ABSTRACT
Yellow rust, caused by Puccinia striiformis f. sp. tritici, is one of the most important diseases of wheat in Ethiopia. Poor knowledge of resistance genes deployed in Ethiopian wheat cultivars is one major factor for recurrent epidemics of rust diseases. Molecular marker based gene identification showed the presence of Yr1, Yr4, Yr6, Yr7, Yr9, Yr10, Yr17 and Yr18 in various frequencies, whereas Yr8 was not detected in any of the tested 74 bread wheat genotypes. Yr7 was the most frequent (74%) followed by Yr6 (56%) and Yr 18 (37%) whereas Yr1 and Yr4 were detected in lower frequency (7 and 14%), respectively. The contribution of each Yr gene was evaluated in yellow rust differential lines possessing various genes. The differential lines carrying Yr9 and Yr8 had the highest average coefficient of infection (ACI) value (83 each) followed by Yr6 and Yr7 with ACI values of 82 and 80, respectively. The lowest ACI value (46.4) was exhibited on a differential line that carried Yr4. The number of Yr genes identified from the tested genotypes varied from 0 to 5. The ACI value exhibited by varieties possessing the maximum number of five resistance genes was 42. The majority (26 genotypes representing 35%) of the genotypes possessed three genes with an average coefficient of infection of 42. Pyramiding of the identified genes does not provide sufficient protection against yellow rust in Ethiopia. Thus, there is urgent need for searching for more effective resistance genes to be incorporated in Ethiopian bread wheat cultivars.
Key words: Average coefficient of infection (ACI), bread wheat, molecular markers, wheat genotypes, Yr genes.
Wheat is one of the staple food crops cultivated by 5 million small scale farmers in Ethiopia. It is ranked fourth in land coverage and total production after teff, maize and sorghum (CSA, 2017). Yellow rust, caused by Puccinia striiformis f. sp. tritici, is one of the most important diseases of wheat that incurs 30 to 69% yield loss in Ethiopia (Badebo et al., 2008). Growing yellow rust resistant cultivars is widely recognized as the most eco-friendly and economically feasible approach. Since the 1970s, more than 100 bread wheat varieties have been released in Ethiopia. However, the resistance is not lasting long due to the development of new virulent races of the pathogen. Poor knowledge of resistant genes deployed in Ethiopian wheat cultivars is one of the major reasons for recurrent epidemics of rust diseases. Those cultivars may be protected by the same resistance gene(s) or combinations, which could increase selection pressure for the corresponding virulent races. To date, more than 67 yellow rust resistant genes have been reported in wheat and its wild relatives (McIntosh et al., 2013). Most of these genes condition race-specific resistance and many have been overcome by the emergence of new races.
The most effective strategy for protecting wheat from rust is to deploy cultivars with combinations of different resistance genes. For this, information on the resistance genes in major cultivars is of paramount importance. The traditional way of gene postulation requires multi-pathotypes testing in which a host cultivar is evaluated against a collection of isolates carrying different avirulence/ virulence gene combinations (pathotypes) on the basis of phenotypic expression in the form of infection types (ITs). As an alternative to gene postulation, presence of resistance genes can be determined by testing host cultivars with molecular markers linked to resistance genes. This approach overcomes gene interactions and plant stage dependent gene expression problems associated with traditional gene postulation (Vanzetti et al., 2011).
Currently, there have been advances in development and mapping of molecular markers that are diagnostic for major Yr genes (Mago et al., 2002; Eriksen, 2004; Li and Niu, 2007; Bansal et al., 2009; Krattinger et al., 2009; Jia et al., 2011; Cabuk et al., 2011; Liu et al., 2014; Yaniv et al., 2015; Ahmad et al., 2015). These markers provide an important tool to plant breeders for marker assisted wheat breeding and also for pyramiding resistance genes in the absence of distinguishable rust virulences (Kaur et al., 2008). This study was conducted to identify yellow rust resistant gene (s) that are present in commercial bread wheat cultivars and elite lines using molecular markers linked to Yr genes, assess the effectiveness of the identified Yr genes to the prevailing races under field conditions in Ethiopia, and to evaluate genetic variations among the wheat cultivars and elite lines.
Plant materials
A total of 58 commercial bread wheat cultivars, 16 elite lines and 15 reference lines with known Yr genes and a negative check, Morocco, with unknown Yr genes and PBW345 were included in this study. All the wheat materials were obtained from the Ethiopian Institute of Agricultural Research (EIAR), KARC, Ethiopia. The list of wheat genotypes together with their additional information is shown in Supplementary Table 1.
Field testing
All wheat genotypes were evaluated for yellow rust reaction in three locations, namely, Mararo, Arsi Robe and Kulumsa under natural infection in 2016 and 2017. The materials were sown in two rows of 1 m length with 0.20 m spacing between rows. For scoring yellow rust severity in the field, the modified Cobb Scale (Peterson et al., 1948) was used to determine the percentage of tissue infected with rust. The host response to infection in the field was scored using ‘‘R’’ or resistant (small uredinia surrounded by chlorosis or necrosis); ‘‘MR’’ or moderately resistant (medium sized uredinia surrounded by chlorosis or necrosis); ‘‘MS’’ or moderately susceptible (medium large compatible uredinia without chlorosis and necrosis); and ‘‘S’’ or susceptible (large, compatible uredinia without chlorosis and necrosis). Disease severity and host response data were combined in a single value called the coefficient of infection (CI). The average coefficient of infection (ACI) was used for host response: immune = 0.0, R = 0.2, MR = 0.4, MS = 0.8, and S = 1.0.
Molecular markers
Two closely linked (usually flaking) markers of each of the major Yr genes were chosen to identify its presence/absence in wheat genotypes/materials, except a few genes for which only one closely linked marker was reported. A total of 31 markers that are linked to yellow rust resistant genes were used to identify Yr genes from the test materials as well as for genetic diversity analysis. Primer names, forward and reverse primer sequences, expected amplified fragment size in base pairs, and annealing temperature are shown in Table 1.
DNA extraction
DNA was extracted from 2 weeks old fresh leaves harvested and pooled from five seedlings of each accession and stored cryogenically at -80°C. Extraction from frozen leaves was performed using the modified cetyl trimethylammonium bromide (CTAB) method described by Doyle and Doyle (1990).
PCR amplification and fragment analysis
Polymerase chain reactions (PCR) were performed in Perkin-Elmer (Norwalk, CT) thermocyclers in a total volume of 25 μl containing 50 to 100 ng each template DNA, 250 nM cy5-labelled forward primer, 250 nM unlabelled reverse primer, 0.2 mM dNTPs, 2.5 μl PCR buffer (10x), 1.5 mM MgCl2, and 1U Taq DNA polymerase. After 3 min at 94°C, 45 cycles were performed with 1 min at 94°C, 1 min at either 50, 55, or 60°C (depending on the individual primer), 2 min at 72°C, and a final extension step of 10 min at 72°C. The PCR product was denatured at 94°C for 2 min and placed on a cold block until use. Each sample of 6 μl (4 μl PCR product and 2 μl internal markers) and one external standard marker of 6 μl were loaded in the preheated gel. For SSR markers, fragments were detected by an Automated Laser Fluorescence (ALF express) sequencer (Amersham Biosciences) and fragment sizes were calculated using the computer program Fragment Analyser 1.02 (Amersham Biosciences) by comparison with internal size standards. The EST-SSR, STM and SCAR markers were resolved in 2.0% agarose gels by loading 15 μl PCR products and the amplified fragments were stained with ethidium bromide and photographed.
Data analysis
Presence of Yr genes in the wheat genotypes was counted for each yellow rust resistance gene based on presence of the fragment sizes of both flaking markers, except for a few genes where one closely linked marker has been reported. This was explained by comparing with the reference lines with known Yr genes. For genetic diversity analysis, amplification profile of markers was recorded with each band representing a different allele, with a particular primer pair. Each allele was scored on the basis of presence of a band (scored as 1) and its absence (scored as 0) for generating a binary matrix which was further used to calculate Jaccard’s similarity coefficients for each pair of parents following NTSYS-PC program (Rohlf, 2000). Allelic Polymorphic Information Content (PIC) for each primer locus and genetic diversity was analysed with Power Marker version 3.25 Software (Liu and Muse, 2005).
Yellow rust reactions in field test
Average coefficient of infection of yellow rust for the wheat genotypes tested at three locations (Kulumsa, Arsi robe and Meraro) during 2016 and 2017 is shown in Table 2. Of the 74 test genotypes, nine cultivars showed resistance (R) with 0 to 10 ACI, 11 cultivars exhibited resistance to moderate resistance (R-MR) with ACI value of 10 to 20 and 14 genotypes showed moderately resistance (MR) with ACI value of 20 to 30, whereas 8, 9 and 23 of the genotypes exhibited moderately resistant to moderately susceptible (MR-MS), moderately susceptible (MS) and susceptible (S) with ACI values in ranges of 30 to 40, 40 to 50, and >50, respectively.
Identification of yellow rust resistance (Yr) genes using molecular markers
Yellow rust resistant gene, Yr1
Yr1 is a seedling resistance gene which is located on chromosome 2AL. An EST-SSR marker, BU099658 and STM marker, Stm673acag (1.1 cM linkage with Yr1 gene) were used to identify this gene in 74 wheat genotypes. The expected fragment size for BU099658 marker was 206 bp (Hasancebi et al., 2014). In the present study, the marker amplified DNA fragment sizes of 157 to 206 bp. The amplified fragment at 206 bp was monomorphic and detected in all the tested materials including the reference line (Yr1/6*Avocet S). The polymorphic DNA fragment size, 162 bp, which was produced in the reference line was used for identification of Yr1 gene. In this regard, the presence of Yr1 was detected in 11 (15%) of the genotypes. The expected fragment size for the second marker, Stm673acag was 120 to 124 bp (Bansal et al., 2009). This marker amplified fragment sizes from 118 to 129 bp in this study. The polymorphic fragment size of 129 bp which was produced in the reference line was used to predict theYr1 gene. Yr1 was identified in 11 (15%) of the tested genotypes. Only 5 (6.7%) of the genotypes exhibited similar fragment size to the reference lines for both markers.
Yellow rust resistant gene, Yr4
Yr4 which is synonymous with Yr4a, and Yr4b was originally derived from common wheat and it is located on chromosome 3B. An SSR marker, Barc075 was located at 2.4 ± 1.2 cM from YrRub/Yr4 (Bansal et al., 2010). This marker amplified a single 105 bp band in the reference line (Hybrid46) and the band was absent in wheat lines without Yr4. After evaluation by this marker, 10 (14%) cultivars, namely, Abola, HAR1018, HAR723, HAR820, Hawi, Hoggana, Jafersson, Madda Walabu, Simba, and Sulla were genotyped to carry Yr4 (Table 2).
Yellow rust resistance gene, Yr6
Li and Niu (2007) reported that the SSR markers Wmc076 and Wmc276 were linked to the Yr6 gene. These two markers amplified 256 and 292 bp fragments, respectively in most lines carrying Yr6 (Li and Niu, 2007). Marker Wmc76 amplified 256, 267 and 271 bp bands in the reference line (Yr6/6*Avocet S). The expected fragment size (256 bp) that indicates for the presence of this gene was used to genotype for Yr6 genes in the tested materials. Based on this marker, 70% of the wheat lines possessed this gene. The second marker, Wmc276 amplified a single band of size 289 bp in the Yr6/6*Avocet S and 292 bp in Pavon 76 (with known Yr6 gene). In this regard, both fragment sizes were considered to identify this gene from the tested lines. Based on this marker, around 63 (85%) of the wheat lines possess Yr6 (Table 2). Of the identified genotypes that possess this gene, 42 (56%) of them showed similar results for both markers.
Yellow rust resistant gene, Yr7
Yr7 was first identified in wheat (Triticum aestivum L.) cultivar ‘Lee’. It is located on the chromosome 2B (Deng et al., 2004). It is one of the Yr genes that was used widely by CIMMYT during the1970s and 1980s, and has been deployed in many commercial cultivars in Ethiopia. An SSR marker, Xgwm556, which is linked at 5.3 cM to the target gene was used to genotype for Yr7. The marker allowed null allele on nine of the tested genotypes. The fragment sizes produced by other genotypes varied between 131 and 160 bp. The marker amplified two different fragment sizes on two of the reference lines (Lee and Yr7/6*Avocet S). Thirty-two of the genotypes showed a fragment size of 157 bp, similar to Yr7/6*Avocet S. A fragment size of 159 bp was observed in 26 genotypes, which was similar to Lee. Six genotypes showed fragment sizes similar to the reference lines. Therefore, 55 (74%) of the genotypes that showed fragment sizes similar to the two reference lines were considered to possess Yr7 gene.
Yellow rust resistant gene, Yr8
Yr8 is located on chromosome 2D. SSR marker Xgwm157 was linked to Yr8 with a map distance of about 1.1 cM. The marker amplified a fragment size of 120 bp in wheat lines that possessed Yr8 genes. In this study, this marker amplified the expected fragment size (120 bp) in the reference line, Yr8/6*Avocet S only, whereas none of the tested wheat genotypes amplified fragment sizes similar to the reference lines, indicating the absence of this gene in all the tested genotypes (Table 3).
Yellow rust resistant gene, Yr9
Yr9 was transferred to wheat through chromosomal translocation of 1B/1R and is linked to the Lr26, Sr31 and Pm8 resistance genes (Zhou et al. 2004). It is common in CIMMYT-originated bread wheat cultivars. SSR marker Xgwm582 and a resistance gene analogue (RGA) clone marker (iag95) were linked to the Yr9 gene (Cabuk et al., 2011; Mago et al., 2002). Marker Xgwm582 amplified three bands (142, 149 and 152 bp) on the reference line. The observed fragment size, of 152 bp, was monomorphic, and it was observed in all the tested wheat genotypes. The fragment size of 142 bp which was amplified on chromosome 1B/1R where the target gene (Yr9) was located was used to haplotype this gene in the tested materials. Based on this, marker Yr9 was detected in 34% of the genotypes. On the other hand, based on RGA iag95 marker (gene specific marker), 36% of the wheat genotypes possess Yr9. Of the identified wheat genotypes, 25 (32%) of them were amplified by both markers (Figure 1).
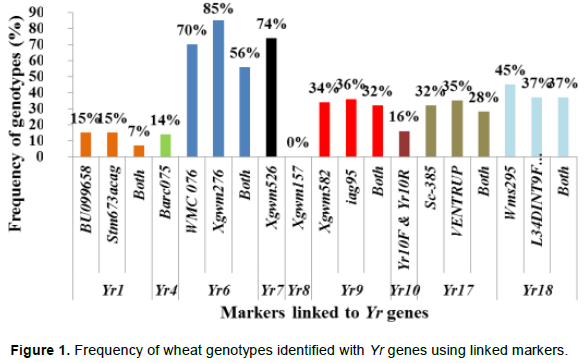
Yellow rust resistant gene, Yr10
Yr10 was originally found in wheat line PI 178383 and located on the short arm of chromosome 1B. Singh et al. (2009) designed two primer pairs (Yr10 F/R and Yr10 F1/R1) based on the Yr10 sequence and produced markers completely linked to Yr10. Genotyping with this marker indicated that Yr10 was identified in 12 (16%) of the commercially released old as well as recently released cultivars such as Alidoro, Enkoy, ET13A2, Galama, Hidassie, K6290 Bulk, K6295-4A, KBG-01, Mada Walabu, Meraro, Mitike and Shina.
Yellow rust resistant gene, Yr17
Wheat yellow rust resistance gene, Yr17, in combination with Lr37 (leaf rust resistant gene) and Sr38 (stem rust resistant gene) are located within a segment of Triticum ventricosum chromosome 2NS translocated to the short arm of wheat chromosome 2A (Helguera et al., 2003). VENTRIUP-LN2 primers were 2NS specific and the 259 bp PCR amplification product was observed only in plants carrying the 2NS translocation. This marker amplified a fragment size of 259 bp on 26 (35%) entries to have Yr17 similar to the reference line (Yr17/6*Avocet S). Likewise, SC-385 amplified a fragment size of 378 pb in the reference line. Based on this marker, Yr17 was identified in 24 (32%) of the tested genotypes. These two markers exhibited a different output for nine of the genotypes. Based on these two markers, 21 (28%) of the genotypes carry this gene.
Yellow rust resistant gene, Yr18
The adult plant resistance gene Yr18 was located on the same chromosome segment containing the Lr34 gene and is tightly linked with it (Singh, 1992b). Additionally, their co-segregation with other traits such as leaf tip necrosis (Ltn1), powdery mildew resistance gene (Pm38), and tolerance to barley yellow dwarf virus (Bdv1) has been reported (Liang et al., 2006; Singh, 1992a; Spielmeyer et al., 2005). Wms298 (Cabuk et al., 2011) and a gene specific marker L34DINT9F, L34PLUSR (Krattinger et al., 2009) were used to haplotype this gene. Of the 74 entries, 33 (45%) and 28 (36%) were found to carry Yr18 according to these markers, respectively. All the wheat genotypes which were genotyped by L34DINT9F, L34PLUSR marker showed the presence of this gene by the second marker, Wms298.
Contribution of the identified yellow rust resistance (Yr) genes
The contribution of each Yr gene to yellow rust resistance was evaluated in differential lines that possessed the individual Yr gene. As shown in Table 2 and Figure 2, the differential lines carrying Yr9 and Yr8 had the highest ACI value (83 each) followed by Yr6 and Yr7 with ACI values of 82 and 80, respectively. The lowest (46.4) value was exhibited on a differential line that carried Yr4. The ACI values in the other differential lines that carried Yr1, Yr10, Yr17 and Yr18 were 62, 60, 77 and 78, respectively. This indicates that the identified resistance genes do not provide sufficient protection to wheat yellow rust if they are used as single genes.
Relationship between the number of pyramided genes and yellow rust resistance
Relationship between the number of pyramided genes and yellow rust resistance is as shown in Figure 3. The number of Yr genes identified from the tested genotypes varied from 0 to 5 genes. Five varieties (Digalu, HAR 820, HAR 934, Hoggana and Mada Walabu) possessed the maximum number of five resistance genes. Among these varieties, the highest (82) and lowest (11.4) ACI values were recorded on Digalu and HAR 820, respectively. These five varieties exhibited ACI values of 42. On the other hand no gene was identified from bread wheat elite line, ETBW6098. The field study indicated that this variety exhibited an ACI value of 66.2. Twenty-six (35%) genotypes possessed three genes with an ACI value of 42 followed by 23 (31%) genotypes possessing two genes with an ACI value of 32.
Genetic diversity
A total of 212 alleles were detected with the 29 markers linked with Yr genes reported on A, B and D genomes. The number of alleles per locus ranged from 1 to 17 with an average of 7.24 alleles per locus. The highest number of alleles (17) was detected for marker Xwms389, which was linked to Yr57 (Table 3). Genetic diversity values ranged from 0.28 to 0.97, with the highest value of 0.98 detected for marker Xwmc389. The lowest genetic diversity value was observed for marker Xwmc198. The mean genetic diversity value was observed to be 0.77. In this study, the polymorphism information (PIC) content value ranged from 0.31 to 0.98 with an average of 0.75. The highest PIC was detected by Xgwm389, which is linked to Yr57, whereas the lowest was detected by SC-385, which is linked to Yr17.
The development of molecular markers for mapping resistance genes to yellow rust and of marker-assisted selection (MAS) has been among the most active areas of research in wheat. The accuracy of MAS is affected by the distance between the target gene and the linked markers. Randhawa et al. (2014) stated that markers used to map a gene may not be suitable for detecting the gene in diverse genetic backgrounds. In the present study, genotyping was performed only for those yellow rust resistance genes where reference lines were available as positive controls. The presence of Yr genes in the wheat genotypes was counted for each yellow rust resistance gene based on the presence of the fragment sizes of two flanking markers, except for a few genes where only one closely linked marker has been reported.
Molecular marker based gene identification showed the presence of Yr1, Yr4, Yr6, Yr7, Yr9, Yr10, Yr17 and Yr18 in various frequencies. On the other hand, Yr8 gene was not detected in any of the tested wheat genotypes. Yr7 is the most frequent (74%) followed by Yr6 (56%), Yr18 (37%) and Yr9 (32%) whereas Yr1 was detected at the lowest frequency (7%), followed by Yr4 (14%). Yr6 and Yr7 are located on chromosomes 7BS and 2BL, respectively, and they confer all stage resistance (ASR) to yellow rust. Yr9 was transferred to wheat through chromosomal translocation of 1B/1R (Zhou et al., 2004). These three genes were used widely by CIMMYT and have been deployed in many commercial cultivars (Van and Rajaram, 1993). Badebo et al. (1990) reported that Yr9 gene was the most frequently (67%) identified gene from Ethiopian commercial and advanced bread wheat genotypes. But in this study, this gene was only detected in 35% of the genotypes, which may indicate the reduction of the previously widely applied Yr9 gene in the country. However, yellow rust resistance in wheat is dominated by few Yr genes, such as Yr7 and Yr6, which were present in 74 and 56% of the tested varieties and lines, respectively. A diversity reduction of resistant varieties is unfavorable for breeding varieties with durable resistance. With extensive deployment of numerous commercial cultivars throughout the world, virulence for these genes was wide spread. Chen et al. (2002) reported occurrence of high virulence frequency to Yr2, Yr6, Yr7, and Yr9 in most wheat producing areas of the world. Virulence frequency as high as 90% was recorded for Yr6 and Yr7 to Pst isolates collected internationally (Sharma-Poudyal et al., 2013). In Ethiopia, virulence frequencies of 92 to 100% were recorded among 107 isolates collected in 2005 from different parts of the country (Dawit et al., 2012). The average coefficients of infection (ACI) exhibited in the present study were 82 (Yr6), 80 (Yr7) and 83 (Yr9), indicating the inefectiveness of these genes in the country.
The adult plant resistance gene Yr18 is located on chromosome 7DL and is tightly linked with leaf rust resistant gene, Lr34 (Singh, 1992a). Additionally, their co-segregation with other traits such as leaf tip necrosis (Ltn1), powdery mildew resistance gene (Pm38), and tolerance to barley yellow dwarf virus (Bdv1) has been reported (Liang et al., 2006; Singh, 1992a; Spielmeyer et al., 2005). This multi-pathogen resistance locus is a valuable source of resistance in wheat breeding. The use of the slow rusting gene pair Lr34/Yr18 in combination with other slow rusting genes has been suggested to contribute to near immunity to leaf and yellow rust infections (Singh et al., 2000). Using a sequence specific marker (L34DINT9F, L34PLUSR) that indicated the presence of the cloned Yr18 in the genomic DNA accurately, this gene was detected in 37% of the tested bread wheat genotypes. Singh (1992a) reported that despite the contribution of this gene in many countries, wheat genotypes with Yr18 displayed inadequate resistance in some locations in Ecuador and Kenya. In the present study, an ACI value of 78 was recorded on the differential line that possessed this gene, which may indicate the ineffectiveness when used alone in Ethiopia. However, more than 67% of the bread wheat genotypes that possessed Yr18 in different Yr gene combinations exhibited ACI values lower than 78. This study is the first report on the presence of the adult plant resistant gene, Yr18, in Ethiopian bread wheat genotypes. Those genotypes that were identified to possess Yr18 may also possess leaf rust resistance gene (Lr34) and other genes linked to Yr18 such as Pm38.
Yellow rust resistance gene, Yr17 together with other genes such as Lr37 and Sr38 are located within a segment of T. ventricosum chromosome 2NS translocated to the short arm of wheat chromosome 2A (Helguera et al., 2003). The resistant gene Yr17 was used in many breeding programs to develop resistant cultivars (Eugene et al., 2015). The yellow rust reaction on wheat seedlings with this gene is influenced by environmental conditions (for example temperature) and genetic background (Eugene et al., 2015). Thus, it is very difficult to apply gene postulation to identify this gene in wheat genotypes. The VENTRIUP-LN2 marker, which is 2NS specific and amplifies a 259 bp PCR product only in plants carrying the 2NS translocation, was used together with the second marker SC-385 for genotyping of this gene. These markers identified 24 (32%) of the entries to have Yr17 in the present study. Those wheat genotypes that are genotyped to have Yr17 may also carry leaf rust (Lr37) and stem rust (Sr38) resistance genes. Earlier reports indicated that Yr17 was postulated to be present only in three (7%) of the tested Ethiopian bread wheat genotypes (Badebo et al., 2008). On the contrary, Yr17 was widely deployed in European wheat cultivars and a virulence frequency close to 100% has been reported in Northern European countries (Villaral et al., 2002). Yellow rust resistance gene Yr17 was effective with regard to the prevailing races in East Africa Badebo and Stubbs, 1995). Similarly, Dawit et al. (2012) reported the effectiveness of Yr17 gene under Ethiopian conditions. In the same report, a virulence frequency of 14% was exhibited on this gene by isolates collected from Ethiopia, but there was no indication for the presence of these genes in the tested Ethiopian wheat cultivars. In the present study, the exhibited ACI on Yr17 was 77, which may indicate that this gene is no more effective to the prevailing races in the country.
In addition to the aforementioned yellow rust resistant genes, this study identified the seedling resistant genes Yr1, Yr4 and Yr10. Yr1 is located on chromosome 2AL. Virulence to Yr1 has been found in several countries of the world (Wan et al., 2017). But, the virulence frequency is high in East Africa where 166 Chinese wheat cultivar originated (Zhan et al., 2016). For instance, virulence frequencies of 50 and 74% were recorded in Kenya and Ethiopia, respectively (Sharma-Poudyal et al., 2013). Similarly, high yellow rust disease intensity with ACI value of 62 was exhibited in the present study. Bansal et al. (2009) reported a molecular marker, stm673acag, that amplified 120 and 124 bp products in most lines carrying Yr1 gene, but in the present study the marker amplified a 129 bp fragment size, which may be due to technical reasons. Hasancebi et al. (2014) reported the second marker, bu099658 that is linked to Yr1 gene. This marker amplified 206 bp only in those lines that possessed Yr1 genes, including Yr1/6*Avocet S. However, this marker amplified 162 bp product on the positive control (Yr1*Avocet S). Both markers were used to haplotype Yr1 in the present study but showed different results. Thus, only 5 (7%) of the genotypes that exhibited a fragment size similar to both markers were considered to possess this gene. According to Bansal et al. (2009) stm673acag is used to haplotype both Yr1 and Sr48, thus those wheat genotypes identified to possess Yr1 may have also stem rust resistant gene Sr48.
Yr4 is originally derived from common wheat and is synonymous with Yr4a and Yr4b, and it is located on chromosome 3B A microsatellite marker, Barc075, which is 2.4 ± 1.2 cM distal to YrRub/Yr4 on chromosome 3BS, was used for genotyping. This marker was initially linked to uncharacterized resistant gene (YrRub) in Australian wheat cultivar Rubric. Later, the marker was tested in genotypes known to carry Yr4 (Hybrid 46 and Avalon). Based on the amplification of the Rubric-specific PCR products at Barc075 loci, it was concluded that YrRub could be Yr4 (Bansal et al., 2010). In the present study, the marker amplified a single 105 bp band in the reference line, Yr4/6*Avocet S. After evaluation by this marker, 11 (14%) of the bread wheat cultivars showed a similar band size to the reference line. Two of the cultivars (HAR723 and HAR820), which were previously reported/postulated to have the Yr4 gene (Badebo, 1990) exhibited a similar fragment size to the reference line. This further confirms the probability of YrRub to be Yr4. Yellow rust resistant gene Yr4 was effective in East Africa with regard to the prevailing races (Badebo and Stubbs, 1995). A low virulence frequency of 6% was recorded in Ethiopia (Dawit et al., 2012). In the present study, Yr4 exhibited an ACI of 50, which may indicate that this gene is no more effective if it is used alone. Yr10 originated from bread wheat and is located on the short arm of chromosome 1B (It is one of the resistant genes that confers high resistance to Pst races in Pakistan (Farrakh et al., 2016) and China (Zheng et al., 2017). In Ethiopia, a yellow rust severity as high as 40% was recorded on the differential lines that possessed this gene. By using a gene specific marker, Yr10 is confirmed to be present in 16% of the tested wheat genotypes.
Gene pyramiding, combining multiple Yr genes in a single genotype is an important strategy to develop durable rust resistant cultivars. The ACI value that was recorded on the genotypes that possessed the maximum number (five) of resistance genes was 42. Cultivars with four and three genes exhibited 36.19 and 42.01 ACI, respectively. This may indicate that pyramiding of those identified genes may not provide sufficient protection against the prevalent races in the country. Thus, there is an urgent need to search for more effective resistance genes to be incorporated in Ethiopian bread wheat cultivars. On the contrary, ACI values ranging from low (8.9) to high (70) were exhibited in cultivars Gassay and Shina, respectively with one gene. Those cultivars with low average coefficient of infection may indicate the presence of additional Yr genes that were not identified in the present study.
The number of alleles per locus ranged from 1 to 17 with an average of 7.24. Genetic diversity values ranged from 0.28 to 0.98, with the highest value of 0.98 detected for markers Xwmc389 and P6M12-P. The mean genetic diversity values were observed to be 0.75. The polymorphic information content (PIC) value ranged from 0.24 to 0.98 with an average of 0.73. The highest PIC value was detected by Xgwm389, which is linked to Yr57, whereas the lowest was by Yr10F & R, linked to Yr10.
The evidence in this study on the basis of genetic diversity and the presence of Yr genes in the improved wheat genotypes will be helpful for developing appropriate breeding strategies to broadening the genetic base in future wheat breeding programs in Ethiopia.
The authors have not declared any conflict of interests.
This research was supported by a fellowship to Woubit Dawit by the German Academic Exchange Service (DAAD). The molecular research was conducted at the Leibniz Institute of Plant Genetics and Crop Plant Research (IPK), Gatersleben, Germany. The authors thank Annette Heber for excellent technical support for genotyping.
REFERENCES
|
Ahmad I, Ullah U, Inamullah, Ahmad H, Muhammad I (2015). Screening of Pakistani wheat landraces for stripe rust resistance using molecular markers. Basic Research Journal of Soil and Environmental Science 3(1):1-3.
|
|
|
|
Badebo A, Stubbs RW, Ginkel MV, Gebeyehu G (1990). Identification of resistance genes to Puccinia striiformis in seedlings of Ethiopian and CIMMYT bread wheat varieties and lines. Netherlands Journal of P1ant Pathology 96:199-210.
|
|
|
|
|
Badebo A, Stubbs RW (1995). Valuable sources of resistance of wheat to the East African yellow rust isolates. In: Breeding for disease resistance with emphasis on durability. In: Proceedings of a regional workshop for Eastern, Central, and Southern Africa Danial DL (eds.), Njoro, Kenya pp. 206-214.
|
|
|
|
|
Badebo A, Fehrmann H, Yahyaoui A (2008). Status of wheat stripe rust (Puccinia striiformis) races and their virulence in major wheat growing areas of Ethiopia. Pest Management Journal of Ethiopia 12:1-7.
|
|
|
|
|
Bansal UK, Hayden MJ, Keller B, Wellings CR, Park RF, Barianaa HS (2009). Relationship between wheat rust resistance genes Yr1 and Sr48 and a microsatellite marker. Plant Pathology 58:1039-1043.
Crossref
|
|
|
|
|
Bansal UK, Hayden MJ, Gill MB, Bariana SH (2010). Chromosomal location of uncharacterized stripe rust resistance gene in wheat. Euphytica, 171:121-127.
Crossref
|
|
|
|
|
Cabuk E, AydinY, Uncuoglu AA (2011). Assessing wheat (Triticum aestivum) genotypes for Yr" resistance genes using conserved regions and simple-sequence motifs. Genetic Molecular Research 10:3463-3471.
Crossref
|
|
|
|
|
Chen XM, Moore M, Milus EA, Long DL, Line RF, Marshall D, Jackson L (2002). Wheat stripe rust epidemics and races of Puccinia striiformis f. sp. tritici in the United States in 2000. Plant Disease 86:39-46.
Crossref
|
|
|
|
|
Central Statistics Authority (CSA) (2017). Report on area and crop production forecast for Major grain crops. Addis Ababa, Ethiopia: statistical bulletin.
|
|
|
|
|
Dawit W, Flath K, Weber WE, Schumann E, Röder MS, Chen X (2012). Postulation and mapping of seedling stripe rust resistance genes in Ethiopian bread wheat cultivars. Journal of Plant Patholology 94:403-409.
|
|
|
|
|
Deng ZY, Zhang XQ, Wang XP (2004). Identification and molecular mapping of a stripe rust resistance gene from a common wheat line Qz180. Acta Botanica 46:36-241.
|
|
|
|
|
Doyle JJ and Doyle JL (1990) Isolation of plant DNA from fresh tissue. Focus 12:13-15.
|
|
|
|
|
Eriksen L, Afsharri F, Christiansen MJ, McIntosh RA, Jahoor A, Wellings CR (2004). Yr32 for resistance to stripe (yellow) rust present in the wheat cultivar Carstens V. Theoretical Applied Genetics 108:567-575.
Crossref
|
|
|
|
|
Eugene AM, Kevin DL, Gina BG (2015). Characterization of stripe rust resistance in wheat lines with resistance gene Yr17 and implication for evaluating resistance and virulence. Phytopathology Society 105:1123-1130.
Crossref
|
|
|
|
|
Farrakh S, Khalid S, Rafique A, Riaz N, Mujeeb-Kazi A (2016). Identification of stripe rust resistant genes in resistant synthetic hexaploid wheat accessions using linked markers. Plant Genetic Resources 14:219-225.
Crossref
|
|
|
|
|
Hasancebi S, Mert Z, Ertugrul F, Akan K, Aydin Y, Akfirat FS, Uncuoglu AA (2014). An EST-SSR marker, bu099658, and its potential use in breeding for yellow rust resistance in wheat. Czech Journal of Genetics and Plant Breeding 50:11-18
Crossref
|
|
|
|
|
Helguera M, Khan IA, Kolmer J, Lijavetzky D, Zhong-qi L, Dubcovsky J (2003). PCR assays for the Lr37–Yr17–Sr38 cluster of rust resistance genes and their use to develop isogenic hard red spring wheat lines. Crop Science 43:1839-1847.
Crossref
|
|
|
|
|
Jia JQ, Li GR, Liu C, Lei MP, Yang ZJ (2011). Characterization of wheat yellow rust resistance gene Yr17 using EST-SSR and rice syntenic region. Cereal Research Communications 39:88-99.
Crossref
|
|
|
|
|
Kaur N, Street K, Mackay M, Yahiaoui N, Keller B (2008). Molecular approaches for characterization and use of natural disease resistance in wheat. European Journal of Plant Patholology 21:387-397.
Crossref
|
|
|
|
|
Krattinger SG, Lagudah ES, Spielmeyer W, Singh RP, Huerta-Espino J, McFadden H, Bossolini E, Selter LL, Keller B (2009). A putative ABC transporter confers durable resistance to multiple fungal pathogens in wheat. Science 323:1360-1363.
Crossref
|
|
|
|
|
Li Y and Niu YC (2007). Identification of molecular markers for wheat stripe rust resistance Gene Yr6. Acta Agriculturae Boreali-Sinica 22:189-192.
|
|
|
|
|
Liu W, Frick M, Huel R, Nykiforuk CL, Wang X, Gaudet DA, Eudes F, Conner RL, Kuzyk A, Chen Q, Kang Z, Laroche A (2014). Stripe rust resistance gene Yr10 encodes an evolutionary-conserved and unique CC–NBS–LRR sequence in wheat. Molecular Plant 7:1740-1755.
Crossref
|
|
|
|
|
Liu K and Muse SV (2005). Power Marker: an integrated analysis environment for genetic marker analysis. Bioinformatics 21:2128-2129.
Crossref
|
|
|
|
|
Mago R, Spielmeyer W, Lawrence GJ, Lagudah ES, Ellis JG, Pryor A (2002). Identification and mapping of molecular markers linked to rust resistance genes located on chromosome 1RS of rye using wheat-rye translocation lines. Theoretical Applied Genetics 104:1317-1324.
Crossref
|
|
|
|
|
McIntosh RA, Yamazaki Y, Dubcovsky J, Rogers WK, Morris CF (2013). Catalogue of gene symbols for wheat. In: 12th International Wheat Genetics Symposium, 8-13 September 2013 Yokohama, Japan. 31p.
|
|
|
|
|
McIntosh RA, Dubcovsky J, Rogers WJ, Morris C, Appels R, Xia XC (2016). Catalogue of gene symbols for wheat: 2013-14 Supplement. Annual wheat newsletter 58.
|
|
|
|
|
Ma JX, Zhou RH, Dong YS, Wang LF (2001). Molecular mapping and detection of the yellow rust resistance geneYr26 in wheat transferred from Triticum turgidum using microsatellite markers. Euphytica 120:219-226.
Crossref
|
|
|
|
|
Peterson RF, Champbell AB, Hannah AE (1948). A diagrammatic scale for estimating rust intensity of leaves and stem of cereals. Canadian Journal of Research 26:496-500.
Crossref
|
|
|
|
|
Randhawa M, Bansal U, Valarik M, Klocova B, Dolezel J, Bariana H (2014). Molecular mapping of stripe rust resistance gene Yr51 in chromosome 4AL of wheat. Theoretical Applied Genetics 127:317-324.
Crossref
|
|
|
|
|
Rohlf FJ (2000). NTSYS-PC: Numerical taxonomy and multivariate analysis system. Exeter Software. New York 44 p
|
|
|
|
|
Sharma-Poudyal D, Chen XM, Wan AM, Zhan GM, Kang ZS, Cao SQ, Jin SL, Morgounov A, Akin B, Mert Z, Shah SJA, Bux H, Ashraf M, Sharma RC, Madariaga R, Puri KD, Wellings C, Xi KQ, Wanyera R, Manninger K, Ganzález MI, Koyda M, Sanin S, Patzek LJ (2013). Virulence characterization of international collections of the wheat rust pathogen, Puccinia striiformis f.sp. tritici. Plant Disease 97:379-386.
Crossref
|
|
|
|
|
Singh RP (1992a). Genetic association of leaf rust resistance gene Lr34 with adult plant resistance to stripe rust in bread wheat. Phytopathology 82:835-838.
Crossref
|
|
|
|
|
Singh RP (1992b). Association between gene Lr34 for leaf rust resistance and leaf tip necrosis in wheat. Crop Science 32:874-878.
Crossref
|
|
|
|
|
Singh RP, Huerta-Espino J, Rajaram S (2000). Achieving near-immunity to leaf and stripe rusts in wheat by combining slow rusting resistance genes. Acta Phytopathologica et Entomologica Hungarica 35:133-139.
|
|
|
|
|
Singh R, Datta D, Priyamvada, Singh S, Tiwari R (2009). A diagnostic PCR based assay for stripe rust resistance gene Yr10 in wheat. Acta Phytopathologica et Entomologica Hungarica 44:1-18.
Crossref
|
|
|
|
|
Spielmeyer W, McIntosh R, Kolmer J, Lagudah E (2005). Powdery mildew resistance and Lr34/Yr18 genes for durable resistance to leaf and stripe rust co-segregate at a locus on the short arm of chromosome 7D of wheat. Theoretical Applied Genetics 111:731-735.
Crossref
|
|
|
|
|
Van Ginkel M and Rajaram S (1993). Breeding for durable resistance to diseases: An international perspective. In: Durability of Disease Resistance. Jacobs T, Parlevliet JE (eds.) Wageningen, The Netherlands. The Netherlands 259 p.
|
|
|
|
|
Villaral LMMA, Lannou C, de Vallavieille-Pope C, Neema C (2002). Genetic variability in Puccinia striiformis f.sp. tritici populations sampled on a local scale during natural epidemics. Applied and Environmental Microbiology 68:6138-6145.
Crossref
|
|
|
|
|
Vanzetti LS, Campos P, Demichelis M, Lombardo LA, Aurelia PR, Vaschetto LM, Bainotti CT, Helguera M, (2011). Identiï¬cation of leaf rust resistance genes in Selected Argentinean bread wheat cultivars by gene postulation and molecular markers. Electronic Journal of Biotechnology 14(3):9-9.
Crossref
|
|
|
|
|
Wan A, Kebede T, Habtemariam Z, Bekele H (2017). Virulence characterization of wheat stripe fungus Puccinia striiformis f.sp. tritici in Ethiopia and Evaluation of Ethiopian wheat germplasm for resistance to races of the pathogen from Ethiopia and the United States. Plant Diseases 101:73-80.
Crossref
|
|
|
|
|
Yaniv E, Raats D, Ronin Y, Korol AB, Grama A, Bariana H, Dubcovsky J, Schulman AH, Fahima T (2015). Evaluation of marker-assisted selection for the stripe rust resistance gene Yr15, introgressed from wild emmer wheat. Molecular Breeding 35:43.
Crossref
|
|
|
|
|
Zhan G, Wang F, Wan C, Han Q, Huang Li, Kang Z, Chen X (2016). Virulence and Molecular Diversity of the Puccinia striiformis f.sp. tritici Population in Xinjiang in Relation to Other Regions of Western China. Plant Disease 100:99-107.
Crossref
|
|
|
|
|
Zheng S, Li Y, Lu L, Liu Z, Zhang C, Ao D, Li L, Zhang C, Liu R, Luo C, Wu Y, Zhang L (2017). Evaluating the contribution of Yr genes to stripe rust resistance breeding through Marker-assisted detection in wheat. Euphytica 213:50-66.
Crossref
|
|
|
|
|
Zhou Y, Zhong HH, Zhang GS, Xia LQ, Chen XM, Gao YC, Jing ZB, Yu GJ (2004). Utilization of 1BL/1RS translocation in wheat breeding in China. Acta Agronomica Sinica 30:531-535.
|
|