oil which is sometimes extracted and often used in the production of margarine and drugs. The residue from the extracted kernel is then used as thickening agent in soup (Arowosege, 2017). The pulp of the I. gabonensis var. gabonensis is sweet, smooth in the mouth and has brittle pulp but its kernel draws less than that of I. gabonensis var. Excelsa (Akusuand and Kiin-Kabari, 2016). I. gabonensis var. gabonensis is highly demanded due to its nutritional, economic and medicinal worth. Due to the high moisture content of the African bush mango, the best method of preserving the I. gabonensis is by sun-drying the seeds which help to extend the shelf life of the Kernels (Vihotogbé et al., 2019).
However, these Kernels of I. gabonensis have been reported to be prone to fungi attack causing the food to become tasteless, loose its thickness, nutritional value and produce mycotoxins (Sanyaolu et al., 2014; Chuku and Aggrey, 2017). Fungi are plant pathogens and major spoilage agents of foods and foodstuffs. During favorable environmental conditions, some fungal strains may release metabolites such as mycotoxins into food hence making it poisonous and unfit for human consumption (Jonathan et al., 2016). Mycotoxins are mainly produced by certain filamentous fungi belonging to Aspergillus, Penicillium and Fusarium genera. The major agro-economic important mycotoxins produce includes aflatoxins, ochratoxins, trichothecenes, zearelenone, fumonisins and tremorgenic. Aflatoxins have been observed as the most toxic because of their highly carcinogenic and hepatotoxic effect, especially Aspergillus flavus and Aspergillus parasiticus (Ubwa, 2014; Ozer et al., 2012; Menza et al., 2015).
MATERIALS AND METHODS
The study was conducted between the months of January to June, 2021. Thirty I. gabonensis var. gabonesis Kernels were randomly collected from display retailers at six different shops in the five major markets in Atiba Local Government Area of Oyo town, Oyo State. All samples were aseptically packaged and transported to the laboratory for analyses.
Enumeration, isolation and identification of microorganisms
One gram of each samples was weighed and mashed in a stomacher bag containing 9 mL of distilled water using a stomacher machine (Seward STOMACHER® 80 Lab System). One mL aliquot from the stomacher bag was pipetted and transferred into a sterile test tube containing 9 mL of 0.1% peptone water. This process was repeated for each of five sets of test tubes until a dilution of 10-6. 1 mL from the dilution 10-5 were plated in duplicate into 15 ml of sterilized and cooled Nutrient Agar (NA), Mac Conkey agar, Eosine Methylene Blue (EMB) agar and Potato Dextrose Agar (PDA). The inverted plates were incubated at 37°C for 24 hours for the bacteria isolates while the PDA plates were inverted and incubated for 5 days at 30°C for the fungal isolates. Distinct colonies were sub-cultured to obtain a pure culture. The inverted plates were incubated for 24 hours in NA, EMB and Mac Conkey agar while the un-inverted PDA plate was incubated for 5 days at 30°C after which the colonies were counted (Kidd et al., 2016). The pure colonies of the bacterial isolates were subjected to gram staining, spore staining, oxidase, catalase and starch hydrolysis. While the pure colonies of the fungal isolates were examined under the microscope after staining with lactophenol cotton blue. The isolates were identified using their morphological characteristics and microscopic structures (Tersoo-Abiem et al., 2020).
Mineral analysis
The mineral analysis of the I. gabonensis Kernels with high microbial load and growth of Aspergillus flavus were done according to the methods of AOAC (2005). A gram of each sample was digested with 10% HNO3 after ashing. The sample was filtered after digestion and the filtrate made up to 100 mL with distilled deionized water. Atomic Absorption Spectrometer (Buck Scientific East Norwalk, USA) was used to determine the concentration of iron, magnesium, zinc and calcium while Flame Photometry (Jenway Ltd, Dunmow Essex UK) was used for the determination of Na.
Proximate analysis
Determination of the proximate composition which include moisture, fats, ash, carbohydrate and protein contents of the I. gabonensis Kernels with high microbial load and growth of A. flavus were done according to the methods of AOAC (2016).
Determination of Moisture content
Moisture content of I. gabonensis Kernels samples with high microbial load and growth of A. flavus were analysed using the gravimetric method reported by AOAC, (2016). A 5 g was measured into a previously measured moisture sampler. The sample in the can was allowed to dry by air over a steam bath and then dried in the 105°C for three hours in the oven. It was cooled in a desiccator and weighed. It was then returned to the oven for proper drying. The sample was further dried, cooled and weighed until a regular weight was achieved. Weight of lost moisture content was obtained by difference and calculated as percentage of the weight of sample analyzed.
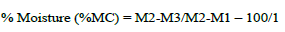
where M1 = Measurement of empty moisture can, M2 = Measurement of can + sample before drying and M3 =Measurement of can + sample after drying
Determination of Ash content
Content if the ash in I. gabonensis Kernels sample was analyzed by the furnace incineration gravimetric process (AOAC, 2005). A 5 g sample was weighed into previously weighed crucible. It was evaporated to dryness over a steam bath and then burnt in a muffle furnace at 550°C until it becomes grey ash. The ashes in the crucible were carefully removed and chill in a desiccator and weighed again. As the measurement increased, the weight of ash was obtained and expressed as percentage of the sample analyzed and calculated using the formula as shown below.
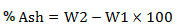
where, W1 = weight of empty crucible and W2 = weight of crucible + ash.
Determination of Protein content
Protein content was done by Greenfield and Southgate (2003) in which the total nitrogen was obtained and multiplied with the factor 6.38. 10 mL of concentrated H2SO4 and 0.5 g of the I. gabonensis var. gabonesis Kernels was boiled with selenium as a catalyst. Digestion was done in a fume cupboard until a clear mixture was achieved. This breakdown was transferred quantitatively to a standard container and diluted with 100 mL distilled water. 10 mL of the breakdown was mixed with the same volume of 45% NaOH solution and distilled in semi micro- Kjeldahl apparatus. The distillate was transferred into 10% boric acid solution and 3 drops of mixed methyl red and bromocresol green indicator. A total of 50 mL distillate was collected and titrated against 0.02 N H2SO4. Titration was done from green colouration to a deep red end- point. Blank was also treated just as described. The Normality (N2) content and protein was calculated as shown below.
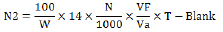
W =weight of sample, N =Normality of titrant, Vf = Total digest volume, Va = Volume of digest analyzed, T = Sample titre, BLK = Reagent Blank titre.
Determination of carbohydrate content of the produced I. Gabonensis kernels
Carbohydrate was calculated as nitrogen free extractives using the formula described by Greenfield and Southgate (2003).
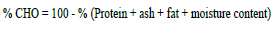
Determination of fat content of the produced ogbono samples
Fat content of I. gabonensis Kernels was carried out by measuring 0.5 g of the sample into a conical flask. 0.88 mL ammonia solution and 10 mL of 95% ethanol was added to it and mixed properly. 25 mL of diethyl ether was added and mixed properly for 1 minute. 25 mL of petroleum ether was properly mixed with it. Mixture separated into phases and after standing for 1 hour. The fat extract (ether phase) was collected and the sample was re-extracted with the same solvent and the extracts was pooled together. The extract was then transferred to a pre-weighed flask and the solvent recovered. The fat in the container was oven dried at 100°C for 30 minutes. The dried samples were cooled in a dessicator and measured. Dried sample was weighed and fat was assayed. The amount was written as a percentage of the sample analysed. It was calculated as shown below (Greenfield and Southgate, 2003).
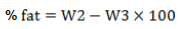
where W1 =weight of flask alone and W2 =weight of flask and extract
Aflatoxin detection and quantification
Detection of aflatoxin levels from I. gabonensis Kernels with high microbial load and growth of A. flavus was carried out by Enzyme-Linked Immunosorbent Assay (ELISA) method. 10 g of each sample was extracted with 20 mL methanol: water (70: 30). The residue was dissolved in 1 mL of methanol: water (3:1, v/v) and 200 ml of diluted extract was applied to the enzyme immuno-sorbent assay (ELISA) plate in order to determine the total aflatoxin content. Each one of the samples and standards were applied in duplicates. Testing for total aflatoxin content was carried out on each sample after the extraction process, using AgraQuant assay kit (Romer Labs) according to the manufacturer's instructions in the ELISA kit. The total aflatoxin concentration was read at 450 to 630 nm. The optical densities (ODs) were compared to those of the standards. Total aflatoxin concentration in each sample was expressed in parts per billion (ppb) (Tersoo-Abiem et al., 2020).
RESULTS
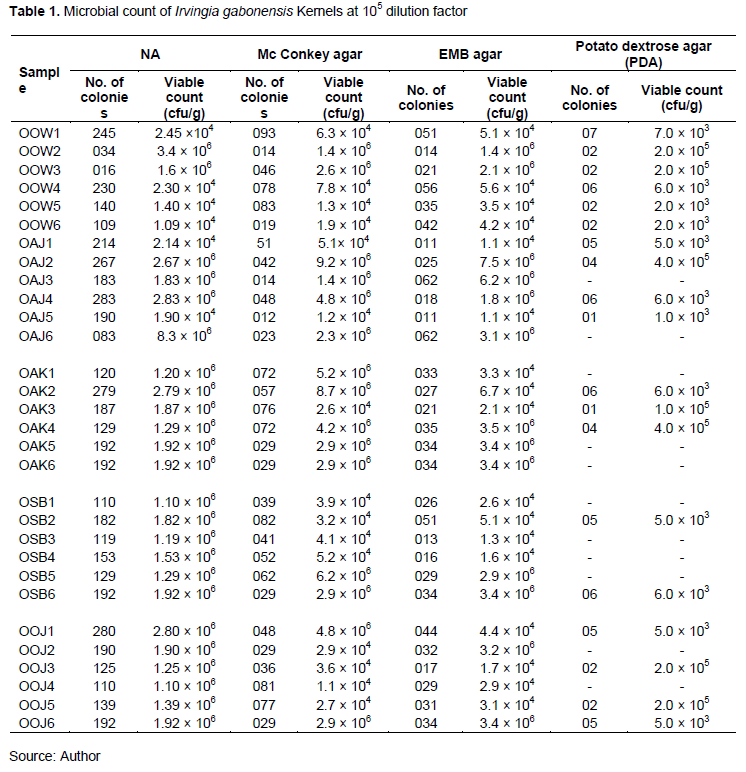
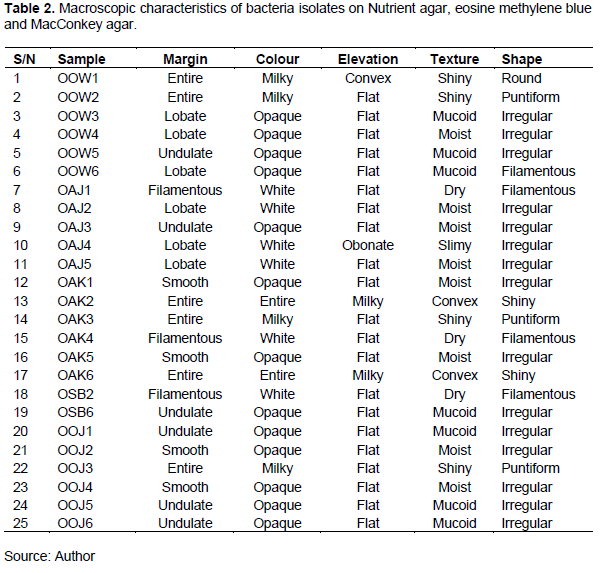
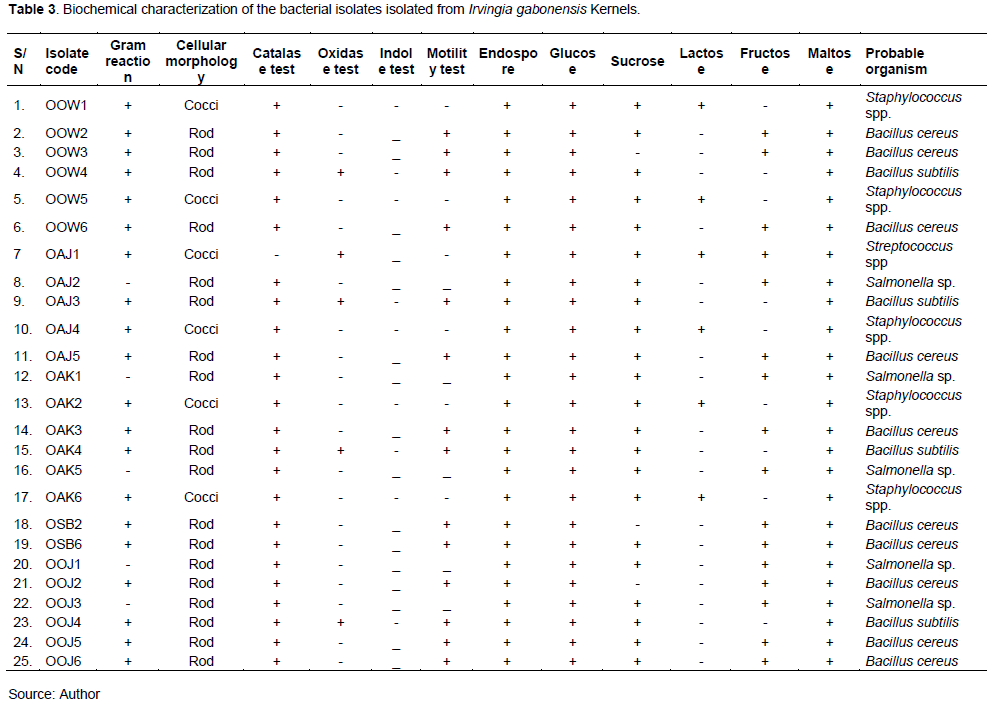
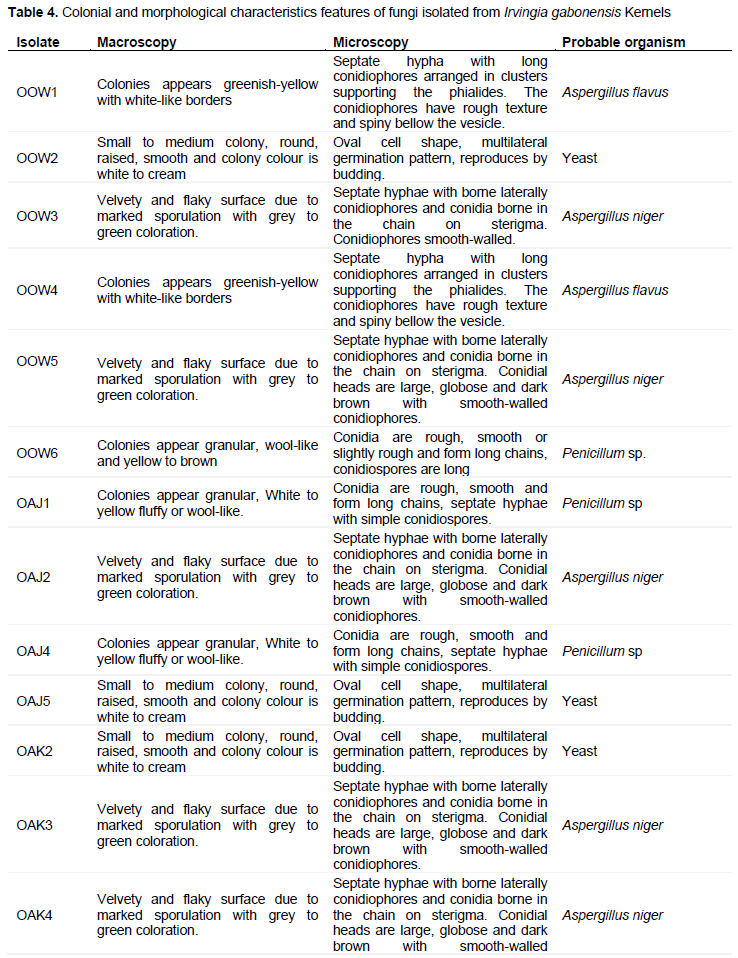
Microorganisms were enumerated from I. gabonensis Kernels samples obtained from five different markets in Oyo town is shown in Table 1. Regardless of the high dilution factor (105) used for the six samples of I. gabonensis Kernels randomly purchased from each market, the highest bacterial count was observed in OOJ1 with 2.80 x 106 cfu/g on Nutrient agar, 4.8 x 106 cfu/g on McMackey agar and 4.4 x 104 cfu/g on Eosine. Methylene Blue agar while OOW1 had high fungal count (7.0 x 103) on Potato Dextrose Agar. Tables 2 and 3 shows the macroscopic characteristics of isolates on Nutrient agar, Eosine Methylene Blue (EMB) and MacConkey Agar. A total of 25 bacteria were isolated. The bacterial isolates showed various colonial appearances on Nutrient agar ranging from smooth surfaces, raised elevation, circular shaped, mucoid colony, pigmented, translucent, opaque, shiny colony, large, medium colonies. Five of the isolates were Gram negative rod while the remaining 20 were Gram positive (4 Gram positive cocci and 21 Gram positive rod). Most of the isolates were indole negative, catalase positive and spore formers. The bacteria isolates were biochemically identified as Bacillus cereus, Bacillus subtillis, Staphylococcus spp., Staphylococcus spp. and Salmonella sp.
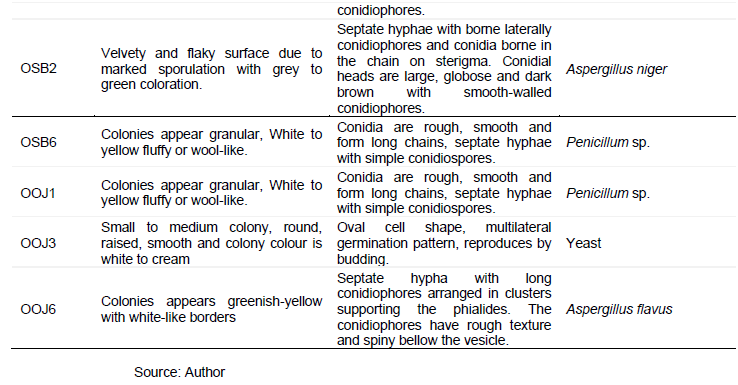
Figure 1 shows the percentage frequency of occurrence of bacteria isolated from I. gabonensis. Table 4 shows the colonial and morphological characteristics features of fungi isolates on PDA. A total of eighteen fungi were isolated. The fungal isolates showed different cultural appearances such as a velvety and flaky surface with grey to black coloration, White and green variants powdery surface growth, dust-like sporulating surface light brown with smooth border, abundant mycelium with pale brown and dark zonation, white air mycelium with quick differentiation, upper side white color with irregularly smooth and fringed. The fungal isolates were identified as Aspergillus flavus, Aspergillus niger, Penicillium sp., and yeast. The percentage frequency of occurrence of fungal isolated from I. gabonensis is shown in Figure 2. 17% of the fungal isolates were A. flavus, 33% were A. niger, 28% were Penicillium sp., while yeast had 22%.
Mineral composition of the Irvingia gabonensis Kernels
Mineral composition, proximate analysis and the detection of aflatoxin were determined using three samples of I. gabonensis Kernels due to their high microbial load and the presence of A. flavus in the karnel. The I. gabonensis Kernels without the growth of A. flavus (OAJ3) was used as control. Table 5 shows the mineral composition of I. gabonensis Kernels. The I. gabonensis Kernels without the growth of A. flavus (OAJ3) had the highest mineral composition as follows 7.5% sodium, 28.2 ppm vitamin C, 87.4 ppm potassium, 37.9 ppm calcium, 13.4 mg/100g iron, 49.9 mg/100g magnesium and 0.03 mg/100g zinc) compare to I. gabonensis Kernels with the growth of A. flavus. Sample OOW1 had the least mineral composition with 5.4% sodium, 20.0 ppm vitamin C, 53.6 ppm potassium, 29.4 ppm calcium, 0.9 mg/100g iron, 34.4 mg/100g magnesium and 0.0.2 mg/100g zinc. However, there are no heavy metals present in any of the samples analyzed.
Proximate composition of I. Gabonensis Kernels
Table 6 shows the mineral composition of I. gabonensis Kernels. The I. gabonensis kernel without the growth of A. flavus (OAJ3) had the 16.3% carbohydrate content, 5.5% moisture content, 7.8% crude protein, 58.9% crude fat, 10.7% crude fibre and 1.8% total ash, 47.1 kg/100g fatty acids with 2589.0 kg/100g metabolized energy. Sample OOW1 had the least mineral composition with 29.9% carbohydrate, 5.5% moisture content, 4.2% crude protein, 50.7% crude fat, 9.1% crude fibre, 1.5% total ash, 40.6 kg/100 g fatty acids and 2340.3 kg/100 g metabolized energy.
Presence of aflatoxin in the I. gabonensis kernels
Aflatoxin level of the I. gabonensis Kernels is shown in Table 7. The I. gabonensis Kernels without the growth of A. flavus (OAJ3) had the no aflatoxin, The I. gabonensis Kernels with the growth of A. flavus (OOW4, OOJ6 and OOW1) had aflatoxin level of 3.47, 3.69 and 5.10 ppb, respectively.
DISCUSSION
The bacterial isolates obtained from this study is similar to the study of Adebayo-Tayo et al. (2006) and Adegbehingbe et al. (2014) who isolated similar bacteria from seeds of I. gabonensis. The fungal isolated from this research include Aspergillus flavus, Aspergillus niger and Penicillum sp. Which is in line with the work of Chibundu et al. (2016). Tersoo-Abiem et al. (2020) also isolated the similar fungal strains from Ogbono samples that were obtained from different markets in Benue states.
The source of microbial contamination in samples could be due to the environment in which the Ogbono samples were sold. Ekundayo et al., (2003) isolated some pathogenic microorganisms from his research and he opined that some of the Ogbono samples could have been stored for a very long period before selling. The presence of Staphylococcus aureus and Bacillus sp. Indicate a potential risk and could be harmful to humans when ingested, due to their ability to produce toxins (Saliu, 2008). Bacillus sp. is normally found in the soil and may have been transported via vegetables. The consumption of these organisms in the Ogbono samples in large numbers could lead to gastrointestinal illness.
The occurrence of S. aureus which are Gram positive cocci, catalase-positive, coagulase-positive,oxidase-negative and facultative anaerobes in most of the samples strongly indicated a high level of poor personal hygiene by the sellers. Although S. aureus is often associated with the skin and mucous glands (especially in the nose of healthy persons) as commensals (Ibrahim, 2017). Since the market is a busy place, particulate matter carrying microorganisms may have been deposited and unhealthy practices carried out in the market could put the unsuspecting public at massive potential risk with strong public health concern of food poisoning.
Mineral composition
The mineral composition of the ogbono samples without the growth of Aspergillus flavus (OAJ3) used as a control had high mineral composition compared to ogbono samples with the growth of Aspergillus flavus which is similar to the work of Oseni and Ekperigin (2007). This result is also line with the work of Ibrahim et al. (2017) and Mgbemena et al. (2019) who recorded higher amount of iron and calcium in Ogbono seeds. They also recommended that the seed of Ogbono seed should be consumed due to the high iron content. Mineral content in food is a measure of the amount of specific inorganic components present within the food. Minerals act as co-factors for enzyme reactions. Sodium, calcium and magnesium are required in major quantities. Sodium acts as charge carriers and is a major factor in extra cellular fluid. It also participates in the functioning of muscle nerve (Mgbemena et al., 2016). Sodium and potassium are needed to help maintain the pH of the body so as to regulate muscles and nerves irritability as well as osmotic balance of the body fluids. Iron content in OAJ3 is higher. This is required for blood formation and normal functioning of the central nervous system. Vitamin C is higher in OAJ3 than other samples. It is a fat-soluble vitamin that serves as a good antioxidant, for healthy vision, skins and other tissues in the body (Onojah et al., 2018).
OOW4, OOJ6 and OOW1 had least mineral composition which could be due to the high microbial load, presence of enteric bacteria and toxin producing microorganisms which made it unfit for consumption (Ezekiel et al., 2016).
The proximate composition of I. gabonensis Kernels
Most of the I. gabonensis Kernels samples with high microbial load were observed to have high moisture content. The carbohydrates and moisture content ranged from 15.3% to 30.0% and 4.5 to 5.5%. Sample OAJ3which is the control has the high moisture content compare to OOJ6 and OOW1 which could be the reason
why the microbial load is higher. The moisture content is however within the range value of most seeds and legumes (Onojah et al., 2018). This is in line with the research of Brooker (2005) who suggested that high moisture content in fruits is an index of its water activity, measure of stability and susceptibility to microbial contamination.
The crude fibre percentage ranged from 10.7 to 9.1%. Sample OAJ3 had the highest crude fibre while OOW had the least, these results are similar to the report of Onojah et al. (2018) who recorded 10.4% crude fibres from Ogbono samples. It is however lower to the result of Aremu et al. (2005) were 15.2% was reported from Bambara groundnut. The intake of dietary fibre can lower cholesterol level, risk of coronary heart disease, diabetes and hypertension (Ramola and Raw, 2003).
The total ash content of I. gabonensis Kernels ranged from 1.5 to 1.8%. Sample OAJ3 was higher than OOW4, OOJ6 and OOW1. The result obtained is related to the work of Efosa et al. (2017). Ash content in food is the inorganic residue left after the removal of moisture and organic matter. It provides the measure of the total amount of minerals within a food. Crude fiber contains indigestible cellulose which helps to absorb water, provide roughage and better functioning of the alimentary system.
The crude fat content of I. gabonensis Kernels ranged from 58.9 to 45.7%. The fat in sample OAJ3 was higher than that of OOW1. This could be due to the infestation of microorganisms in the Kernels. The high value of the crude fat in OOJ3 suggests that the Kernels may be a source of vegetable oil for industrial uses.
The crude protein in I. gabonensis Kernels samples ranged from 4.2 to 7.8%. The crude protein value for Sample OAJ3 is higher than the ogbono samples obtained from OOW4, OOJ6 and OOW1. The crude protein value of OOW1 is low compared to some commonly consumed plant protein in Nigeria and this does not qualify the seed as a protein rich food. The lowvalue obtained could also be as a result of the long storage period before been purchased for this research (Onojah et al., 2018). Protein contents contribute positively to the requirement for biomolecules needed for repair and maintenance of the body tissues as well as synthesis of vital hormones for the body (Soetan et al.,
2010).
The calculated fatty acid value for I. gabonensis Kernels ranged from 47.1 to 36.6 kg/100g. The results suggest that oil gotten from samples OAJ3, OOW4 and OOJ6 are suitable as edible oil and can be used for industrial purposes. Sample OOW1 had lower fatty acid value due to the presence of some pathogenic microorganisms in the Kernels.
The calculated metabolized energy ranged from 2589.0 to 2340.3 kg/100g which shows that the sample have good concentration of energy.
The detection of aflatoxins in the I. gabonensis Kernels reveals the production of toxins by A. flavus. Aspergillus species such as A. flavus and A. parasiticus, these doubles as the most notorious fungi commonly isolated from I. gabonensis seed due to their high potential for producing aflatoxins (Osibona et al., 2018). Several factors such as moisture contents, high relative humidity, temperature, substrate composition and the presence of competing microorganisms influenced mold growth on the seeds (Adebayo-Tayo et al., 2006). The environmental conditions in some part of Nigeria favours the growth of fungi and aflatoxin production in foods. Some measures of precautions should be taken when handling and processing dry foods. The growth of molds on I. gabonensis seeds is a pointer to the potential health risk associated with its consumption (Osibona et al., 2018). In Nigeria, several foods including nuts, cereals, dry fish, spices, and melon seeds among other food substances, are susceptible to contamination with aflatoxins due to the critical conditions such as temperature and humidity which is known to favour the growth of aflatoxin-producing molds (Ubwa et al., 2014, Chigoziri and Ekefan, 2013).
CONCLUSION
This study revealed different microorganisms associated with I. gabonensis Kernels sold in different markets within Oyo Town, Oyo State, Nigeria. Microorganisms isolated include Bacillus cereus, Bacillus subtillis, Staphylococcus spp. Staphylococcus spp., Enterobacteriaceae, Aspergillus flavus, Aspergillus niger, Penicillium spp and yeast. The I. gabonensis seeds with the growth of A. flavus had low mineral composition and proximate value. Although, the total aflatoxin levels of the samples analysed were below the maximum acceptable limits specified by International Regulatory Agencies in food and agricultural products (20 ppb), frequent and prolonged intake of the I. gabonensis Kernels could result in health hazards and reduced the economic value of the food. This study showed that some of the I. gabonensis Kernels samples used had been stored for a longer period. Inadequate elimination of moisture and exposure to dirty environment (markets), made them lose some of their nutrients as well as minerals.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interest.
REFERENCES
|
Adebayo-Tayo BC, Onilude AA, Ogunjobi AA, Gbolagade JS, Oladapo MO (2006). Detection of fungi and aflatoxin in shelved bush mango seeds stored for sale in Uyo, Eastern Nigeria. African Journal of Biotechnology 5(19):1729-1732 |
|
|
Adegbehingbe KT, Adetuyi FC, Akinyosoye FA (2014). Effect of fermentation on nutrient and antinutrient contents of ground-cooked lima bean (Phaseolus lunatus) seeds using Bacillus subtilis and Bacillus pumilus. British Microbiology Research Journal 4(11):1285-1298. |
|
|
Akusuand OM, Kiin-Kabari DB (2016). Effect of frozen storage on the chemical stability, functional and sensory properties of 'ogbono' (Irvingia garbonensis var. excelsa) and 'egusi' (Citrullus vulgaris Schrad) seed flours. American Journal of Food Science and Technology 4(1):20-24. |
|
|
Association of Official Analytical Chemists (AOAC) (2016). Official Methods of Analysis, 20th Ed. Washington DC, USA, 120 p. |
|
|
Association of Official Analytical Chemists (AOAC) (2005). Official Methods of Analysis, 18th ed. Washington, DC, USA. |
|
|
Arowosoge OGE (2017). Marketing and utilization of Irvingia kernels 'ogbono' in Ado-Ekiti metropolitan area of Ekiti State. Journal of Agriculture and Ecology Research International 13(1):1-10. |
|
|
Azuonwu O, Azuonwu CT, Ndah A (2019). Isolation and identification of potential high risk pathogens from blenders used in grinding some food stuffs in a local community market in rivers state: a public health concern. Journal of Microbiology and Experimentation 7:4. |
|
|
Bamidele OP, Ojedokun OS, Fasogbon BM (2015). Physicochemical properties of instant ogbono (Irvingia gabonensis) mix powder. Food Science and Nutrition 3(4):313-318. |
|
|
Brooker DJ (2005). Quality assurance for corn, wheat flour tortilla manufacturing, AACC International, Inc. Elsvier Inc., pp. 97-123. |
|
|
Chibundu NE, Micheal S, Yinka S, Foluke IO, Stella UN, Afeez TB, Rudolf K (2016). Mold and mycotoxin exposure assessment of melon and bush mango seeds, two common soup thickeners consumed in Nigeria. International Journal of Food Microbiology 237:83-91. |
|
|
Chigoziri E, Ekefan EJ (2013). Seed borne fungi of Chilli Pepper (Capsicum frutescens) from pepper producing areas of Benue State, Nigeria. Agriculture and Biology Journal of North America 4(4):370-374. |
|
|
Chuku EC, Aggrey H (2017). Fungi Associated with Seeds of Irvingia gabonensis is and their Effect on Shelf Life. International Journal of Agriculture and Earth Science 3(8):69-74. |
|
|
Efosa GE, Obosa EE, Usunomena U (2017). Proximate composition, mineral content and amino acid profile of Irvingia gabonensis O'Rorke Bail leaf. International Journal of Scientific World 5(1):23-27. |
|
|
Ekundayo FO, Oladipupo OA, Ekundayo EA (2003). Studies on the effects of microbial fermentation on Bush Mango (Irvingia gabonensis) seed cotyledons. African Journal of Microbiology Research 7:4363-4367. |
|
|
Ezekiel CN, Sulyok M, Somorin Y, Odutayo FI, Nwabekee SU, Balogun AT, Krska R (2016). Mold and mycotoxin exposure assessment of melon and bush mangoseeds, two common soup thickeners consumed in Nigeria. International Journal of Food Microbiology 237:83-91. |
|
|
Greenfield DTA, Southgate DAT (2003). Food composition data, production, management and use, 2nd ed. Rome, FAO. 5-199. |
|
|
Ibrahim HO, Osilesi O, Adebawo OO, Onajobi FD, Karigidi KO, Mohammed LB (2017). Nutrients compositions and phytochemical contents of edible parts of Chrysophyllum albidum fruit, Journal of Nutrition and Food Sciences 7(2):1-9. |
|
|
Jonathan SG, Adeniyi MA, Asemoloye MA (2016). Fungal Biodeterioration, Aflatoxin Contamination, and Nutrient Value of (Suya Spices). Hindawi Publishing Corporation Scientifica. |
|
|
Kidd S, Halliday C, Alexiou H, Ellis D (2016). Descriptions of medical Fungi. 3rd edition. Newstyle printing, South Australia. |
|
|
Menza NC, Muturi WM, Kamau ML (2015). Incidence, Types and Levels of Aflatoxinin Different Peanuts Varieties Produced in Busia and Kisii Central Districts, Kenya. Open Journal of Medical Microbiology 5(4):209-221. |
|
|
Mgbemena NM, Ilechukwu I, Okwundolu FU, Chukwurah JO, Lucky IB (2019). Chemical composition, proximate and phytochemical analysis of Irvingia gabonensis and Irvingia wombolu peels, seed coat, leaves and seeds. Ovidius University Annals of Chemistry 30(1):65-69. |
|
|
Mgbemena NM, Obodo GA, Anusiem ACI, Ezigbo CI, Ilechukwu I, Mgbo VO, Enoo O (2016). Fundamental aspects of chemistry, M. and J. Grand Orbit Communications Port Harcourt Nigeria pp. 67-72. |
|
|
Onojah PK, Musa F, Ugwuowo EN (2018). Comparative Studies on the Proximate Composition and Anti-Nutrient Content of the Cotyledons of Two Species of Irvingia (Ogbono) Sold in Anyigba Main Market, Kogi State, Nigeria. Journal of Chemical Society of Nigeria 43(2):98-107. |
|
|
Oseni OA, Ekperigin M (2007). Studies on biochemical changes in maize wastes: Fermented with Aspergillus niger. Biokemistri 19(2):75-79. |
|
|
Osibona AO, Ogunyebi OO, Samuel TO (2018). Storage fungi and mycotoxins associated with stored smoked catfish (Clarias gariepinus). Journal of Applied Sciences and Environmental Management 22(5):643-646. |
|
|
Ozer H, Basegmez HO, Ozay G (2012). Mycotoxin risks and toxigenic fungi in date, prune and dried apricot among Mediterranean crops. Phytopathologia Mediterranea 51(1):148-157. |
|
|
Ramola P, Raw PU (2003). Dietary fibre content of fruit and leafy vegetables. Nutrition News 24(3):1-6. |
|
|
Saliu JK (2008). Effect of smoking and frozen storage on the nutrient composition of some African fish. Advances in Natural and Applied Sciences 2(1):16-20. |
|
|
Sanyaolu AA, Adeniyi AA, Adedotun OA (2014). The Effect of post harvestmyco-deterioration on the proximate composition of Irvingia gabonensis seeds. International Journal of Phytopathology 3(1):41-48. |
|
|
Soetan KO, Olaiya CO, Oyewole OE (2010). The importance of mineral elements for humans, domestic animals and plants; a review. African Journal of Food Science 4(5):200-222. |
|
|
Tersoo-Abiem EM, Mnguchivir E, Onyejeche IB, Gwadza PM (2020). Fungal and Aflatoxin Contamination of Smoke Dried Catfish and African Bush Mango Seeds (Ogbono) Sold in Markets in Selected Processing Zones in Benue State, Nigeria. European Journal of Nutrition and Food Safety 12(9):43-51. |
|
|
Ubwa ST, Abah J, Atu BO, Tyohemba RL, Yande JT (2014). Assessment of total aflatoxins level of two major nutsconsumed in Makurdi Benue State, Nigeria. International Journal of Nutrition and Food Sciences 3(5):397-403. |
|
|
Vihotogbé R, Raes N, van den Berg, RG, Sinsin B, Sosef MS (2019). Ecological niche information supports taxonomic delimitation of Irvingia gabonensis and I. wombolu (Irvingiaceae). South African Journal of Botany 127:35-42. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0