ABSTRACT
Heavy metals are transition elements whose presence at mild doses in water bodies is deadly. Most effectively harnessed technique of their remediation is the microbial approach, also known as bio-remediation. This study is hereby aimed to investigate the potential of Klebsiella species isolated from diesel polluted soil in bioremediating the heavy metals present in effluent water. A sample of diesel polluted soil was obtained from an automobile repair shop in Ile-Ife, Osun State Nigeria. Bacterial strains from the sample were isolated, characterized and identified by biochemical techniques. Three of the isolates were found to be Klebsiella species. Each of the strains and their consortium were administered in a 16-day bioremediation study, into sterile digested effluent water from a stream in Ile-Ife, Osun State, Nigeria. Each of the strains was tolerant to the presence of the heavy metals compared to the consortium, except with Chromium which gave consistency in both administrations. The Klebsiella species were very tolerant to Chromium. Cadmium follows suit with copper in better tolerance while there seem much resistance to Nickel. The concentration of Chromium was reduced from 2.718 to 0.046 mg L-1, 0.039, 0.041 and 0.047 mg L-1 by Klebsiella edwardsii, Klebsiella oxytoca, Klebsiella pneumoniae and the consortium of the strains, respectively. Cadmium was also reduced from 0.027 to 0.002 mg L-1, 0.001, 0.003 and 0.002 mg L-1 by K.edwardsii, K. oxytoca, K. pneumoniae and the consortium of the strains, respectively. The same list of organisms respectively reduced copper from 0.173 to 0.022 mg L-1, 0.025 0.018 and 0.030 mg L-1, and reduced Nickel from 0.103 to 0.019 mg L-1, 0.020, 0.017, and 0.017 mg L-1, respectively. It can be concluded that the trend of each microbe’s and the consortium’s affinity for the heavy metals uptake is predictably in the trend Cr > Cd > Cu > Ni.
Key words: Bio-remediation, diesel polluted soil, effluent water, tolerant, resistant, heavy metals, Klebsiella species.
The main threats to human health from heavy metals are associated with exposure to lead, cadmium, mercury and arsenic amongst others. These metals have been extensively studied and their effects on human health are regularly reviewed by international bodies such as the World Health Organization (WHO). Heavy metals have been used by humans or involved in most human activities such as mining operations, metal smelting, foundry works, metal moulding operations, etc. for thousands of years (Järup, 2003).
Although, several adverse health effects of heavy metals have been known for a long time, and exposure to these metals is increasing every day. This is being experienced in some parts of the world, in particular in less developed countries (Järup, 2003). The commonest techniques of removal of heavy metals include chemical precipitation, oxidation or reduction, filtration, ion-exchange, reverse osmosis, membrane technology, evaporation and electrochemical treatment. These techniques become ineffective when the concentrations of heavy metals are less than 100 mg L-1 hence, need for the use of microbes for remediation (Ahluwalia and Goyal, 2007).
Indigenous diesel polluted sites are potential sources of heavy metals degraders other than the hydrocarbon itself (Akpoveta and Osakwe, 2010). Diesel, a refined petroleum fraction, is known to contain a known range or limit of heavy metals as part of its constituents. Hence, continual spillage of the diesel on both soil and water bodies, makes the host bodies prone to having accumulating doses of these heavy metals.
Heavy metals are evident constituents of petroleum diesel for heavy duty engines, which constitutes significant content of exhausts’ particulate matter (Akpoveta and Osakwe, 2010; Bharathi et al., 2005; Sharma et al., 2005). Amongst the many inadvertent use of the diesel is its spillage at many automobile workshop centres on both the soil and water drainages as well as through pipeline bunkering. Hence, increase in bioavailability of these heavy metals in these host bodies is inevitable.
Due to these activities, heavy metals in diesel oil can be deposited in the soil and enter the human food web thereby constituting risk to the ecosystem, as they tend to bio-accumulate which can be transferred from one food chain to another. Consequently they are discovered in various food chains, where the results are usually detrimental to plants, animals and humans alike (Abdu et al., 2007).
Most of the deleterious cases of heavy metals contamination are treated biologically, based on the safe metabolic pathways peculiar to the degrading agents and the environmental friendliness of the process involved, leaving no pollutants to the risk of the sustainable environment (Mulligan et al., 2001; Gavrilescu, 2004; Rezaee et al., 2005; Elouzi et al., 2012).
Biological treatment is an innovative technology available for heavy metal polluted wastewater. Since microorganisms have developed survival strategies in heavy metal polluted habitats, their different microbial detoxifying mechanisms such as bio-accumulation, bio- transformation, bio-mineralization or biosorption can be applied either ex situ or in situ to design economical bioremediation processes (Dixit et al., 2015; Lin and Lin, 2005; Malik, 2004). Many are the cases of the heavy metals removal by biosorption with further transformation thereafter (Volesky, 1990).
Uptake of heavy metals into the cellular structures of the microbial agent is dependent on the tolerance of the organism for the particular heavy metal (Mustapha and Halimoon, 2015; Rajendran et al., 2003). According to Dixit et al. (2015), the uptakes of heavy metals in the microorganisms are actively (bio-accumulation) and/or passively (adsorption).
The microbial cell walls, which mainly consist of polysaccharides, lipids and proteins, offer many functional groups that can bind heavy metal ions, and these include carboxylate, hydroxyl, amino and phosphate groups. Among various microbe-mediated methods, the biosorption process seems to be more feasible for large scale application compared to the bio-accumulation process, because microbes will require addition of nutrients for their active uptake of heavy metals, which increases the biological oxygen demand or chemical oxygen demand in the waste.
Several microorganisms have been extensively studied for heavy metal bio-remediation. These include the fungi of genera Penicillium, Aspergillus and Rhizopus; and the bacterial species being Bacillus and Pseudomonas species (Huang and Huang, 1996, Volesky and Holan, 1995). However, amongst bacterial strains of unique and significant tolerance for heavy metals, which are yet to be researched extensively are the Klebsiella species. They are gram-negative, facultative, non-motile, usually encapsulated rod-shaped bacteria belonging to the family Enterobacteriaceae (Niemelä and Väätänen, 1982). These are often found in pristine and hydrocarbon polluted sites, such that they can degrade the heavy metals tolerable to them and the hydrocarbons as well (Kumaran et al., 2011; Nwinyi et al., 2014; Rodrigues et al., 2009).
Klebsiella pneumoniae and Klebsiella oxytoca are well-known strains with high tolerance for cadmium and arsenic, respectively (Shakoori et al., 2010, Shamim and Rehman, 2012). This study is hereby aimed to investigate the potential of Klebsiella species isolated from diesel polluted soil in bioremediating the heavy metals present in effluent water obtained from Ile-Ife community, Osun State Nigeria..
The effluent water bio-remediated for heavy metals, are obtained from the stream in Ile-Ife community, having its source from automobile workshops and filling station. Reagents and salts used were all of analytical grade.
Microbial isolation, characterization and identification
The soil sample was prepared by suspending 0.5 g of the diesel-oil-polluted soil sample, in 50 ml of sterile distilled water, in a 100 ml conical flask in which some glass chips were inserted. The flask was carefully agitated to obtain a uniform suspension. 0.1 ml of the suspension was taken and added to 9.9 ml of distilled water in test tube A, then 0.1 ml of the suspension in test tube A was taken and added to 9.9 ml of distilled water in test tube B. 1.0 mm of each dilution in tubes A and B was transferred into properly labelled petri dishes, respectively. Culturing was done by making use of 20 ml molten sterile Eosin Methylene Blue (EMB) agar. The culture plate was carefully rotated to mix the cell suspension with the medium. The culture plates were allowed to stand for the EMB agar to set. They were incubated at 35°C for 48 h. Colonial characteristics of the mixed culture obtained were observed. The differential colonies were isolated by sub-culturing into nutrient agar slants and labelled accordingly for use. The 18 h cultures were gram stained, to study the morphological characteristics of the cultures (i.e. cell shape, cell arrangement and gram reaction) and to ensure their purification.
Gram staining process
According to Gunasekaran (2007), microscope slides were soaked in chromic acid, washed properly and cleaned with cotton wool soaked in ethanol. The slides were labelled. A sterile inoculating loop was used to transfer a loopful of the broth culture and spread on a slide to obtain a thin film (smear). The smear was heat fixed, flooded with crystal violet for 60 s and rinsed off with gram iodine solution for absorption to take place. The iodine was left to act for 1min. Decolourization of the smears was done by adding 95% ethanol for 60 s and rinsed off with tap water. The slides were counterstained with 1% carbolfuschsin for 1 min after which, they were rinsed with tap water to stop the reaction and blot dried. The prepared slides were then viewed under a microscope and the results recorded.
Biochemical characterization of bacterial isolates
The bacterial isolates were subjected to various biochemical procedures for characterization, according to Collins (1989). These are:
1. Triple sugar ion test
2. Sulphide-indole-motility test
3. Catalase test
4. Citrate utilization test
5. Methyl red test
6. Voges-proskauer test
7. Nitrate reduction
8. Oxidation fermentation test
9. Oxidase test
10. Sugar fermentation test
Heavy metal resistance test
Molten sterile nutrient agar containing the heavy metals (Cu2+, Ni2+, Cd2+ and Cr3+) were prepared in varying concentrations and a loopful of each bacteria isolate was collected and streaked on the surface of the heavy metal enriched medium. This procedure was carried out for each of the isolated microorganisms. Thereafter, the plates were incubated at 37°C for 24 h and monitored for growth.
Digestion of water samples for bioremediation
A digestion process was carried out on 10 ml of effluent water sample. 10 mm of aqua regia and 1 ml of perchloric acid were added into the measured water sample, in a conical flask. The mixture was then placed in a water bath at 80°C.
After total digestion and subsequent cooling, the resulting solution was diluted to 50 ml mark of a 50 mm standard volumetric flask with de-ionized water and analysed for heavy metals using atomic absorption spectrophotometer, AANALYST400 (Ogoyi et al., 2011). The heavy metals analysed were Cu, Ni, Cr and Cd.
Inoculum preparation
A suspension of standardized bacterial-saline medium was constituted for each isolate and the consortium. Each of the isolates was grown in nutrient broth for 18 hours, and the bacterial cells pelleted by centrifugation at 4000 rpm for 15 min at 4
oC using Eppendorf Centrifuge 5804R, New Brunswick, New Jersey, USA. The pellet was then rinsed twice and resuspended using 0.85 % NaCl to give an OD
600nm of

0.5. The consortium culture was prepared by mixing each of the single isolates in equal portion and top up with 0.85 % NaCl to give a final reading of OD
600nm 
0.5, corresponding to approximately 1

10
7 colony-forming units per ml as determined by spread plate method. This was used as the starting culture in all subsequent experiments (Basha and Rajaganesh, 2014; Wong et al., 2015).
Bio-remediation of heavy metals
250 mm of Bushnell-haas medium was prepared and 10 ml was distributed into four conical flasks, each of which contains 10 ml of diesel-polluted-water. Sterilization by autoclaving was done at 121°C, 15 psi for 15 min.
The sterile digested water sample was aseptically inoculated with 5 ml of the inoculum, of pure and mixed cultures of Klebsiella species. The flasks were incubated on a New Brunswick Gyratory shaker for 16 days. Samples were withdrawn for four days interval for analysis, using the atomic absorption spectrophotometer.
Isolation, gram staining, biochemical characterization of isolates and heavy metals resistance tests
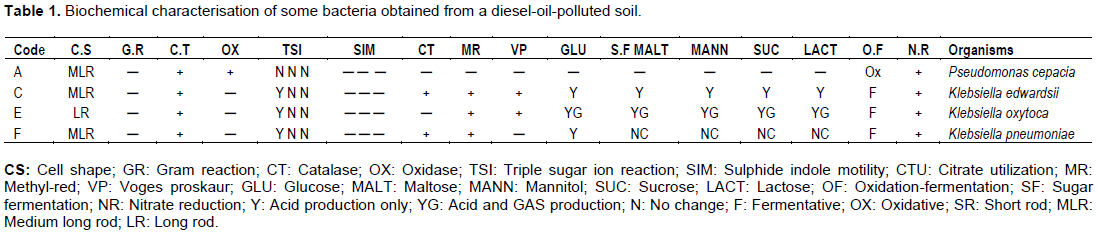
Four bacterial isolates were obtained from the diesel-oil-polluted soil with each of them, being given an isolate code. Colonial characteristics of the individual isolates showed that Isolate A was large, regular, slightly raised and violet at the centre on EMB agar. Isolate C was large, regular, raised and sticky. Isolate E was irregular, spread, slightly raised, mucoid and sticky while organism F was seen to be large, regular, flat, rough and yellowish in colour. Gram staining result showed long gram negative bacilli chains.
Table 1 shows the results of the morphological characterization of the isolates. Based on the colonial, morphological and biochemical characteristics, the isolates were identified as Pseudomonas cepacia (isolate A), Klebsiella edwardsii (isolate C), Klebsiella oxytoca (isolate E) and Klebsiella pneumoniae (isolate F). This result was in agreement with the work of Adeyemo et al. (2013) in which, Klebsiella species was isolated from a diesel contaminated soil. Isolates C, E, and F showed better growth on the sterile EMB agar than isolate A. P. cepacia, K. edwardsii, K. oxytoca and K. pneumoniae were resistant to Ni and Cd which is corroborated by the work of Kumaran et al. (2011).
Bio-remediation of heavy metals
Using Klebsiella edwardsii
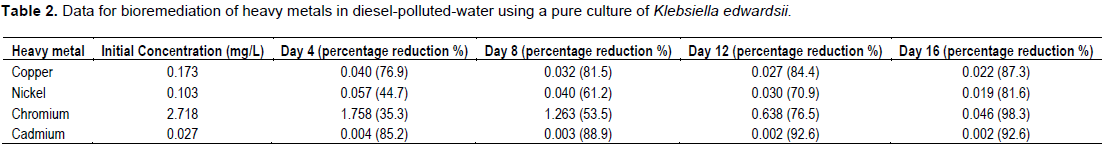
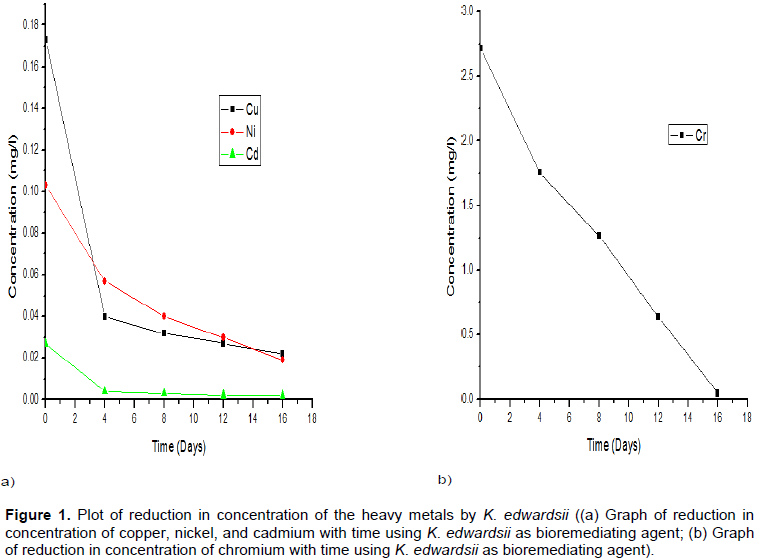
The obtained results of the determined concentrations of each heavy metals analysed are as stated in Table 2. The Table 2 further shows the reduction in heavy metal concentration at intervals of 4 days. The results indicate that, K. edwardsii has an increasing tolerance of all the metals but most for Chromium through the 16 days of the study.
Using Klebsiella oxytoca
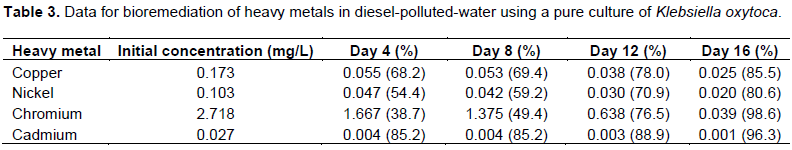
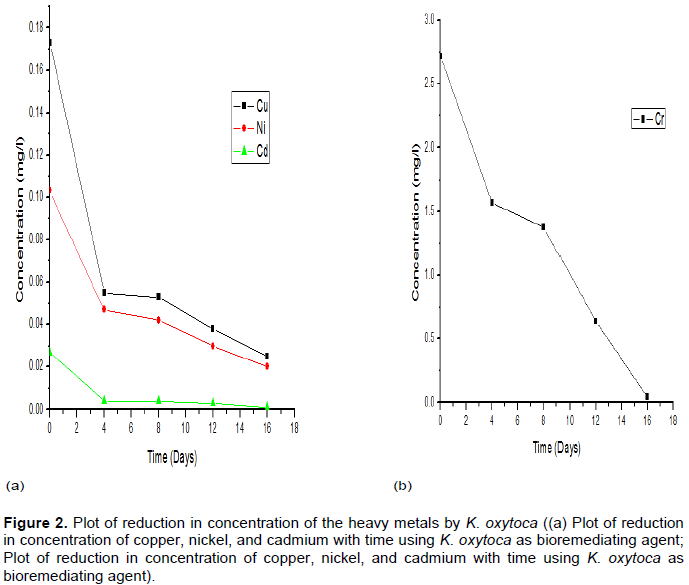
Similarly, K. oxytoca gave results as contained in Table 3. The results in Table 3 show that the tolerance of K. oxytoca after the first 4 days was least for Chromium among the metals but most after the complete 16 days.
The strain showed a progressive affinity for the metals in the order of Cr>Ni>Cu>Cd. The result also shows that K. oxytoca significantly reduced cadmium just within the first 4 days. The trend of the reduction in concentration is also depicted in Figure 2.
Using Klebsiella pneumonia
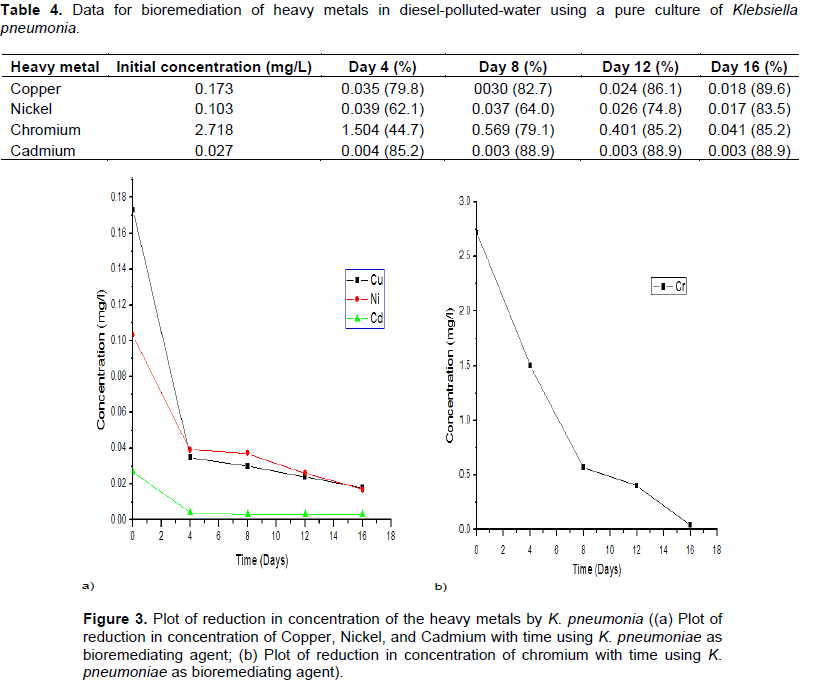
K. pneumoniae showed high tolerance for selected heavy metals in such a way that, it was most for Chromium but least with Cadmium after the first 4 days. Table 4 and Figure 3 reveal the trend of degradation.
Using the consortium of the Klebsiella species
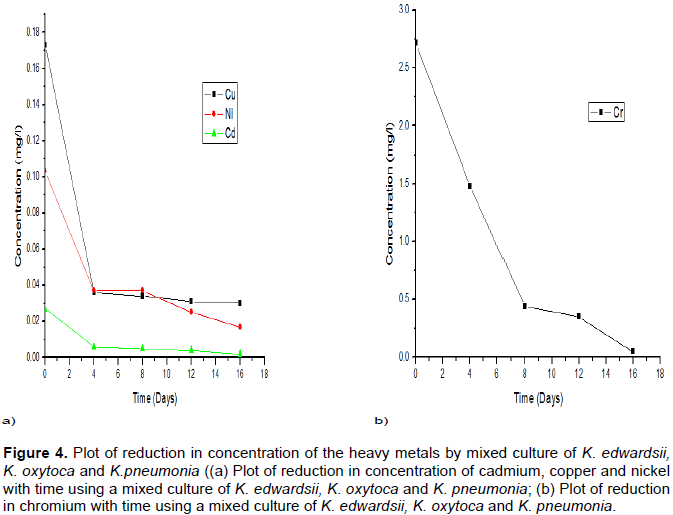
The potency of the consortium, except Chromium in the bioremediation was observed to be relatively low compared to the significant reduction by the use of each Klebsiella species isolates as seen in Tables 2 to 4 and Figures 1 to 3.
The results are contained in Table 5 and Figure 4. It is seen in each that, the tolerance of the strains dropped with the individual strain’s interference on the metabolic paths. This implies that the interaction of the metals or metal ions on their sorption properties by the strains of study reduces the remediation potential of the organisms. However, the use of the mixed strains proved more tolerant for Chromium as higher reduction in its concentration was obtained across the first 12 days of study before the obvious desorption. The affinity for Cadmium was in line with the result of Shamim and Rehman (2012).
All the gram negative Klebsiella species isolates were tolerant to the heavy metals, though with variations. The isolates were capable of reducing the selected heavy metals’ concentration unlike the known facts about gram positive and a few gram negative bacterial isolates according to Lima e Silva et al. (2012). The sensitivity of each of the isolates and their consortium was greatest for chromium..
A few heavy metals tolerant- and degrading-bacteria exist in the niche of soil collection. The pure isolates were P. cepacia, K. edwardsii, K. oxytoca and K. pneumoniae. The trend of each microbe’s and the consortium’s affinity for the heavy metals uptake is, predictably in the trend Cr > Cd > Cu > Ni.
The author(s) have not declared any conflict of interests.
REFERENCES
|
Abdu N, Muazu M, Olufajo O, Omokore D, Akpa G (2007) Trace metals accumulation in urban soil: effects of municipal waste fertilization. Proceedings of The 41st Annual Conference of The Agricultural Society of Nigeria, pp. 289-293.
|
|
|
|
Adeyemo I, Agbolade J, Olufunmilola O (2013). Microflora associated with diesel powered generators contaminated soil arena of obafemi awolowo and oduduwa universities in ile–ife, Osun State, Nigeria. Glob. J. Biol. Agric. Health Sci. 2:65-73.
|
|
|
|
Ahluwalia SS, Goyal D (2007). Microbial and plant derived biomass for removal of heavy metals from wastewater. Bioresour. Technol. 98:2243-2257.
Crossref
|
|
|
|
Akpoveta O, Osakwe S (2010). Determination of heavy metal content in refined petroleum products sold in agbor metropolis, Delta State, Nigeria. 33rd International Conference of the Chemical Society Of Nigeria. Kuto, Abeokuta, Ogun State, Nigeria, 10.
|
|
|
|
Basha SA, Rajaganesh K (2014). Microbial bioremediation of heavy metals from textile industry dye effluents using isolated bacterial strains. Int. J. Curr. Microbiol. Appl. Sci. 3:785-794.
|
|
|
|
Bharathi K, Dwivedi D, Sharma M, Agarwal AK (2005). Diesel exhaust particulate characterization for poly aromatic hydrocarbons and benzene soluble fraction. SAE Technical Paper.
Crossref
|
|
|
|
Collins C (1989). Collins and Lyne's microbiological methods, 6th edn Butterworths, London.
|
|
|
|
Dixit R, Malaviya D, Pandiyan K, Singh UB, Sahu A, Shukla R, Singh BP, Rai JP, Sharma PK, Lade H (2015). Bioremediation of heavy metals from soil and aquatic environment: an overview of principles and criteria of fundamental processes. Sustainability 7:2189-2212.
Crossref
|
|
|
|
Elouzi AA, Akasha AA, Elgerbi AM, El-Baseir M, El Gammudi BA (2012). Removal of heavy metals contamination by bio-surfactants (rhamnolipids). J. Chem. Pharm. Res. 4(9):4337-4341.
|
|
|
|
Gavrilescu M (2004). Removal of heavy metals from the environment by biosorption. Eng. Life Sci. 4:219-232.
Crossref
|
|
|
|
Gunasekaran P (2007). Laboratory manual in microbiology, New Age International.
|
|
|
|
Huang C, Huang C (1996). Application of aspergillus oryze and rhizopus oryzae for cu (ii) removal. Water Res. 30:1985-1990.
Crossref
|
|
|
|
Järup L (2003) Hazards of heavy metal contamination. Br. Med. Bull. 68:167-182.
Crossref
|
|
|
|
Kumaran N, Sundaramanickam A, Bragadeeswaran S (2011). Absorption studies on heavy metals by isolated bacterial strain (Pseudomonas sp.) from uppanar estuarine water, southeast coast of india. J. Appl. Sci. Environ. Sanit. 6:471-476.
|
|
|
|
Lin CC, Lin HL (2005). Remediation of soil contaminated with the heavy metal (cd 2+). J. Hazard. Mater. 122:7-15.
Crossref
|
|
|
|
Malik A (2004). Metal bioremediation through growing cells. Environ. Int. 30:261-278.
Crossref
|
|
|
|
Mulligan C, Yong R, Gibbs B (2001). Remediation technologies for metal-contaminated soils and groundwater: an evaluation. Eng. Geol. 60:193-207.
Crossref
|
|
|
|
Mustapha MU, Halimoon N (2015). Screening and isolation of heavy metal tolerant bacteria in industrial effluent. Proc. Environ. Sci. 30:33-37.
Crossref
|
|
|
|
Niemelä S, Väätänen P (1982). Survival in lake water of Klebsiella pneumoniae discharged by a paper mill. Appl. Environ. Microbiol. 44:264-269.
|
|
|
|
Nwinyi OC, Kanu IA, Tunde A, Ajanaku KO (2014). Characterization of diesel degrading bacterial species from contaminated tropical ecosystem. Braz. Arch. Biol. Technol. 57:789-796.
Crossref
|
|
|
|
Ogoyi D, Nguu E, Mwita C, Shiundu P (2011). Determination of heavy metal content in water, sediment and microalgae from Lake Victoria, East Africa. Open Environ. Eng. J. 4:156.
Crossref
|
|
|
|
Rajendran P, Muthukrishnan J, Gunasekaran P (2003). Microbes in heavy metal remediation. Ind. J. Exp. Biol. 41:935-944.
|
|
|
|
Rezaee A, Derayat J, Mortazavi S, Yamini Y, Jafarzadeh M (2005). Removal of mercury from chlor-alkali industry wastewater using Acetobacter xylinum cellulose. Am. J. Environ. Sci. 1:102-105.
Crossref
|
|
|
|
Rodrigues D, Sakata S, Comasseto J, Bicego M, Pellizari V (2009). Diversity of hydrocarbonâ€degrading klebsiella strains isolated from hydrocarbonâ€contaminated estuaries. J. Appl. Microbiol. 106:1304-1314.
Crossref
|
|
|
|
Shakoori FR, Aziz I, Rehman A, Shakoori A (2010). Isolation and characterization of arsenic reducing bacteria from industrial effluents and their potential use in bioremediation of wastewater. Pak. J. Zool. 42:331-338.
|
|
|
|
Shamim S, Rehman A (2012). Cadmium resistance and accumulation potential of Klebsiella pneumoniae strain cbl-1 isolated from industrial wastewater. Pak. J. Zool. 44:203-208.
|
|
|
|
Sharma M, Agarwal AK, Bharathi K (2005). Characterization of exhaust particulates from diesel engine. Atmos. Environ. 39:3023-3028.
Crossref
|
|
|
|
Volesky B (1990). Biosorption of heavy metals, CRC Press.
|
|
|
|
Volesky B, Holan Z (1995). Biosorption of heavy metals. Biotechnol. Prog. 11:235-250.
Crossref
|
|
|
|
Wong KK, Quilty B, Hamzah A, Surif S (2015). Phenol Biodegradation and metal removal by a mixed bacterial consortium. Bioremediat. J. 19(2):104-112.
Crossref
|