ABSTRACT
Actinomycetes are aerobic and gram-positive spore forming bacteria. They belong to the order actinomycetales and are characterized by substrates and aerial mycelium growth. They are the most abundant microorganisms in soil. They play important roles in the cycling of organic matter and inhibit the growth of several plant pathogens in the rhizosphere. Due to the presence of enzymes such as proteases and chitinase, actinomycetes have been studied as a natural controller of insects and phytopathogenic fungi that cause considerable losses in agriculture. Additionally, the facilities for the industrial manipulation of cultures, and the diversity of metabolites produced make actinomycetes preferred for the control of pests. Furthermore, actinomycetes constitute a “green” alternative for controlling insects and fungi, since they do not contaminate the environment, and are natural members of the soil. They also contribute to the sustainability of soil by formation and stabilization of compost piles, due to their degrading capabilities, and ability to form stable humus. Moreover, they can be associated with other soil microorganisms to degrade recalcitrant residues like celluloses to maintain biotic soil equilibrium.
Key words: Actinomycetes, compounds, agriculture, fungi, insect.
Historically, discoveries and developments of a class of new bioactive compounds with antimicrobial and anti-parasitic activities have frequently emerged from natural sources (Chin et al., 2006; Ganesan, 2008). These natural bioactive compounds produced serve as a model for the synthesis of synthetic and semi-synthetic drugs. These developments are directly linked to the screening of natural producers such as microorganisms and plants, which require employment of biotechnological techniques (Marinelli and Marconi, 2011; Newman and Cragg, 2012). Particularly, the use of microbial sources for the investigations of novel natural bioactive compounds has proved to be productive during the last two decades and was emphasized extensively in review articles (Berdy, 2005; Balts, 2007; Naine, 2011; Raja and Prabakarana, 2011). Microbial natural products are biotechnologically preferable due to their remarkable appropriate pharmacological activities and facility for controlling variables in bioprocess (Sanchez and Demain 2002). Well-known as the source of several drugs such as antibiotics, antitumor, immunosuppressants, antiviral and antiparasitic agents, microorganisms are responsible for the production of about 23,000 bioactive secondary metabolites (dos Reis Feitosa et al., 2014; Subbanna et al., 2018). Despite this large amount, only 150 of these compounds have been employed in pharmacology, agriculture or other fields (Brzezinska et al., 2014). The filamentous bacteria group actinomycetes alone are the main producers responsible for the production of over 10,000 of these compounds, representing 45% of all bioactive microbial metabolites discovered (Brzezinska et al., 2014). When considering only these compounds in practical use, it may represent about 80% (Olano et al., 2008). This bacterial group represents the most economically and biotechnologically worthwhile microorganisms (Baltz, 2007; Naine et al., 2011; Raja and Prabakarana, 2011).
Actinomycetes are a group of gram-positive branching unicellular filamentous bacteria belonging to the order actinomycetales; it is so called, because it has a fancied similitude with the radiating rays of the sun when seen in tissue ruptures. It is characterized by high content of G+C in DNA, presence of LL-Diaminopimelic acid (LL-DAP) and the presence or absence of characteristic sugars in the cell wall. Members of this group are ubiquitous and the discovery of new actinomycete taxa from diverse habitat with unique metabolic activity implies, generally, discovery of novel bioactive compound. Their reproduction is based on fission of hyphae or by means of special spores (conidia). They may also form branching threads or rods, and their hyphae are generally nonseptate. Septa, when existing, may be observed in some forms (Manulis et al., 1994). The chemical composition of cell wall is similar to that of Gram-positive bacteria but because of their well-developed morphological (hyphae) and cultural characteristics, actinomycetes have been considered as a group, well separated from other bacteria. Cell wall maintains cell shape, preventing bursting due to osmotic pressure. This wall, as distinctive in prokaryotic organisms, consists of a thick layer of peptidoglycan, a structure composed of glycan (polysaccharides) chains of alternating Nacetyl-d-glucosamine (NAG) and N-acetyl-d-muramic acid (NAM) and diaminopimelic acid (DAP). Teichoic and teichuronic acids are chemically bonded to peptidoglycan (Bhatti et al., 2017; De Schrijver and De Mot, 1999). Although historically referred to as the ray fungi due to the mycelia of branching filaments (hyphae), actinomycetes, unlike the true fungi, have thin hyphae (0.5 1.5 mm in diameter) with genetic material coiled inside as free DNA (Bhatti et al., 2017).
Linked polymers containing short chains of amino acids and long chains of amino sugars are found in cell wall of the hyphae. The actinomycetes cell wall composition is of considerable taxonomic significance, varying considerably among different groups. Therefore, there are at least, four major cell wall types based on the three features of peptidoglycan composition and structure: (i) diaminopimelic acid isomer on tetrapeptide side chain position 3, (ii) sugar content of peptidoglycan and (iii) the presence of glycine in interpeptide bridges (Davenport et al., 2000). Interactions between actinomycetes and plants in soil rhizosphere make bacterial species essential for the micro-environment, characterizing them as plant growth-promoting rhizobacteria. Increase in population and food prices concomitant with the reduction in agricultural activities has become a global food security concern. On the other hand, losses of U$$ 120 billion (representing 20 to 40%) have been attributed to insect pests and fungal attack (FAO, 2010). Approximately, 70,000 different insect species damage food crops across the world (Vijayabharathi, 2013). Among them, the species belonging to Lepidoptera order are the major cause of crop losses (Qin et al., 2009). Bacteria belonging to actinomycetes group also present several mechanisms especially useful in the development of potential anti-fungal drugs based on anti-fungal bioactive metabolites, due to their versatility in the production of extracellular enzymes and a variety of these secondary metabolites. Many of these anti-fungal bioactive compounds have been characterized and employed in agriculture (Arasu et al., 2008). The aim of the present study is to show the potentialities of actinomycetes for producing biofungicide and bioinsecticide important in agriculture. This study also describes the general mechanisms of action and status of main commercial products derived from bioactive compounds.
ACTINOMYCETES AND BIOCONTROL OF INSECTS IN AGRICULTURE
Since the application of chemical insecticides on crops for controlling deleterious insects has become hazardous to environment and human health, many efforts have been oriented in order to amend their use for a more ecofriendly and safe alternative control methods (Bream et al., 2001). The bacterial species, Bacillus thringiensis, for example, is the most successful microorganism employed as a commercial insecticide for biological control, replacing conventional chemical insecticides in some areas of application. Several other varieties of microorganisms including fungi and nematodes have been reported as strategies to biologically control insect pests, but, actinomycetes especially, play an important role in the biological control of insects through the production of a large variety of insecticidally active compounds against different order of insects (Hokkanen and Lynch, 1995). Hussain et al. (2002) confirmed the very high mortality of larval and pupal stages of Musca domestica reaching up to 90% of mortality, after actinomycetes treatments, and Sundarapandian et al. (2002) verified actinomycetes effectiveness against Culex quinquefasciatus. The effective action of actinomycetes against insects is not only attributed to the production of bioactive compounds, but especially attributed to their capacity to produce chitinase enzyme, which degrades the insect chitin surface, allowing penetration of bioactive toxic lethal compound in the insect body (Brzezinska et al., 2014). Therefore, the action of actinomycetes in insects is combined, depending on the action of two or more mechanisms (Gadelhak et al., 2005).
A large variety of compounds that act in isolation or generally in combinations with other molecules is related to actinomycetes and it justifies the ability or even the potentiality of several of them to be employed as insect plagues controllers. Some typical actinomycetes and their major role as biocontrol agent are depicted in Table 1. Cotton bollworms represent a highly polyphagous insect species (Lepdotera order), attacking crops such as tomato, cotton, pigeon pea, chickpea, rice, sorghum and cowpea. Other vegetal hosts include groundnut, okra, peas, field beans and soybeans. The high mobility, high fecundity, and facultative diapause contribute to the dispersion of these species and, therefore, over 181 plant species of economically important crops are potentially infested (Shad et al., 2012). The second most important polyphagus lepidopteran pest, Spodoptera litura, causes 25 to 100% yield loss on economically important crops such as cotton, groundnut, chilli, tobacco, caster and okra. In the last two decades, actinomycetes have been shown to be a vast source of novel agents having considerable potential for the biocontrol of insect pests. Many secondary metabolites produced by the members of this group of bacteria show insecticidal activity. Hence, the possibility of using these metabolites in controlling insects had been achieved by several researchers (Dutton and Gueguen, 1999; Sazonova et al., 1993; Dindo, 1993; Spindler and Spindler-Barth, 1994; Lasota and Dybas 1991; Scholl et al., 1992; Campbell et al., 1983; Vijayan and Balaraman 1991; Takahashi et al., 1989; Mishra et al., 1987).
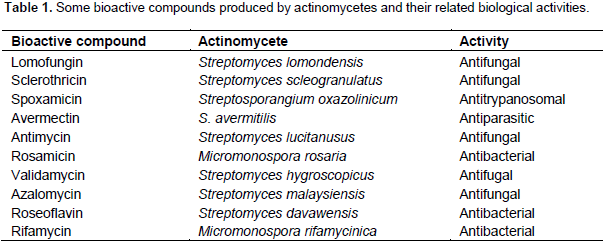
ACTINOMYCETES’ MAIN BIOACTIVE COMPOUNDS USED AGAINST PHYTOPATHOGENIC INSECTS
As discussed, a large variety of metabolites found in actinomycetes species may contribute to the control of insects in plants. As a rule, most of them act based on combined effects.
Chitinase as a coadjutant
Chitinase is an enzyme used by insects to degrade the structural polysaccharide “chitin” during the molting process (Gupta et al., 1995; Zhang et al., 2002). The largest chitinase activity among bacteria has been observed in species of Streptomyces. Gadelhak et al. (2005) isolated streptomycete and non-streptomycete actinomycetes and tested their capacity to produce chitinase enzyme on colloidal chitin agar (CCA). These experiments confirmed the high ability of Streptomyces to produce chitinase. Species of Streptomyces show high multiplicity of chitinase genes.
Spinosyn as the first specific insect pest controller
The tobacco budworm, Heliothis virescens (F.) is a well-known tobacco plague in United States of America. As reported by Sparks and co-workers (1981, 1982, 1983), this species and others belonging to the same genus developed resistance to a wide variety of insecticides including DDT, methyl-parathion and the pyrethroids. In some areas, the resistance spectrum of H. virescens expands and incorporates many of the newer organophosphorus and carbamate insecticides (Sparks et al., 1993). The search on new insect control agents includes an assortment of random or directed screening scenarios, sometimes referred to as the trial and error approach (Sparks and Hammock, 1983; Hammock, 1985; Hammock et al., 1986). Discovery of new insect control agents is still a common and hard effective development, particularly when the focus is the natural product source as well as synthetic organic chemistry. In this context, in 1985, a program to screen fermentation broths from soil microorganisms for pharmaceutically and agriculturally useful compounds was conducted by scientists at Lilly Research Laboratories (Indianapolis, IN, USA). Those scientists discovered a new species of Actinomycete, Saccharopolyspora spinosa (Mertz and Yao 1990) isolated from soil sample. Extracts of this fermentation broth were active against mosquitos (Kirst et al., 1992) and southern armyworm, Spodoptera eridania (Cramer), larvae in early screening assays. Consequently, they discovered this activity was associated with a new bioactive compound classified in the group of macrocyclic lactones (Kirst et al., 1992) called the spinosyns (Sparks et al., 1998).
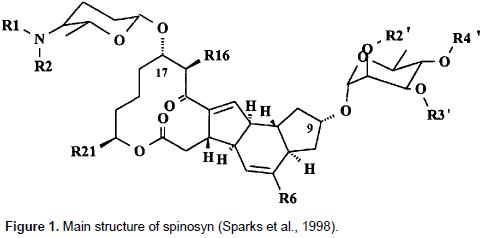
Spinosyn A (1 H-as-Indaceno [3,2-d] oxacyclododecin-7,15-dione, 2-[(6-deoxy-2,3,4-tri-O- meth yl-a- L- man nopyranosyl)oxy]-13-[[5-(dimethyl-amino)tetrahydro-6-methyl-2H-pyran-2-yl]oxy]-9-ethyl 2,3,3a,5a,5b,6,9,l0,1l,12,13,l4,l6a,l6b-tetradecahydro-14-methyl-,[2R-[2R*,3aS*,5aR*,5bS*,9S* 13S*(2R*,5S*,6R*),l4R*,16aS*,16bR*]]-[9CI]) are the principal components of spinosad™ (Tracer Naturalyte Insect Control, Indianapolis, IN), and other spinosyns active against larvae of H. virescens. Spinosyns are a macrolide insecticide belonging to the family of tetracyclic lactones to which are attached an amino-sugar (D-forosamine) and a neutral sugar (L-rhamnose).Members of the family differ in the extent of N- and O-methylation on the sugars, or C-methylation on the polyketide nucleus (Ichikawa et al., 2013; Kirst, 2010; Sparks et al., 1998). Their activity against mosquitos (mosquito larvicidal activity) was evidenced. In complementary works, Strobel and Nakatsukasa (1993) identified a strain A83543, as S. spinosa, and confirmed it as a new species of actinomycete which produces a large family of macrolide compounds. Particularly, spinosyns A and D are the two active ingredients. Most of the genes involved in spinosyn biosynthesis are clustered in an 74 kb region of the S. spinosa genome. This region has been characterized by DNA sequence analysis and targeted gene disruptions. The spinosyn biosynthetic gene cluster contains five large genes encoding a type I polyketide synthase, and 14 genes involved in modification of the macrolactone, or in the synthesis, modification and attachment of the deoxysugars. Four genes required for rhamnose biosynthesis (two of which are also required for forosamine biosynthesis) are not present in the cluster. A pathway for the biosynthesis of spinosyns was proposed by Waldron et al. (2000). Figure 1 shows the main structure of spinosyn.
The economic significance of fungal biotechnology cannot be overstated; indeed, fungi have been exploited to yield a range of valuable products, some of which have proved invaluable to mankind. Since ancient times, fungi have been utilized for simple food processing. In the last century, the development of fungal biotechnology for the subsequent production of valuable commodities such as antibiotics, enzymes, vitamins, pharmaceutical compounds, fungicides, plant growth regulators, hormones, and proteins were seen (Wiley, 2018); however, fungi can be detrimental to agriculture, especially foods of largest commercial importance. Fungi represent one of the major threats for biodeterioration of cereals and pulses during storage, causing economic losses to growers by increasing the free fatty acid content of seeds and decreasing germination ability of the plants (Dhingra et al., 2001; Kedia et al., 2014). In addition, the mycotoxins secreted by different food borne molds cause qualitative losses of commodities, potentially inducing various health problems in consumers. Some species of Aspergillus are highly aflatoxigenic, particularly in tropical and subtropical countries, secreting high level of aflatoxins. Furthermore, aflatoxin is classified as group 1 human carcinogen by the International Agency for Research on Cancer (Mishra et al., 2013). Food commodities are frequently contaminated by fungi, and the associated toxins generated by some of them during storage, transportation and post-harvest processing cause significant losses in quality, quantity, nutrient composition, and thereby reduce market value.
According to the Food and Agriculture Organization (FAO), about 1000 million metric tons of food is spoiled globally each year due to mycotoxins produced by fungi (Bhat et al., 2010; Prakash et al., 2005; Prakash et al., 2014). Commodities are intensive products in natural state (primary) or with a small degree of industrialization. This category involves agricultural products (raw and/or processed), minerals (raw and/or industrialized) and energy (Veríssimo and Xavier, 2015). In the food sector of Brazil, among the main commodities, crops such as soybean, corn and cotton can be mentioned. Among the most consumed grains is soy, which is considered as a functional food; it provides nutrients to the body, prevents chronic degenerative diseases and is also an excellent source of minerals. Corn, in turn, is also of great importance for human consumption, as it is an energetic, digestible food with high starch content. It is the raw material of many industrialized products including animal feed, in which corn and soybeans complement each other (Ferrarini, 2004; Balini et al., 2015). Corn has high energy content and soy has rich protein value (Oliveira et al., 2004 Balini et al., 2015). Corn is one of the oldest food grains considered as one of the three major cereal crops in the world, together with rice and wheat. Brazil produced 56.3 million tons of corn in the 2011-2012 harvest, and exported 8.5 million tons (Godfray and Garnet, 2014; De Rossi, 2015). From the process of cultivation till distribution, the seeds of these grains are conditioned to fungus contamination.
This is because the grains present food components of these microorganisms, in addition to several other factors. The most frequently found genera are Aspergillus and Penicillium; the fungi are so-called because they grow in seeds and store grains with moisture contents within the range of 8 to 18%. Among these fungi, the genus Aspergillus is a frequent contaminant of soybean and corn (Balini et al., 2015). Cotton (Gossypium hirsutum L.) is among the most important fiber crops in the world. Each year, approximately 35 million hectares of cotton are planted worldwide. World cotton trade moves about $ 12 billion annually and involves more than 350 million people in its production, from farms to logistics, ginning,processing and packaging. Currently, cotton is produced by more than 60 countries, where the five countries that produce the most are: China, India, the United States, Pakistan and Brazil (USDA, 2017). Throughout the world, there are reductions in cotton productivity due to outbreaks of disease. In Brazil, with the introduction of cultivars adapted to the Cerrado and with higher yield of fiber, cotton cultivation has intensified. In this ecosystem, climatic conditions are favorable to the development of various diseases caused by fungi, resulting in increased production costs (Borém and Freire, 2014).
ACTINOMYCETES’ ACTION AGAINST PHYTOPATHOGENIC FUNGUS
Actinomycetes are important enzyme producers, such as quitinases, proteases, peptidases and cellulases. Quitinases are the most important in the process of phytopathogenic fungi control. Similar to insect control mechanisms, the control of actinomycetes in fungi is a result of combined and complementary action. Firstly, the cell wall chitin is degraded by enzymes, and consequently the cell is assessed and antibiotics and others active compounds are released in the cell. Fungal plant diseases management by Streptomyces has been well documented, but few commercial products are in the market using specific strains of the microorganism or its metabolites. While it is unmanageable for massive production, the potential microorganisms like S. plicatus used in biocontrol programmes connecting their enzymatic properties seems to be practical to develop methods for production and extraction of secondary metabolites, or the use of extract of broth directly (Sinha et al., 2014). Investigations on the potential of actinomycetes on biological control have been reported since the 80s, as shown in the classical work of Tahvonen (1982b), who tested strains of Streptomyces isolated from peat for control of soil and seedborne disease in peat culture. A key illustration of Streptomyces biocontrol agent is the action of a strain of Streptomyces griseoviridis, reported by Tahvonen (1982a). In that paper, the author described a strain originally isolated from light coloured Sphagnum peat as antagonistic to a variety of plant pathogens together with Alternaria brassicola (Schw.) Wiltsh., Botrytis cinerea Pers., Fusarium avenaceum Sacc and Fusarium culmorum. The species, S. griseoviridis has been used in root dipping or growth nutrient treatment of cut flowers, potted plants, greenhouse cucumbers, and different alternative vegetables (Bhatti et al., 2017).
Fungal pathogens pose serious problem worldwide and cause a number of plant diseases including rusts, smuts, rots, wilt, anthracnose causing severe damage to crops (Pakdeevaraporn et al., 2005; Ashokvardhan et al., 2014); in this context and in the natural world, no microorganism can survive independently, and micro-organisms can interact with each other by a number of ways. Interactions between microorganisms can be divided into mutually beneficial, neutral and harmful such as mutualism, neutralism, amensalism, antagonism, parasitism, etc. So, interactions between micro-organisms can be positive, negative or no effects (Moënne-Loccoz, 2014). Biological control of postharvest diseases by antagonistic microorganisms seems to be a promising alternative to fungicides. Understanding the methods of action of antagonisms is essential to allow the use of antagonists under partial conditions and to enhance their biological control while protecting human health and the environment. Several modes of action have been documented for the antagonistic activity of biological control agents: they act by multiplying on the fruit surface or within wounds on the fruit; this is done by competing for space and nutrient at an infection court on the product, by antibiosis, by restricting the action of hydrolytic enzymes produced by pathogen, by producing enzyme to degrade pathogen cell walls, and/or by direct parasitism of the pathogen (Long et al., 2005; Shojaee et al., 2014). Soil actinomycetes have revealed their wide antifungal activity (Ventura et al., 2007; Sharma et al., 2014; Tinatin and Nuzrat, 2006). They have been shown to protect several plants to various degree of soil borne fungal pathogens.
Actinomycetes as biocontrol agent produce Urauchimycins which is a member of antimycin class, a set of well-identified antifungals, that act by inhibiting the electron flow in the mitochondrial respiratory chain of a phytopathogenic fungus and have been identified in Streptomyces isolated from the integument of attine ants (Schoenian et al., 2014). More recently, Dias et al. (2017) isolated strains of Streptomyces sp. (a very promising genus of the order Acinomicetales) of sediment from an urban mangrove located at the city of São Luís MA and verified antimicrobial activity of these bacteria in front of organisms of clinical and agricultural interest. Costa et al. (2017) isolated species of actinomycetes of biotechnological interest from soil contaminated with agrochemicals and verified, already in isolation, the antibiosis potential of these bacteria. Furthermore, biofungicides such as MYCOSTOP® are produced using actinomycetes for the control of seed- and soil-borne plant pathogens (Fusarium, Alternaria, Phytophthora and Pythium) which cause damping-off and root diseases, Actinovate® isolated from streptomycetes species. Streptomyces lydicus WYEC108 is a strain of this species which has been formulated to control fungal plant pathogens effectively for fresh market tomatoes, PRESTOP® is used for controlling damping-off and root diseases (Pythium, Fusarium, Phytophthora and Rhizoctonia) as well as for the control of Botrytis grey mould and Didymella (Mycosphaerella) gummy stem blight in cucumber (Kamara and Gangwar, 2015), which are all available commercially. After undergoing various biological processes, Streptomyces are able to successfully control plant pathogenic fungi including P. oryzae by hydrolyzing their cell walls (Kavitha et al., 2010; Awla et al., 2016).
It is of great importance to know the diversity of bioactive compounds produced by soil microorganisms. Understanding the mechanisms of action of these bioactive is crucial for its efficient application in agriculture. In this sense, actinomycetes are preferable due to their predominance in soil, and enormous potential to adapt and produce a variety of bioactive metabolites with activity against important phytopathogenic fungi and insects. Although many biofungicides and bioinsecticides have been produced and successfully applied to cultivars, many other potentially useful molecules with bioactivities against such organisms need more investigations to confirm their efficiency. Additionally, many other compounds need to be commercially available, and the production must be intensified. The status of research worldwide, points out to the necessity of tests involving new metabolites and further investigations, to find new species and optimize production process conditions.
The authors have not declared any conflict of interests.
REFERENCES
|
Arasu M, Duraipandiyan V, Agastian P, Ignacimuthu S (2008). Antimicrobial activity of Streptomyces spp. ERI-26 recovered from Western Ghats of Tamil Nadu. J. Mycol. Méd. 18:147-153.
Crossref
|
|
|
|
Ashokvardhan T, Rajithasri AB, Prathyusha P, Satyaprasad K (2014). Actinomycetes from Capsicum annuum L. Rhizosphere Soil Have the Biocontrol Potential against Pathogenic Fungi. Int. J. Curr. Microbiol. Appl. Sci. 3(4):894-903.
|
|
|
|
|
Awla HK, Kadir J, Othman R, Rashid TS, Wong MY (2016). Bioactive Compounds Produced by Streptomyces sp. Isolate UPMRS4 and Antifungal Activity against Pyricularia oryzae. Am. J. Plant Sci. 7:1077-1085.
Crossref
|
|
|
|
|
Balini LC, Luis AS, Soares LC, Vendruscolo ECG, Fiorini A (2015). Identificação Pela Técnica De Pcr-Rflp, De Aspergillus Spp. Isolados De Grãos De Soja E Milho. J. Chem. Infect. Model. 53(9):1689-1699.
Crossref
|
|
|
|
|
Baltz RH (2007). Antimicrobials from actinomycetes: Back to the future. Microbe 2:125-131.
|
|
|
|
|
Berdy J (2005). Bioactive microbial metabolites. J. Antibiot . 58:1-26.
Crossref
|
|
|
|
|
Bhat R, Rai RV, Karim AA (2010). Mycotoxins in food and feed: present status and future concerns. Compr. Rev. Food Sci. Food Saf. 9:57-81.
Crossref
|
|
|
|
|
Bhatti AA, Haq S, Bhat RA (2017). Actinomycetes benefaction role in soil and plant health. Microb. Pathog. 111:458-467.
Crossref
|
|
|
|
|
Borém A, Freire EC (2004). Algodão: do plantio a colheita. 1ed. Minas Gerais: UFV, 2014-312.
|
|
|
|
|
Bream AS, Ghazall SA, El-Aziz ZK, Ibrahims SY (2001). Insecticidal Activity of Selected Actinomycete Strains Against the Egyptian Cotton Leaf Worm Spodoptera Littoralis (Lepidoptera: Noctuidae). Mededelingen Faculteit Landbouwkundige en Toegepaste Biologische Wetenschappen Universiteit Gent. 66(2a):503-544.
|
|
|
|
|
Brzezinska MS, Jankiewicz U, Burkowska A, Walczak M (2014). Chitinolytic microorganisms and their possible application in environmental protection. Curr. Microbiol. 68(1):71-81.
Crossref
|
|
|
|
|
Campbell WC, Fisher MH, Stapley EO, Albers SG, Jacob TA, Schonberg G (1983). Ivermectin: a potent new antiparasitic agent. Science 221(4613):823-828.
Crossref
|
|
|
|
|
Chin Y, Balunas MJ, Chai HB, Kinghorn AD (2006). Drug discovery from natural sources. Am. Assoc. Pharm. Sci. J. 8:E239-E253.
|
|
|
|
|
Costa MS, Araujo TRR, Martins AJ, Silva QAS, Costa CH, Miranda RCM (2017). Isolation of Microorganisms of Biotechnological interest from Areas Contaminated with Agrochemicals. Rev. Investig. Biomed. 9:17-23.
|
|
|
|
|
Davenport TP, Curtis M, Goodfellow FM, Stainsby MB (2000). Quatitative use of fluorescent in situ hybridization to examine relationships between mycolic acid-containing actinomycetes and foaming in activated sludge plants. Appl. Environ. Microbiol. 66:1158-1166.
Crossref
|
|
|
|
|
De Rossi RL, Reis EM, Brustolin R (2015). Conidial morphology and pathogenicity of Exserohilum turcicum isolates of corn from Argentina and Brazil. Summa Phytopathol. 41(1):58-63.
Crossref
|
|
|
|
|
De Schrijver A, De Mot RA (1999). Subfamily of MalT-related ATP-dependent regulators in the LuxR family. Microbiology 145:1287-1288.
Crossref
|
|
|
|
|
Dhingra OD, Mizubuti ESG, Napoleao IT, Jham G (2001). Free fatty acid accumulation and quality loss of stored soybean seeds invaded by Aspergillus ruber. Seed Sci. Technol. 29:193-203.
|
|
|
|
|
Dias, LRL, Bastos, DKL, Lima, NS, Silva, MRC, Miranda RCM (2017). Bioprospecting of Microorganisms with Biotechnological Interest Isolated in Mangrove Ecosystem. Rev. Investig. Biomed. 9:24-30.
|
|
|
|
|
Dietz A, Currie S, Jennie CHC, Angela B (1996). Chapter 5 - Actinomycetes. Maintaining cultures for biotechnology and industry. San Diego: Academic Press. pp. 85-99.
Crossref
|
|
|
|
|
Dindo ML (1993). The potential of plant compounds in insect control. Difesa delle Piante (Italy). 16(1):23-44.
|
|
|
|
|
Donadio S, Monciardini P, Sosio M (2007). Polyketide synthases and non-ribosomal peptide synthetases: The emerging view from bacterial genomics. Nat. Prod. Rep. 24:1073-1109.
Crossref
|
|
|
|
|
Dutton R, Gueguen F (1999). A new insecticide from Dow AgroSciences. Proceedings of the Fifth International Conference on Pests in Agriculture, Part I, Montpellier, France, 7-9 December, 191-200.
|
|
|
|
|
Feitosa TR, Arruda FVF, Cavalcanti FJRN, Baptista NMQ, Callou MJA, SILVA T, Miranda RCM, Gusmão NB (2014). Antimicrobial activity of fungi isolated from the water of the sky high, Recife-PE supply against bacteria of clinical interest system. Afr. J. Microbiol. Res. 8:2999-3007.
Crossref
|
|
|
|
|
Ferrarini H (2004). Determinação de teores nutricionais do milho por espectroscopia no infravermelho e calibração multivariada. Dissertation - Curso de Pós graduação em Química, Universidade Federal do Paraná, Curitiba, Brazil.
|
|
|
|
|
Food and Agriculture Organization of the United Nations (FAO) (2010). The State of Food Insecurity in the World - Addressing food insecurity in protracted crises. Rome, Italy ISBN 978-92-5-106610-2.
|
|
|
|
|
Gadelhak G, EL-Tarabily KA, Al-Kaabi FK (2005). Insect control using chitinolytic soil actinomycetes as biocontrol agents. Int. J. Agric. Biol. 7(4):627-633.
|
|
|
|
|
Ganesan A (2008). The impact of natural products upon drug discovery. Curr. Opin. Chem. Biol. 12:306-317.
Crossref
|
|
|
|
|
Godfray HCJ, Garnett T (2014). Food Security and Sustainable Intensification. Phil. Trans. R. Soc. B. 369(1639):20120273.
Crossref
|
|
|
|
|
Gupta R, Saxena RK, Chaturvedi P, Virdi JS (1995). Chitinase production by Streptomyces viridificans: its potential in fungal cell wall lysis. J. Appl. Bacteriol. 78:378-383.
Crossref
|
|
|
|
|
Hammock BD (1985). Regulation of juvenile hormone titer: Degradation. In. G. A. Kerkut and L. I. Gilbert [eds.], Comprehensive insect physiology, biochemistry and pharmacology. Insect control. Pergamon, New York. 7:431-472.
|
|
|
|
|
Hammock BD, Abdel-Aa YAI, Ashour A, Buehler A, Hanzlik TN, Newit R, Sparks TC (1986). Paradigms for the discovery of new insect control agents. In. M. Sasa, I. Yamamoto, S. Matsunaka, and K. Ohsawa leds], Human Welfare and Pest Control Chemicals. Proceedings of the XVlll Pesticide Science Society Symposium and Public Lectures. Nissas Science Foundation, Tokyo. pp. 53-72.
|
|
|
|
|
Hokkanen HMT, Lynch JM (1995). Biological Control: Benefits and Risks. Cambridge University Press, New York.
Crossref
|
|
|
|
|
Hussain AA, Mostafa SA, Ghazal SA, Ibrahim SY (2002). Studies on antifungal antibiotic and bioinsecticidal activities of some actinomycete isolates. Afr. J. Mycol. Biotechnol. 10:63-80.
|
|
|
|
|
Ichikawa N, Sasagawa M, Yamamoto M, Komaki H (2013). Dobiscuit: A database of secondary metabolite biosynthetic gene clusters. Nucleic Acids Res. 41:D408-D414.
Crossref
|
|
|
|
|
Kamara V, Gangwar M (2015). Original Research Article Antifungal Activity of Actinomycetes from Rhizospheric Soil of Medicinal plants against phytopathogenic fungi. Int. J. Curr. Microbiol. Appl. Sci. 4(3):182-187.
|
|
|
|
|
Kavitha A, Vijayalakshmi M, Sudhakar P, Narasimha G (2010). Screening of Actinomycete Strains for the Production of Antifungal Metabolites. Afr. J. Microbiol. Res. 4:027-032.
|
|
|
|
|
Kedia A, Prakash B, Mishra PK, Dubey NK (2014). Antifungal and antiaflatoxigenic properties of Cuminum cyminum (L.) seed essential oil and its efficacy as a preservative in stored commodities. Int. J. Food Microbiol. 7:168-169.
Crossref
|
|
|
|
|
Kirst HA (2010). The spinosyn family of insecticides: realizing the potential of natural products research. J. Antibiot. 63:101-111.
Crossref
|
|
|
|
|
Kirst HA, Michel KH, Mynderse JS, Chio EH, Yao RC, Nakatsukasa WM, Boeck LD, Occolowitz JL, Paschal JW, Deeter JB, Thompson GD (1992). Discovery, isolation, and structure elucidation of a family of structurally unique, fermentation-derived tetracyclic macrolides. In: Baker DR., Fenyes JG & Steffens JJ (Eds) Synthesis and Chemistry of Agrochemicals III. American Chemical Society, Washington, DC. pp. 214-225
Crossref
|
|
|
|
|
Lasota JA, Dybas RA (1991). Avermectins, a novel class of compounds: implications for use in arthropod pest control. Annu. Rev. Entomol. 36:91-117.
Crossref
|
|
|
|
|
Long CA, Wu Z, Deng BX (2005). Biological Control of Pennicillium italicum of citrus and Botrytis cinerea of grape by strain 34-9 of Kloeckera apiculata. Eur. Food Res. Technol. 221(1-2):197-201.
Crossref
|
|
|
|
|
Manulis S, Shafir H, Epteinamnon E, Barash I (1994). Biosynthesis of indole-3-acetic acid via the indole-3-acetarnide pathway in Streptomyces spp. Microbiology 140:1045-1050.
Crossref
|
|
|
|
|
Marinelli F, Marcone GL (2011). Microbial secondary metabolites. Comprehensive biotechnology (second edition). Burlington, American: Academic Press. pp. 285-297.
Crossref
|
|
|
|
|
Mertz FP, Yao RC (1990). Sacclwropolyspora spinosa sp. nov. isolated from soil collected in a sugar mill. Int. J. Syst. Evol. Microbiol. 40(1):34-39.
|
|
|
|
|
Mishra AK, Tsachaki M, Rister J, Ng J, Celik A, Sprecher SG (2013). Binary cell fate decisions and fate transformation in the Drosophila larval eye. PLoS Genet. 9(12):e1004027.
Crossref
|
|
|
|
|
Mishra SK, Keller JE, Miller JR, Heisey RM, Nair MG, Putnam AR (1987). Insecticidal and nematicidal properties of microbial metabolites. J. Ind. Microbiol. 2(5):267-276.
Crossref
|
|
|
|
|
Moënne-Loccoz Y, Mavingui P, Combes C, Normand P (2014). Microorganisms and biotic interactions. In. Bertrand JC, Caumette P, Lebaron P, Matheron R, editors. Environmental microbiology: fundamentals and applications. Dortrecht, Netherlands: Springer. pp. 395-444.
|
|
|
Naine J, Srinivasan MV, Devi SC (2011). Novel anticancer compounds from marine actinomycetes: A review. J. Pharm. Res. 4:1285-1287.
|
|
|
|
Newman DJ, Cragg GM (2012). Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 75(6):311-335.
Crossref
|
|
|
|
|
Olano C, Lombo F, Mendez C, Salas JA (2008). Improving production of bioactive secondary metabolites in Actinomycetes by metabolic engineering. Metab. Eng. 10:281-292
Crossref
|
|
|
|
|
Pakdeevaraporn P, Wasee S, Taylor PWJ, Mongkolporn O (2005). Inheritance of resistance to anthracnose caused by Colletotrichum capsici in Capsicum. Plant Breed. 124(2):206-208.
Crossref
|
|
|
|
|
Prakash S, Johnson RE, Prakash L (2005). Eukaryotic translesion synthesis DNA polymerases: specificity of structure and function. Annu. Rev. Biochem. 74:317-353.
Crossref
|
|
|
|
|
Prakash B, Kedia A, Mishra PK, Dubey NK (2014). Antifungal and antiaflatoxigenic properties of Cuminum cyminum (L.) seed essential oil and its efficacy as a preservative in stored commodities. Int. J. Food Microbiol. 168:1-7.
|
|
|
|
|
Qin S, Li J, Chen H, Zhao G, Zhu W, Jiang C, Xu L, Li W (2009). Isolation, Diversity, and Antimicrobial Activity of Rare Actinobacteria from Medicinal Plants of Tropical Rain Forest in Xishuangbanna, China. Appl. Environ. Microbiol. 75:6176-6186.
Crossref
|
|
|
|
|
Raja A, Prabakarana P (2011). Actinomycetes and drug-an overview. Am. J. Drug Discov. Dev. 1(2):75-84.
Crossref
|
|
|
|
|
Sanchez S, Demain AL (2002). Metabolic regulation of fermentation processes. Enzyme Microb. Technol. 31:895-906.
Crossref
|
|
|
|
|
Schoenian, I, Spiteller MM, Manoj MJ (2014). Chemical basis of the synergism and antagonism in microbial communities in the nests of leaf cutting ants. Proc. Natl. Acad. Sci. USA. 108(5):1955-1960.
Crossref
|
|
|
|
|
Scholl PJ, Guillot FS, Wang GT (1992). Moxidectin: systemic activity against common cattle grubs (Hypoderma lineatum) (Diptera: Oestridae) and trichostrongyle nematodes in cattle. Vet.Parasitol. 41(3-4):203-209.
Crossref
|
|
|
|
|
Shad SA, Fazal S, Saleem MA, Zaka MA (2012). Field evolved resistance to carbamates, organophosphates, pyrethroids, and new chemistry insecticides in Spodoptera litura Fab. (Lepidoptera: Noctuidae). J. Pest Sci. 85(1):153-162.
Crossref
|
|
|
|
|
Sharma M, Dangi P, Choudhary M (2014). Actinomycetes: Source, Identification, and Their Applications. Int. J. Curr. Microbiol. Appl. Sci. 3(2):801-832.
|
|
|
|
|
Shojaee N, Shahidi BGH, Shahdaei S, Leyla SB (2014). Biological control of citrus green mould, Penicillium digitatum, by antifungal activities of Streptomyces isolates from agricultural soils. Afr. J. Microbiol. Res. 8(14):1501-1509.
Crossref
|
|
|
|
|
Sinha K, Hegde R, Kush A (2014). Exploration on native actinomycetes strains and their potential against fungal plant pathogens Int. J. Curr. Microbiol. Appl. Sci. 3(11):37-45.
|
|
|
|
|
Sparks TC (1981). Development of insecticide resistance in Heliothis zea and Heliothis virescens in North America. Bull. Entomol. Soc. Am. 27:186-192.
Crossref
|
|
|
|
|
Sparks TC, Graves JB, Leonard BR (1993). Insecticide resistance and the tobacco budworm: Past, present and future. In: R. M. Roe and R. J. Kuhr leds. Reviews in pesticide toxicology. Toxicology Communications, Raleigh, NC. 2:149-183.
|
|
|
|
|
Sparks TC, Hammock BD (1983). Insect growth regulators: Resistance and the future. In: G. P. Georghiou and T. Saito Ieds. Pest resistance to pesticides. Plenum, New York. pp. 615-668.
Crossref
|
|
|
|
|
Sparks TC, Shour MH, Wellemeyer EG (1982). Temperature-toxicity relationships of pyrethroids on three lepidopterans. J. Econ. Entomol. 75:643-646.
Crossref
|
|
|
|
|
Sparks TC, Thompson GD, Kirst HA, Hertlein MB, Larson LI, Worden TV, Thibault ST (1998). Biological Activity of the Spinosyns, New Fermentation Derived Insect Control Agents, on Tobacco Budworm (Lepidoptera: Noctuidae) Larvae. J. Econ. Entomol. 91(6):1277-1283.
Crossref
|
|
|
|
|
Spindler KD, Spindler-Barth M (1994). Inhibition of chitinolytic enzymes from Streptomyces griseus (bacteria), Artemia salina (Crustacea), and a cell line from Chironomus tentans (Insecta) by allosamidin and isoallosamidin. Pest Manage. Sci. 40(2):113-120.
Crossref
|
|
|
|
|
Strobel RJ, Nakatsukasa WM (1993). Response surface methods for optimizing Saccharopolyspora spinosa, a novel macrolide producer J. Ind. Microbiol. 11:121-127.
Crossref
|
|
|
|
|
Subbanna ARNS, Rajasekhara H, Stanley J, Mishra KK, Pattanayak A (2018). Pesticidal prospectives of chitinolytic bacteria in agricultural pest management. Soil Biol. Biochem. 116:52-66.
Crossref
|
|
|
|
|
Sundarapandian S, Sundaram MD, Tholkappian P, Balasubramanian V (2002). Mosquitocidal properties of indigenous fungi and actinomycetes against Culex quinquefasciatus Say. J. Biol. Control 16:89-91.
|
|
|
|
|
Tahvonen R (1982a). Preleminary experiments into the use of Streptomyces spp. isolated from peat in the biological control of soil and seedborne disease in peat culture, in peat culture. J. Agric. Sci. Finl. 54:357-369.
|
|
|
|
|
Tahvonen R (1982b). The suppressiveness of Finnish light coloured Sphagnum peat. J. Agric. Sci. Finl. 54:345-356.
|
|
|
|
|
Takahashi A, Kurasawa S, Ikeda D, Okami Y, Takeuchi T (1989). Altemicidin, a new acaricidal and antitumor substance. I. Taxonomy, fermentation, isolation and physicochemical and biological properties. J. Antibiot. 42(11):1556-1561.
Crossref
|
|
|
|
|
Tinatin D, Nurzat T (2006). Biodiversity of Streptomyces of high-mountainous ecosystems of Kyrgystan and its biotechnological potential. Antonie Leeuwenhoek 89:325-328.
Crossref
|
|
|
|
|
United States Department of Agriculture (USDA) (2017). Agriculture Outlook Forum, Cotton Outlook, Fev, 2017.
|
|
|
|
|
Vaishnav P, Demain AL (2010). Unexpected applications of secondary metabolites. Biotechnol. Adv. 29:223-229.
Crossref
|
|
|
|
|
Ventura M, Canchaya C, Tauch A, Chandra G, Fitzgerald GF, Chater KF, Van Sinderen D (2007). Genomics of Actinobacteria: Tracing the evolutionary history of an ancient phylum. Microbiol. Mol. Biol. Rev. 71:495-548.
Crossref
|
|
|
|
|
Veríssimo MP, Xavier CL (2015). Tipos de commodities, taxa de câmbio e crescimento econômico: evidências da maldição dos recursos naturais para o Brasil. Rev. Econ. Contemp. Rio de Janeiro 18(2):267-295.
|
|
|
|
|
Vijayabharathi R, Kumari BR, Sathya A, Srinivas V, Abhishek R, Sharma HC, Gopalakrishnan S (2014). Biological activity of entomopathogenic actinomycetes against lepidopteran insects (Noctuidae: Lepidoptera), International Crops Research Institute for the Semi-Arid.
|
|
|
|
|
Vijayan, V, Balaraman K (1991). Metabolites of fungi & actinomycetes active against mosquito larvae. Indian J. Med. Res. 93:115-117.
|
|
|
|
|
Waldron C, Madduri K, Crawford K, Merlo DJ, Treadway P, Broughton MC, Baltz RH (2000). A cluster of genes for the biosynthesis of spinosyns, novel macrolide insect control agents produced by Saccharopolyspora spinosa. Antonie van Leeuwenhoek 78:385-390.
Crossref
|
|
|
|
|
Wiley J (2018). Fungi: Biology and Applications, Third Edition Book. Edited by Kevin Kavanagh. John Wiley & Sons, Inc. Publishers.
|
|