ABSTRACT
In the study reported herein, we aimed to isolate a trypsin inhibitor from Ricinus communis leaves through chromatographic and spectrometric techniques and evaluate its toxic effects on the development of Spodoptera frugiperda larvae. Plant extracts were submitted to fractionation in adsorption column. The fraction 10, which showed the highest inhibitory activity, were incorporated into an artificial diet at the concentrations of 0, 0.06, 0.12, 0.25 and 0.5%, and offered to S. frugiperda larvae. Fresh weight of larvae, food consumed and weight of eliminated faeces were registered. Based on these parameters the following nutritional index were calculated: Relative Consumption Rate (RCR), Relative Metabolic Rate (RMR), Relative Growth Rate (RGR), Approximated Digestibility (AD), Efficiency of Ingested Food Conversion (EIC), Efficiency of Digested Food Conversion (EDC) and the Metabolic Cost (100 - EDC). The inhibitor at 0.5% concentration was deleterious to S. frugiperda, extending the larval stage in 11 days, with higher RCR and ECD, and lower RGR, ECI and ECD. Therefore, the trypsin inhibitor from leaves of R. communis affected the S. frugiperda larval development, being promising in studies of alternative and sustainable control methods for lepidopteran pest species.
Key words: Castor beans, enzymatic inhibition, integrated pest management, plant defense against herbivory.
Abbreviation:
RCR, Relative consumption rate; RMR, relative metabolic rate; RGR, relative growth rate; AD, approximated digestibility; EIC, efficiency of ingested food conversion; EDC, efficiency of digested food conversion.
The fall armyworm, Spodoptera frugiperda (Smith, 1797) (Lepidoptera: Noctuidae), is one of the most important insect pest of maize in Brazil, attacking the plant at different growth stages, when the larvae burrow into the plant whorl causing severe defoliation and 37% loss in production (Cruz, 2002). Due to the polyphagous feedingbehavior, fall armyworm may attack and damage many other agricultural crops such as rice, sorghum, cotton, soybean and many others (Alvarez et al., 2009). Current control of S. frugiperda populations relies mainly on spraying chemical insecticides, which have been overused in recent years, resulting in insect resistance (Yu et al., 2003), environmental contamination (Starner and Goh, 2012) and affecting the human health (Loewenherz et al., 1997). Due to those factors, organic cultivation has been increased, replacing the use of chemical insecticides by alternative methods of pest control (Uchino et al., 2015). A promising alternative to control fall armyworm is the use of plants secondary metabolites (Tavares et al., 2009; Alves et al., 2014). These chemicals compounds produced by plants can induce deleterious effects on insects such as weight loss, fecundity and fertility reduction, increasing duration of immature stages, feeding deterrence, ultra structural modification on tissues, changes in some nutritional parameters, and inhibition of digestive enzymes, which is lethal to some insects (Malau and James, 2008; Nathan et al., 2008; Correia et al., 2009). The use of proteinase inhibitors (PIs), a class of substances involved in plant defense is an example of these new alternative. Levels of PIs in plant leaves are usually low and can be increased to high levels if the plants are attacked by insects, suffer mechanical damage or exposed to plant hormones (Rakwal et al., 2001).
Plant proteinase inhibitors forms and complex structures in which proteolysis is limited and extremely slow (Tiffin and Gaut, 2001). Proteinase inhibitors also causes an amino acid deficiency in insects influencing growth, development and eventually causing its death by inhibiting gut proteinases or due to a massive over-production of the digestive enzymes, reducing the availability of essential amino acids for the production of other proteins (Jongsma and Bolter, 1997; Pompermayer et al., 2001). Based on these facts, Ricinus communis L. (Euphorbiaceae) was highlighted as a promising plant to be used on integrated pest management. Santiago et al. (2008) observed bioactivity of aqueous extract from green fruits of R. communis when added to an artificial diet, reducing the period of immature stages and the larvae weight of S. frugiperda. Bigi et al. (2004) identified toxic compounds from R. communis to the leaf cutting ant Atta sexdens rubropilosa (Forel, 1908) (Hymenoptera: Formicidae) and concluded that the metabolites responsible for this activity were mainly the fat acids and the ricinin. Consequently, the aim of this study was to isolate a trypsin inhibitor from leaves of R. communis and evaluate its effect on the development of S. frugiperda larvae.
Insect rearing
The experiment was conducted in a climatized room at 25 ± 2°C with 12 h photofase and 70 ± 10% relative humidity. The insects were reared on the artificial diet developed by Karster-Junior et al. (1978).
Collection of the plant and preparation of extracts
Leaves of R. communis were collected in a field located in Lavras County (21°13’29.73’’ S; 49°58’43.93’’ W) state of Minas Gerais, Brazil, and the voucher specimen was deposited in the herbarium of the Federal University of Lavras (UFLA). All of the plant extracts were prepared in the Biochemistry laboratory at UFLA. Leaves, without petiole, central and secondary nervures, were cut in 1 cm² pieces, and placed on an Erlenmeyer with ethanol (pa > 99.8%) respecting the proportion of 1:7 (weight of leaves/volume of extractor). The remained moisture was stored away from the light for 20 days, and filtered after this period in a Büchner funnel with filter paper and a rotary evaporator connected to a vacuum pump. After filtration, the extract was oven-dried at 45°C to eliminate all of the solvent.
Chromatographic conditions and enzyme inhibition assays
Chromatographic analysis was performed using a 50 cm long column, and 2.5 cm inner diameter packed with silica Kieselgel 60 (0.040 to 0.063 mm; 230 to 400 mesh) Merck®. The eluents were hexane, chloroform, ethyl acetate, ethanol, methanol and acetic acid. All the fractions collected showed different volume (around 100 mL) and the solvent exchange occurred based on the observations of spots in the column. Seventeen fractions were obtained from this column, which were concentrated in rotary evaporator until complete eluent evaporation. All fractions were resuspended separately in 5 mL of ethanol and subjected to trypsin inhibition in vitro testing. Inhibitor activity was determined as described by Kakade et al. (1974) by the comparison between a kinetic assay of trypsin activity in the inhibitor presence and absence, over four time intervals (Erlanger et al., 1961). Results, obtained in three replicates, were expressed in milliunits of inhibited trypsin (mUIT). This unit represents the amount of light absorbed by 1 nMole p-nitroanilide produced from the trypsin activity on N – alpha – benzoyl – DL – arginine - p - nitroanilide (BapNA) substrate after one minute of reaction with 1 g leaves of R. communis. Fifth instar larvaes of S. frugiperda were immobilized on ice and dissected. Midgut was removed and macerated in a Potter homogenizer in the proportion of 1 midgut to 4 mL of water at 4°C. The homogenized solution was filtered through 100 µm nylon mesh and centrifuged for 10 min at 14,000 x g (4°C). Supernatant was used in the inhibitor activity assays. The fraction that exhibited higher trypsin inhibition in in vitro tests was submitted to chromatographic separations, which were achieved using a High Performance Liquid Chromatographer Shimadzu LC 20A, equipped with an automatic injector (injection volume 20 µL) and a UV-VIS detector. A Agilent – Zorbax Eclipse AAA-C18 column (150 mm × 4.6 mm containing 5 μm spherical particles) and an Agilent – Zorbax Eclipse AAA pre-column (12.5 × 4.6 mm, 5 μm) were used. Oven temperature was 45°C. Analyses were realized through elution gradient, in which Eluent A (Metanol HPLC grade) was added in the eluent B (Water MiliQ) by a linear gradient (20 to 100% in 45 min), returning to initial concentration in the last 5 min. Fractions with 1 mL of volume were collected at every minute in glass tubes for 50 min. Fifty fractions were obtained and used in trypsin inhibition assays following Kakade et al. (1974). Control assays were performed to avoid variation caused by different eluent concentrations. These assays were driven through the injection of ethanol (solvent used to solubilize the inhibitor) in the HPLC in the same conditions of the samples. Fractions that showed enzyme inhibition were injected in a mass spectrometer. Mass spectrometry was performed using Photo-Fenton UV/H2O2 process. The spectrawere obtained in the negative mode in a mass spectrometer model LC/MS Trap Agilent 1100 under the following conditions: air flow 5 µL/min; capillary voltage of -3500 V; dry temperature of 325°C; and nebulizer pressure 10 psi. The analysis was carried in triplicates.
Assay with larvae of caterpillars of S. frugiperda
Fraction obtained were also utilized in vivo bioassays with larvae of S. frugiperda. The semi-purified inhibitor (preparative HPLC) was added in the artificial diet (Kasten-Junior et al., 1978), at the concentrations of 0, 0.06, 0.12, 0.25 and 0.5% (mass/volume). Diets were weighted (5 g) and offered to five-days-old larvae in a closed individual acrylic recipient. Bioassay was conducted in a complete randomized design (CRD) with six replicates per treatment. Each replicate contained six individually reared larvae totalizing 36 larvae per treatment. The biological characteristics evaluated were: larvae weight each three days, which were used to develop a growth curve; larvae survival and length of larval and pupae stages. The amount of food consumed was calculated with a correction factor for the water losses ([1-a/2][W-(L+bL)]), where: e = initial diet weight; b = aliquot average loss; W = introduced diet weight; L = non-eaten food weight (Cohen, 2004). Growth curve values were analyzed by the DRC statistical package of R® software (R Development Core Team, 2009), using the logistic model and lack-of-fit test. Amount of food consumed, larvae weight, pupae weight, and length of larval and pupae stages were submitted to Shapiro-Wilk’s test, using the Mvnormteste package of R® software ® (R Development Core Team, 2009. Data were submitted to Analysis of Variance (ANOVA) and Scott-Knott test, using the Laercio package of R® software ® (R Development Core Team, 2009).. The mortality data were evaluated counting the number of dead individuals during the assay period and corrected by the Abbott’s formula ([M = me - mb/1 - mb] where: M = Mortality, me = Treatment mortality, mb = Control mortality).
Chromatographic and spectrometric analyses
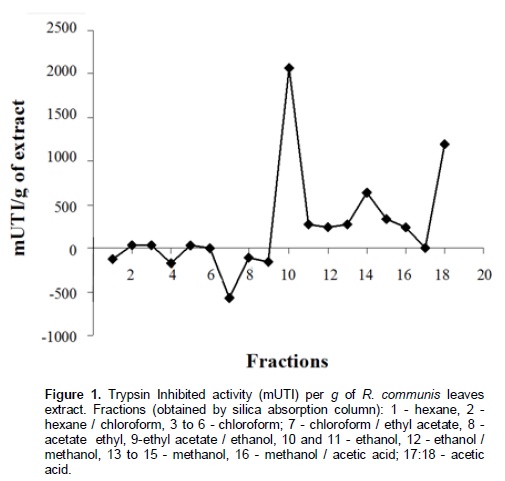
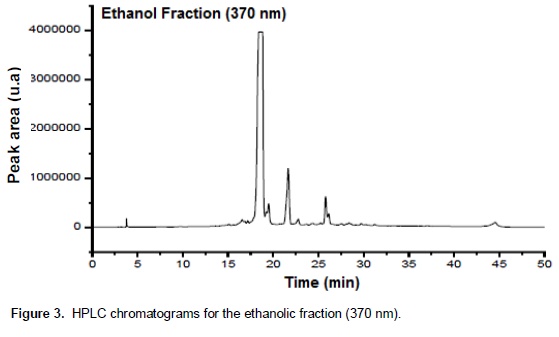
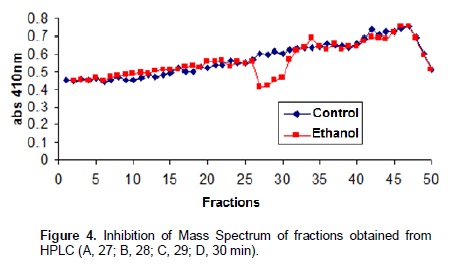
Chromatographic fractions soluble in hexane, chloroform and ethyl acetate (fractions from 1 to 9) showed no activity in the trypsin inhibition tests. The fraction 7, corresponding to the eluent chloroform/ethyl acetate, had one activity peak and the fractions soluble in ethanol, methanol and acetic acid (fractions 10 to 18) showed trypsin inhibition. The higher inhibition activity was registered at the 10th ethanol fraction (ethanol – 10), which was selected for HPLC analysis and in vivo bioassays (Figure 1). Fractions originated from HPLC retention time of 26 to 29 min (Figures 2 and 3) inhibited trypsin from the S. frugiperda digestive system. Aiming to decrease the influence of eluents in the trypsin inhibition analysis, a control test containing only ethanol was injected in the HPLC. All mass spectra (Figure 4) were obtained from the HPLC fractions (26 to 29 min) that inhibited tryspin from the lepidopteran digestive system. Mass spectrometry did not allow quantifying the inhibitor mass. However, repeated peaks related with trypsin inhibition were identified in all mass spectra.
Biological assay
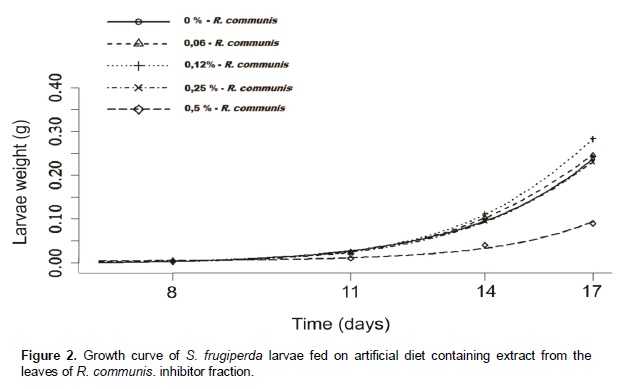
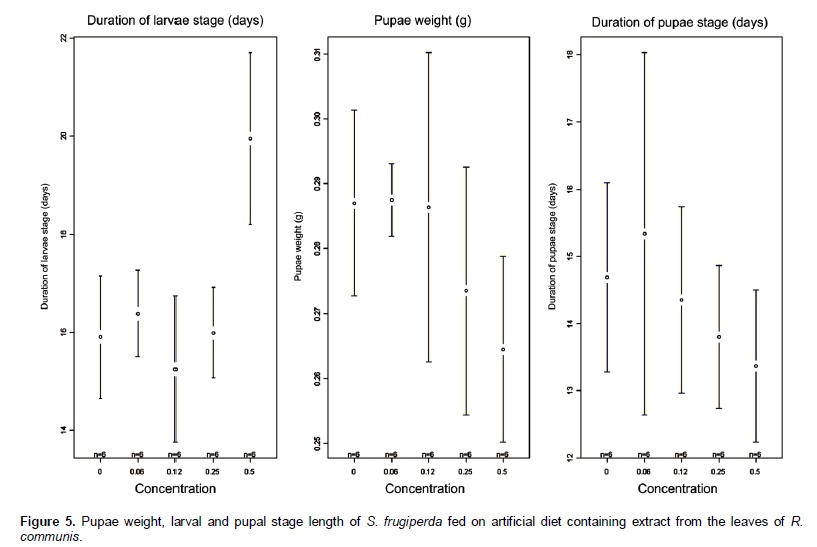
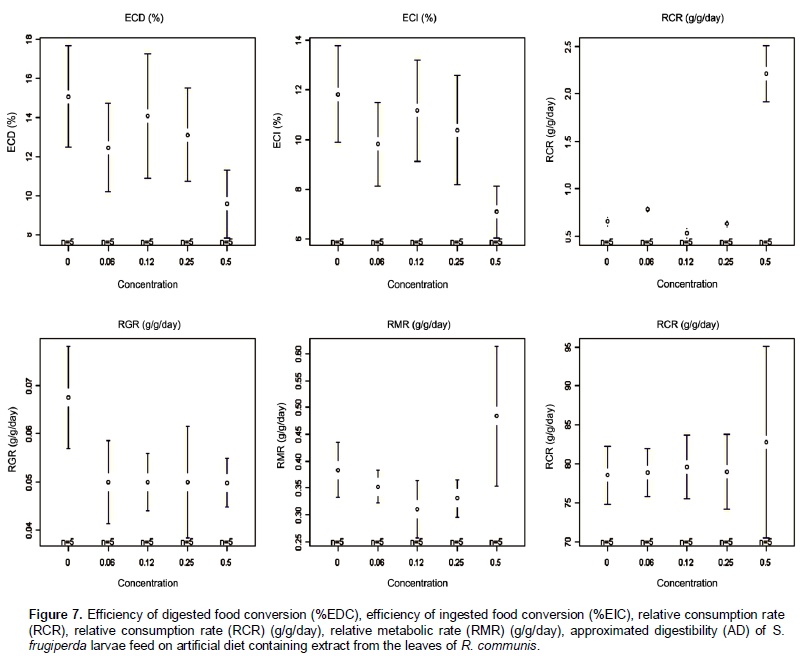
Castor oil trypsin inhibitor showed toxic characteristic to S. frugiperda larvae only at the concentration of 0.5%. Insects fed with 0 and 0.06% of inhibitor developed normally. In the treatments with inhibitor concentrations at 0.12 and 0.25% the larvae reached its maximum weight at the 23rd day, whereas in the treatment with inhibitor concentration at 0.5% the larvae development was problematic, extending the larval stage and decreasing weight average (Figure 5). In the treatment with inhibitor concentration at 0.5%, larval stage was ten days higher than the control treatment. Rates of larvae and pupae survival were not affected by the treatments, ranging from 63.3 to 87.7 and 12.0 to 14.4 days, respectively (Figure 6). Among the nutritional indexes evaluated (Figure 7), 0.5% of inhibitor concentration resulted in higher relative consumption rate (RCR), lower relative growth rate (RGR), efficiency of ingested food conversion (EIC) and efficiency of digested food conversion (EDC). Approximated digestibility (AD) and relative metabolic rate (RMR) showed no significant differences among treatments (Figure 7). The Abbott formula (Abbott 1925) of efficacy for the concentrations of 0.5, 0.25 and 0.12% provided values of 25, 12 and 4.17%, respectively. The concentration of 0.06% showed no efficiency.
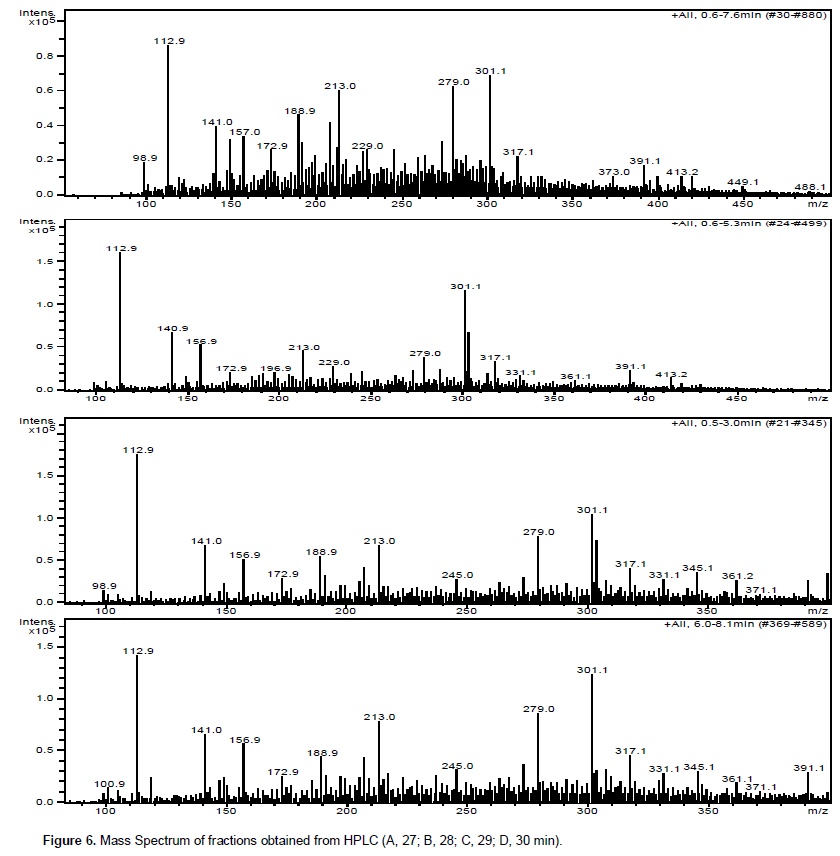
Trypsin inhibitors are extensively studied in several aspects aiming biochemistry characterization, three dimensional structures, interactions with multiple protease classes and effects on pests and pathogens (Abd El-Latif, 2014; Kuwar et al., 2015; Pontual et al., 2014; Zhu-Salzman and Rensen, 2015). Previous works performed with castor oil leaves suggested that trypsin inhibitor is a non-proteic organic molecule (Rossi et al., 2012) differing from other trypsin inhibitors described in literature. Precipitations with acetone and saturated ammonium solution, high temperature exposition and inhibition assays in the presence of de β-mercapto-ethanol were performed demonstrating the molecule non-proteic characteristic and suggesting different ways to extract and purify this trypsin inhibitor. Aiming to isolate the inhibitor, two chromatographic methods were utilized in the present study. Previously, adsorption chromatographywith silica was carried out to separate the organic compounds present in the extract, based on the molecule polarity. Subsequently all fractions obtained were subjected to in vitro analysis. Trypsin from the S. frugiperda digestive system was inhibited by the ethanol and methanol fractions. Several inhibition peaks were observed (Figure 1), suggesting the presence of more than one trypsin inhibitor in castor oil leaves. A trypsin activator molecule was also detected in the fraction chloroform/ethyl acetate. Due to the higher trypsin inhibition activity (Figure 1) only the ethanol fraction – 10 was analyzed in the HPLC. Inhibition assays showed presence of inhibitor moleculesat four retention times (26 to 29 min) (Figure 2 and 3). These fractions were submitted to mass spectrometry (Figure 5). More studies are needed to identify the compound responsible for the inhibition. However, based on the repeated peaks observed in the mass spectrometry, this molecule likely has a common main structure, differing only in some ramifications. Inhibitor activity can be evidenced by adding these molecules to artificial diets offered to the target insect. Although in vitro assays are initial tests to indicate insecticide activity, many works confirmed their results through in vivo assays on larvae of pest insects (Alves et al., 2014; Tavares et al., 2009). Several works on literature reported in vivo effect of Kunitz and Bowman-Birk proteinase inhibitors on larval development (Shade et at.,1986), pupae weight (Duan et al., 1996), total viability of insects cycle (Shade et al., 1986) and insect adaptation mechanisms (Brioschi et al., 2007; Paulillo et al., 2000). However, to the best of our knowledge there is no study exploring these responses for a non-proteic trypsin inhibitor.
In this study, the castor oil trypsin inhibitor (COTI) activity was analyzed during the larval development of S. frugiperda, an important pest of the Brazilian agriculture. For this, COTI was added in artificial diet in crescent concentrations. Among all the biological parameter analyzed only the length of larval stage and pupae weight showed significant results when compared to the control (Figure 6). An increase of ten days was registered in S. frugiperda larval stage length for the treatment with inhibitor concentration at 0.5%, proving the deleterious effect of this inhibitor. Probably, an essential amino acid deficiency may have affected the insect development, delaying the stage conclusion (Ryan, 1990). Decreased pupae weight was registered in the treatments with inhibitor concentration of 0.25% and 0.5%. This parametersuggested reduced food ingestion, probably caused by the inhibitor presence. Parameters as length of the pupae stage, food ingestion, feces weight, larvae and pupae survival rates showed no variations with the inhibitor inclusion on the diet (Figure 6). Differences between in vitro and in vivo assays are frequently reported in the literature (Bolter and Jongsma, 1995). In this study, COTI reached 66% of trypsin inhibition in vitro, whereas in vivo assays showed deleterious effect in few biological parameters. This result is completely understandable due to the pest capacity to adapt to the inhibitor presence on diets or genetically modified plants (Bolter and Jongsma, 1995; Ishimoto et al., 1996; Jongsma and Boulter, 1997). Pest adaptation capacity can be explained by two hypotheses: 1) Higher quantity of digestive enzyme expressed in presence of inhibitor; 2) different proteinase classes expressed to overcome the inhibitor effect. According to Broadway et al., (1986), insect larvae can overproduce protease in the presence of proteinase inhibitor in the digestive system. In the inhibitor concen-tration of 0.5%, the growth curve presented to the larval stage 12 days was higher compared to the control treatment, showing an evident deficit in larvae develop-ment. Pupae formation was also affected, delaying in three days compared with control treatment. Remaining treatments were not affected comparing with control, demonstrating a regular development. However, at the concentration of 0.12 and 0.25% presented larval stage three days higher compared with control treatment (Figure 5).
According to Martinez and Endem (2001) increasing the larval stage and growth inhibition can be triggered by reduced food ingestion and lower food conversion, caused by the presence of one or more inhibitor in the diet. Larvae of S. frugiperda fed with concentration of 0.5% also showed alterations in the nutritional indexes (Figure 7) as higher relative consumption rate (RCR), lower relative growth rate (RGR), lower efficiency of ingested food conversion (EIC) and lower efficiency of digested food conversion (EDC) with more metabolic cost. These results attest the potential of COTI, since similar studies on larvae of H. virescens fed on tobacco plants expressing potato proteinase inhibitors (PIN-2), showed no variation in nutritional parameters evaluated (Brito et al., 2001). Approximated digestibility (AD) and relative metabolic rate (RMR) showed no significant differences among treatments (Figure 7). The efficacy at the concentrations of 0.5, 0.25 and 0.12% were 25, 12 and 4.17%, respectively. The concentration of 0.06% showed no efficiency. In this study, the non-proteic castor oil trypsin inhibitor at the concentration of 0.5% impaired S. frugiperda larvae development changing their nutritional parameters. These results can be used to develop an alternative and more sustainable method to control lepidopteran pest species through trypsin inhibition. Future works should investigate the inhibitor molecule characteristics, activities on enzyme catalysisand evaluate its kinetic properties.
The author(s) did not declare any conflict of interest.
The authors gratefully acknowledge the financial support provided by the Coordination for the Improvement of Higher Education Personnel (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - CAPES, Brazil), National Council for Scientific and Technological Development (Conselho Nacional de Desenvolvimento Científico e Tecnológico - CNPq, Brazil) and State of Minas Gerais Research Support Foundation (by Fundação de Amparo à Pesquisa do Estado de Minas Gerais - FAPEMIG, Brazil).
RCR, Relative consumption rate; RMR, relative metabolic rate; RGR, relative growth rate; AD, approximated digestibility; EIC, efficiency of ingested food conversion; EDC, efficiency of digested food conversion.
REFERENCES
Abbott, WS (1925). A method of computing the effectiveness of an insecticide. J Econ Entomol, 18:265-266.
Crossref |
|
|
Abd El-latif AO (2014) In vivo and in vitro inhibition of Spodoptera littoralis gut-serine protease by protease inhibitors isolated from maize and sorghum seeds. Pest Biochem. Physiol. 116(1):40-48.
Crossref |
|
|
Alvarez A, Pera Lm, Loto F, Virla Eg, Baigori, Md (2009). Insecticidal crystal proteins from native Bacillus thuringiensis: numerical analysis and biological activity against Spodoptera frugiperda. Baigori Biotechnol. Lett. 31(1):77-82.
Crossref |
|
|
Alves APC, Corrêa AD, Alves DS, Saczk AA, Lino Jéssica BR, Carvalho GA (2014). Toxicity of the phenolic extract from jabuticabeira (Myrciaria cauliflora (Mart.) O. Berg) fruit skins on Spodoptera frugiperda. Chil. J. Agric. Res. (Online). 74(2):200-204.
Crossref |
|
|
Bigi MFM, Torkomian VLV, Groote STCS, Hebling MJA, Bueno OC, Pagnocca FC, Fernandes JB, Vieira PC, Silva MFGF (2004). Activity of Ricinus communis (Euphorbiaceae) and ricinine against the leaf-cutting ant Atta sexdens rubropilosa (Hymenoptera: Formicidae) and the symbiotic fungus Leucoagaricus gongylophorus. Pest Manag. Sci. 60(9):933-938.
Crossref |
|
|
Bolter CJ, Jongsma MA (1995). Colorado potato beetles (Leptinotarse decemlineata) adapt to proteinase inhibitors induced in potato leaves by methyl jasmonate. J. Insect Physiol. 41(12):1071-1078.
Crossref |
|
|
Brioschi D, Nadalini LD, Bengtson MH, Sogayar MC, Moura DS, Marcio Silva-Filho C (2007) General up regulation of Spodoptera frugiperda trypsins and chymotrypsins allows its adaptation to soybean proteinase inhibitor. Insect Biochem. Mol. Biol. 37(12):1283-1290.
Crossref |
|
|
Brito LO, Lopes AR, Parra JRP, Terra WR, Silva-Filho MC (2001). Adaptation of tobacco budworm Heliothis virescens to proteinase inhibitors may be mediated by the synthesis of new proteinases. Comp. Biochem. Physiol. B. 128(2):365-375.
Crossref |
|
|
Broadway RM, Duffey SS, Pearce G, Ryan CA (1986). Plant proteinase inhibitors: a defense against herbivorous insects? Entomol. Exp. Appl. 41:33-38.
Crossref |
|
|
|
Cohen AC (2004). Insect diets science and technology. CRC press, Boca Raton, Florida, USA. |
|
|
Correia AA, Teixeira VW, Teixeira AAC, Oliveira JV, Torres JB (2009). Morphology of the alimentary canal of Spodoptera frugiperda (J E Smith) Larvae (Lepidoptera: Noctuidae) fed on neem-treated leaves. Neotrop. Entomol. 38(1):83-91.
Crossref |
|
|
Duan X, Li X, Xue Q, Abo-El-Saad M, Xu D, Wu R (1996). Transgenic rice plants harboring an introduced potato proteinase inhibitor II gene are insect resistant. Nat. Biotechnol. 14:494-498.
Crossref |
|
|
Erlanger BF, Kokowsky N, Cohen W (1961). The preparation and properties of two, new chromogenic substrates of tripsina. Arch. Biochem. Biophys. 95(1): 271-278.
Crossref |
|
|
Ishimoto M, Sato T, Chrisperls MJ, Kitamura K (1996). Bruchid resistance of transgenic azuki bean expressing seed α-amilase inhibitor of common bean. Entomol. Exp. Appl. 79:309-315.
Crossref |
|
|
Jongsma, MA, Bolter C (1997). The adaptation of insects to plant protease inhibitors. J. Insect Physiol. 43(10): 885-895.
Crossref |
|
|
|
Kakade ML, Rackis JJ, Mcghee JE, Puski G (1974). Determination of trypsin inhibitor activity of soy bean products: a collaborative analysis of an improved procedure. Cereal Chem. 51(1): 376-382. |
|
|
|
Kasten-Junior P, Precetti AACM, Parra JPP (1978). Dados biológicos comparativos de Spodoptera frugiperda (J.E. Smith, 1797) em duas dietas artificiais e substrato natural. Rev. Agric. 53(2):68-78. |
|
|
Kuwar SS, Pauchet Y, Vogel H, Heckel DG (2015). Adaptive regulation of digestive serine proteases in the larval midgut of Helicoverpa armigera in response to a plant protease inhibitor. Insect Biochem. Mol. 59:18-29.
Crossref |
|
|
Loewenherz C, Fenske RA, Simcox NJ, Bellamy G, Kalman D (1997). Biological monitoring of organophosphorus pesticide exposure among children of agricultural workers in central Washington State. Environ. Health Perspect. 105(12):1344-53.
Crossref |
|
|
|
Malau MB, James DB (2008). Evaluation of larvicidal properties of some plant On Simulium Damnosum Complex. Internet J. Toxicol. 4:2. |
|
|
Martinez SS, Emden HF (2001). Growth disruption, abnormalities and mortality of Spodoptera littoralis (Boisduval) (Lepidoptera: Noctuidae) caused by Azadirachtin. Neotrop. Entomol. 30(1):113-125.
Crossref |
|
|
Nathan SS, Choi, MY, Seo HY, Paik CH, Kalaivani K (2008). Toxicity and behavioral effect of 3beta,24,25-trihydroxycycloartane and beddomei lactone on the rice leaffolder Cnaphalocrocis medinalis (Guenée) (Lepidoptera: Pyralidae). Ecotoxicol. Environ. Saf. 72(4):1156-1162.
Crossref |
|
|
Paulillo LCMS, Lopes AR, Cristofoletti PT, Parra JRP, Terra WR, Silva-Filho MC (2000). Changes in midgut endopeptidase activity for Spodoptera frugiperda (Lepidoptera: Noctuidae) are responsable for adaptation to soybean proteinase inhibitors. J. Econ. Entomol. 93(3):892-896.
Crossref |
|
|
Pompermayer P, Lopes AR, Terra WR, Parra JRP, Falco MC, Silva-Filho MC (2001). Effects of soybean proteinase inhibitor on development, survival and reproductive potential of the sugarcane borer, Diatraea saccharalis. Entomol. Exp. Appl. 99(1):79-85.
Crossref |
|
|
Pontual EV, Napoleão TH, Assis CRD, Bezerra RS, Xavier HS, Navarro DMAF, Coelho LCBB, Paiva PMG (2012). Effect of Moringa oleifera flower extract on larval trypsin and acethylcholinesterase activities inAedes aegypti Arch. Insect Biochem. Physiol. 79:135-152.
Crossref |
|
|
|
R Development Core Team (2009). R - A language and environment for statistical computing, version 2.10.1, 1st edition. R Foundation for Statistical Computing. |
|
|
Rakwal R, Agrawal GK, Jwa NS (2001). Characterization of a rice (Oryza sativa L.) Bowman-Birk proteinase inhibitor: tightly light regulated induction in response to cut, jasmonic acid, ethylene and protein phosphatase 2A inhibitors. Gene 263(1-2):189-198.
Crossref |
|
|
Rossi GD, Santos CD, Carvalho GA, Alves DS, Pereira LLS, Carvalho GA (2012) Biochemical Analysis of a Castor Bean Leaf Extract and its Insecticidal Effects Against Spodoptera frugiperda (Smith) (Lepidoptera: Noctuidae). Neotrop. Entomol. 41:503-509.
Crossref |
|
|
Ryan CA (1990) Proteinase inhibitors in plants: genes for improving defenses against insects and pathogens. Annu. Rev. Phytopathol. 28:425-449.
Crossref |
|
|
Santiago GP, Pádua LEM, Silva PRR, Carvalho EMS, Maia CB (2008). Effects of plant extracts on the biology of Spodoptera frugiperda (J. E. Smith, 1797) (Lepidoptera: Noctuidae) maintained under artificial diet. Cienc. Agrotec. 32(3):792-796.
Crossref |
|
|
Shade RE, Murdock LL, Foard DE, Pomeroy MA (1986). Artificial seed system for bioassay of Cowpea weevil (Coleoptera: Bruchidae) growth and development. Environ. Entomol. 15(6):1286-1291.
Crossref |
|
|
Starner K, Goh KS (2012). Detections of the Neonicotinoid Insecticide Imidacloprid in Surface Waters of Three Agricultural Regions of California, USA, 2010-2011. Bull. Environ. Contam. Toxicol. 88(3): 316-321.
Crossref |
|
|
Tavares WS, Cruz I, Petacci F, Assis Júnior SL, Freitas SS, Zanuncio JC, Serrão JE (2009). Potential use of Asteraceae extracts to control Spodoptera frugiperda (Lepidoptera: Noctuidae) and selectivity to their parasitoids Trichogramma pretiosum (Hymenoptera: Trichogrammatidae) and Telenomus remus (Hymenoptera: Scelionidae). Ind. Crop Prod. 30(3):384-388.
Crossref |
|
|
Tiffin P, Gaut BS (2001). Molecular evolution of the wound-induced seine proteinase inhibitor wip1 in zea and related genera. Mol. Biol. Evol. 18(11):2092-2101.
Crossref |
|
|
Uchino H, Iwama K, Jitsuyama Y, Yudate T, Nakamura S, Gopal J (2015). Interseeding a Cover Crop as a Weed Management Tool is More Compatible with Soybean than with Maize in Organic Farming Systems. Plant Prod. Sci. 18(2):187-196.
Crossref |
|
|
Yu SJ, Nguyen SN, Abo-Elghar GE (2003). Biochemical characteristics of insecticide resistance in the fall armyworm, Spodoptera frugiperda (J.E. Smith). Pestic. Biochem. Physiol. 77(1):1-11.
Crossref |
|
|
Zhu-Salzman K, Zeng R (2014). Insect Response to Plant Defensive Protease Inhibitors. Annu. Rev. Entomol. 60:233-252.
Crossref |