ABSTRACT
Four soil samples and water effluents each within the mining environment were tested in this study. Twenty six bacteria were isolated using nutrient agar and acidified nutrient agar. Gram staining and endospore staining were carried out to determine the Bacillus species used for bioremediation. Speciation of the microorganisms using 16S rRNA sequencing showed the organisms to be Bacillus cereus NK1, Lysinibacillus species TAI-282, Lysinibacillus fusifomis, Bacillus aryabhattai PM1 and Bacillus megaterium from the samples. The temperature of the water effluents ranged from 29.00 to 34.70°C. Sample C from beneficiation area had the highest temperature of 34.70°C. The pH ranged from 6.70 to 9.77 with effluent from primary crushing area two (PC2) having the highest of 9.77. Bioremediation of the water samples were carried out for 6 days using the identified Bacillus species from the mine site. For all the effluents treated, there was an increase in the concentration of magnesium with effluent from PC2 treated with L. fusifomis increasing to 7.453±0.004 from 4.278±0.003 and these values were significantly different at p≤0.05. Concentration of calcium in effluent from iron ore storage area increased from 1.350 ±0.002 to 15.450±0.004 after treatment with B. aryabhattai PM1, and the values were significantly at p≤0.05. Bacillus spp. from PC2 reduced the concentration of iron from 179.738 to 0.091 ppm on effluent from PC1, the concentration was significantly different at p≤0.05. The indigenous microorganisms from within the iron ore mining site had bioremediation potentials reducing the concentrations of most of the heavy metals present.
Key words: Iron ore, molecular analysis, bioremediation, Bacillus species.
Heavy metals are difficult to remove from the environment and are ultimately indestructible unlike many other pollutants that can be chemically or biologically degraded (Ozaki et al., 2003). Severe damage is caused to aquatic life when such metals are present and microorganisms are killed during biological water purification process (Vinodhini and Narayanan, 2008). Contamination of metals is a major environmental problem and especially in the aquatic environment some of which at low concentration are toxic or carcinogenic. Metals remaining in contaminated sediments may accumulate in microorganisms which in return enter into the food chain eventually affecting human wellbeing (Shakeri and Moore, 2010).
The iron ore deposits in Nigeria are located at the middle belt geopolitical zone which is characterized by alternating layers rich in chart, a form of silica (SIO2) and layers rich in iron minerals such as heamatite (Fe2O3),
magnetic (Fe3O4), iron silicate, chamosite and siderite (Fagade et al., 2010). These deposits are found in vast reserves of chemical and classic rocks such as sedimentary, igneous and metamorphic for over three thousand years (Morris, 2012). Itakpe iron ore is located in Latitude 07°36’20’’N and longitude 6°18’35’’E in Okehi Local Government Area of Kogi State, Nigeria (Ifeanyi et al., 2013).
The presence of contaminants in an environment leads to an increase in the numbers of microbes able to degrade such. The residues for the treatment are usually harmless products and include carbon dioxide, water and cell biomass (Sardrood et al., 2013).
The aim of this study was to isolate and identify Bacillus species from water effluents and soil samples from Itakpe iron ore mining site using Polymerase Chain Reaction (PCR) and bioremediate samples with some of the isolates.
Study location
The study location was Itakpe iron ore mine site, in Kogi State, Nigeria. Four water samples and four soil samples were collected within the mining site at different locations aseptically in August, 2017 (Figure 1). Soil samples were collected using soil auger to a depth of 5 cm and put into polythene bags. Water samples were collected using Grab sampling method. The samples were transported to the laboratory for analysis within 12 h (Nafanda, 2005).
The temperatures of the samples were taken using mercury-bulb thermometer and the pH determined with Hanna pH 211 Model on site.
Determination of colour and presence of particles
Visual examination of the water samples was used to determine the colour and presence of particles.
Determination of odour
The water effluent containers were shaken vigorously for about 5 s. The covers were removed aseptically and the odour quickly determined by inhalation of air near the mouth of the plastic containers.
Determination of total dissolved solids (TDS) was according to the method of Jamal et al. (2015). Biochemical oxygen demand (BOD) was determined using the method of Thompson and Stevenson (1984) and the chemical oxygen demand (COD) was carried out by using the photometer method (Thompson and Stevenson, 1984).
Metal analyses
The Atomic Absorption Spectrophotometer (AAS) Buck Scientific model 210 was used to determine the metal and mineral concentrations of the samples using the calibration plot method.
Isolation of bacteria
One gram each of the soil samples and 1 ml each of effluent from the primary crushing area one, primary crushing area two, beneficiation area and Iron ore storage area were used. Pour plate method according to Thompson and Stevenson (1984) was used. The samples were serially diluted using sterile distilled water as diluents according to the method of Murugalatha et al. (2018). Nutrient agar and acidified nutrient agar were used for the isolation of bacteria. Sub culturing was carried out until pure cultures were obtained.
Identification of bacterial isolates
Preliminary identification of the bacterial isolates was carried out. Gram staining and endospore staining were carried out using the methods of Bergey and John (2000).
DNA isolation and purification
This procedure was carried out using the QIAamp DNA mini kit (Qiagen, #51306). Isolates used were cultured in broth overnight, and DNA isolation was carried out according to manufacturer’s instruction. Using a NanoDrop ND1000 (Thermo Scientific, USA) machine, DNA was quantified by calibrating the machine with 1 μl of water, followed by 1 μl blank (Tri-EDTA buffer) and then 1 μl of the DNA sample to be quantified (Kumar et al., 2016).
Polymerase chain reaction (PCR)
PCR procedure was carried out as described by Mullis et al. (1986), using a pair of primers, 16SF (GTGCCAGCAGCCGCGCTAA) and 16SR (AGACCCGGGAACGTATTCAC). Denaturation was achieved at 94°C for 5 min, and subsequently for 30 s; annealing at 56°C for 30 s; extension at 72°C for 45 s. The processes occurred in 36 cycles and the final extension was at 72°C for 7 min. The products were further purified by adding 2 volumes (20 µl) of absolute ethanol, incubated at room temperature for 15 min, and then centrifuged at 10,000 rpm for 15 min. Supernatant was discarded and 2 v (40 µl) of 70% ethanol added, centrifuged again at 10,000 rpm for 15 min, supernatant was discarded and product air dried. Final product was held at 10°C for further analysis (Kumar et al., 2016).
Gene sequencing
The amplicons from the polymerase chain reaction were subjected to sequencing reactions using BigDye Terminator v3.1 Cycle Sequencing Kit, following manufacturer’s guidelines. The products were loaded unto 3130xl Genetic Analyzer (Applied Biosystems, 2010) to generate the molecular sequences of each amplicon.
Base sequence analysis
The base sequences generated from each amplicon were analyzed by a combination of Basic Local Alignment Search Tool (BLAST) and Fast Alignment (FASTA) (Donkor et al., 2014). Sequences were submitted as query at http://www.ncbi.nlm.nih.gov/Blast.cgi for comparison with database sequences using the NCBI nucleotide BLAST. Isolates were identified based on DNA-DNA similarity at 99%.
Phylogenetic analysis
The analysis involved 7 nucleotide sequences. There were a total of 42 positions in the final dataset. All positions containing gaps and missing data were eliminated. Phylogenetic analysis was carried out by maximum parsimony (MP) method based on the partial sequences of 16S rRNA gene of representative isolates in this study. The best substitution model that described the sequence data set was obtained and 1000 bootstraps values were used to determine the confidence interval of the resultant tree. The evolutionary history was inferred using the Neighbor-Joining method (Saitou and Nei, 1987). Evolutionary analyses were conducted in MEGA7 (Kumar et al., 2016).
Bioremediation of water samples using identified Bacillus spp.
The modified method of Fagade et al. (2010) was employed using seven isolates. These isolates were grown on nutrient broth for 48 h at 37°C and read on a spectrophotometer at 610 nm. The cultures were standardized at 0.00 optical densities. 1000 ml of each sample was used and 5 ml of identified Bacillus spp. was inoculated into the different samples. They were incubated at 28°C for seven days. Bioremediation of the samples were carried out using the following Bacillus spp. isolated from the soil samples and water effluents: Bacillus cereus NK1, Lysinibacillus species TAI-282, Lysinibacillus fusifomis, Bacillus spp. 1, Bacillus aryabhattai PM1, Bacillus megaterium and Bacillus spp. 2.
Statistical analysis
Data obtained were subjected to Analysis of Variance (ANOVA) using SPSS version 20 at P≤0.05 level of significance.
A total of eight samples from different locations were used for this study. Samples A, B, C and D were water samples and samples E, F, G and H were soil samples.
Physicochemical parameters of samples
Table 1 shows the physicochemical parameters of the effluents. The temperatures varied from 30.27 to 34.75°C and were within the standard limit for effluent which is <40°C. The temperature of Sample B from primary crushing area two (PC2) was 29.00°C and the lowest while that of Sample C from beneficiation area was 34.70°C and the highest. Particles were present in all samples. The color of sample A, effluent from primary crushing area (1) was brownish with no odor while sample B was colorless with an objectionable odor pH of 9.21. Sample D from the iron ore storage area had the highest pH of 9.77.
Biochemical characteristics of isolates
Table 2 shows the biochemical characteristics of isolates from both soil and water samples. All selected isolates were Gram positive and endospore formers. Isolate S1 from soil around primary crushing area one (PC1) appeared flat with entire edge, circular and creamy in color. Isolate H1 from effluent around primary crushing area one appeared flat with entire edge, circular and greenish in color.
Phylogenetic tree of bacterial isolates
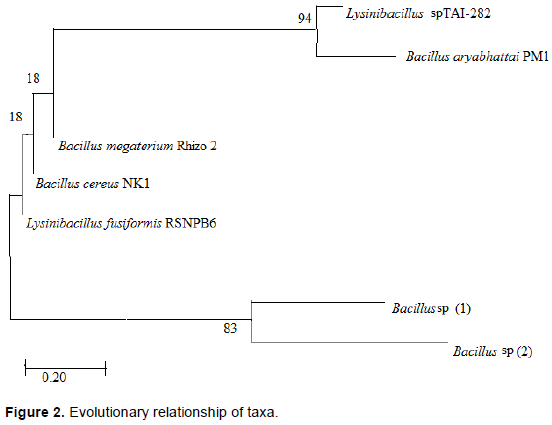
Figure 2 shows the evolutionary relationship of bacterial isolates in this study. The phylogenetic tree revealed the evolutionary relationships among the isolates in this study. The optimal tree with the sum of branch length = 3.68169715 is shown. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap tests (1000 replicates) are shown next to the branches. The tree was drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree.
The tree showed 2 sister clades sharing common ancestors; Lysinibacillus spp. TAI-282, B. aryabhattai PM1 and Bacillus spp.-Bacillus spp. (2) at 94 and 83%, respectively. Other isolates however showed weak relationships at <50% bootstrap replicates. B. megaterium Rhizo 2 and B. cereus NK1 had the lowest evolutionary relationship with 18% each.
Elemental and mineral composition of effluents from PCI before and after treatment with Bacillus isolates
There was a reduction by Bacillus spp. of iron concentration from 179.738 to 0.049 ppm. Bacillus aryabhattai PMI reduced lead concentration from 0.314 to 0.093 ppm and L. fusifomis reduced manganese from 1.255 to 0.007 ppm (Table 3).
Statistical analyses at P≤ 0.05 showed that calcium present in water was significantly different from other water samples after treatment with selected isolates. Control was 5.200f±0.003 while the isolate with the highest amount of value was H2 Bacillus spp. from water sample from primary crushing area two, which indicated an increase in calcium after treatment. However, isolate S1 B. megaterium with a value of 7.600e ±0.001 was statistically the same with isolate H1 B. cereus with value of 7.750e±0.002 after treatment of mine water. The value for untreated lead was statistically different from other effluents after treatment with the selected isolates. The control had a value of 0.314a±0.000 and was statistically different from water sample treated with isolate S1* B. aryabhattai with a value of 6.61h±0.002. However, manganese present in water sample treated with isolate S1 B. megaterium was statistically the same with manganese present in water sample treated with isolate H1 L. fusifomis with values of 8.711a±0.001 and 8.199a±0.002, respectively.
Elemental and mineral composition of effluents from PC2 before and after treatment with Bacillus spp.
Statistical analysis showed that the concentration of iron before treatment was 70.368a±0.118 and significantly different statistically after treatment with Bacillus spp. The concentration was reduced to 0.144c±0.002. The concentrations of cadmium present in the effluent when treated with B. aryabhattai PM1 and B. megaterium were not significantly different at P≤0.005. The values recorded were 0.001e±0.004 and 0.013e±0.001, respectively (Table 4).
Elemental and mineral composition of effluents from Beneficiation area before and after treatment with Bacillus isolates
Statistical analyses at P≤0.05 showed that lead present in the control was not statistically different with that present in water sample treated with Bacillus spp. with values of 0.010b±0.000 and 0.178b±0.001. Iron present in water sample treated with B. aryabhattai PM1 was not different significantly from water sample treated with B. megaterium with values of 0.078b±0.003 and 0.078b±0.001. However, nickel present in water samples treated with B. aryabhattai PM1, B. megaterium, L. fusifomis and Bacillus spp. were all not statistically different (Table 5).
Elemental and mineral composition of effluents from Iron ore storage area before and after treatment with Bacillus isolates
Table 6 shows the elemental composition of effluent from Iron ore storage area before and after treatment. Statistical analysis at P≤0.05 shows that iron present in the control was significantly different from that present in water treated with Bacillus spp. with values of 2.176a±0.0047 and 0.029f±0.004, respectively. Also, arsenic present in the control was significantly different from that present in water treated with B. megaterium with values of 0.124a±0.002 and 0.087d±0.0021. In addition, cadmium present in water sample treated with B. megaterium, L. fusifomis and Bacillus spp. were all statistically not different with values of 0.015c±0.002, 0.012c±0.002 and 0.018c±0.003.
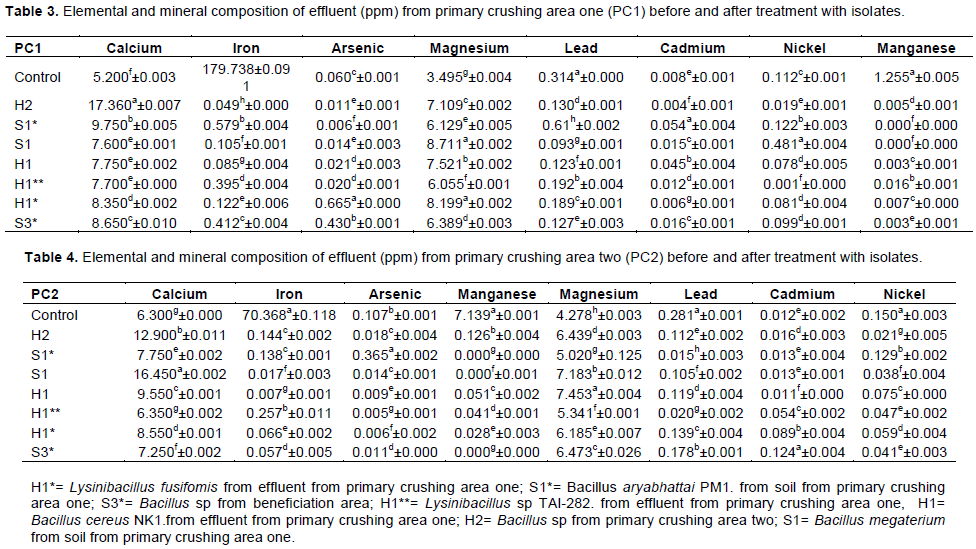
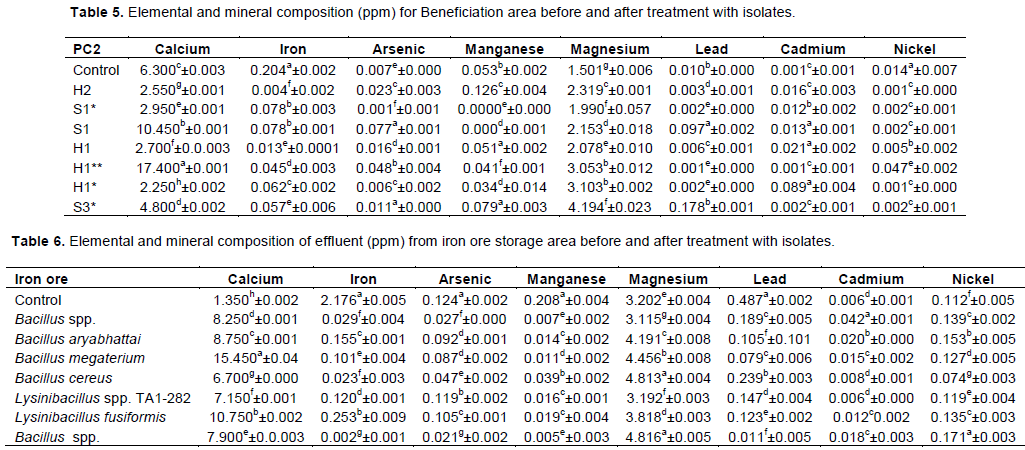
Table 7 shows the physico-chemical constituents of effluent from primary crushing area two (PC2). There was a reduction in all the parameters after treatment for five days. Statistical analyses at P≤0.05 show that BOD for the control was statistically different from all samples treated with selected isolates; the value of the control was 125.80g mg/l. The BOD value obtained when B. cereus NK1 was used was not statistically different from that obtained with the use of L. fusifomis. The values were 4.27d and 4.50d mg/l, respectively. The BOD value after treatment with B. aryabhattai PM1 was the lowest at 2.20a mg/l against the control value of 125.80g mg/l making B. aryabhattai PM1 the most effective in reducing BOD in the water sample from Primary crushing area two (PC2). The COD for the control was 260.17h mg/l and statistically different from sample treated with B. aryabhattai PM1 with a value of 4.40a mg/l. B. megaterium has the highest COD value after treatment with different isolates with a value of 26.23g mg/l and statistically different to Bacillus spp. with a COD value of 17.30f.
Statistical analyses at P≤0.05 showed that the control of PC2 had the highest TDS value of 1780.00g mg/l and statistically different from other samples treated with selected isolates. B. megaterium with TDS value of 314.67f mg/l was statistically different from B. cereus NKL1 with value of 113.00b mg/l. In addition, Lysinibacillus spp. TAI-282 with value of 235.00e mg/l was statistically different to B. megaterium with a value of 314.67f mg/l and the highest value after
treatment.
Isolation of Bacillus spp. from soil samples and water effluents from Itakpe iron mining site was carried out. Eight different locations were used for sampling namely with soil and effluents collected; Primary crushing area 1 (PC1), Primary crushing area 2 (PC2), Beneficiation area and Iron ore storage area. The temperatures of the samples varied from 29.00 to 34.70°C. Jamal et al. (2015) recorded similar temperatures of 29.80 to 39.00°C from acid mine drainage. The pH of samples varied from 6.70 to 9.77 with effluent from Iron ore storage area having the highest pH value of 9.77 while that from Beneficiation area (Sample C) had the lowest pH of 6.70. A study carried out by Jiang and Xu (2017) on process water in iron flotation of Yuanjiacum iron mine site recorded a pH of 9.12 for the tailings wastewater. This is similar to that recorded in this study. The temperature and pH values obtained in this study were within the Federal Environmental Protection Agency (FEPA) limits for discharge of effluents into water bodies.
Total Dissolved Solids (TDS) recorded for all water samples were below the FEPA limit of 2000 mg/l; values between 45.00 and 1780.00 mg/l. This was different from the study conducted by Jamal et al. (2015) on heavy metals from acid mine drainage (AMD) where TDS values ranged between 2213 and 2908 mg/l. B. aryabhattai PM1 and B. megaterium were effective in reducing the TDS in beneficiation (BENEF) sample from the initial TDS of 181.67f to 45.00a mg/l and 45.00a mg/l, respectively, the values were not significantly different statistically from each other. According to APHA (1998), high levels of TDS were as a result of the presence of potassium, chlorides and sodium in water. However, these ions have been found to have little or no effect but in the presence of toxic ions such as lead, cadmium, nitrate and arsenic in water, the result will be more hazardous to the ecosystem (APHA, 1998).
Biochemical oxygen demand (BOD) value ranged between 2.20 and 125.80 mg/l. The initial BOD of effluent from Primary crushing area two (PC2) was 125.80 mg/l and reduced to 2.20 mg/ml by B. aryabhattai PM1 and this result was different from the report on study conducted by Hammer and Hammer (2004) on water and waste water technology where BOD values between 130 and 200 mg/l were recorded.
The chemical oxygen demand (COD) values of the effluents were between 4.40 and 260.17 mg/l. B. megaterium reduced the COD value from the initial reading of 260.17 to 4.40 mg/l. According to Nafanda (2005), high COD value indicates the presence of high organic matter in effluent, so with the reduction in COD value, the microorganisms were effective in bioremediation. This result was similar to that obtained from study carried out by Fagade et al. (2010) where decrease in COD from initial 714.05 to 281.60±49.78 mg/l was recorded. Also, Jiang and Xu (2017) reported a reduction in COD of tailings wastewater from131 to 21 mg/L in their study. There was a decrease in TDS in this study with values of 181.67 to 45.00 mg/l for sample obtained from Beneficiation area but an increase in the TDS was reported by Fagade et al. (2010) with initial TDS value of 54.1 to 160.6 mg/l.
The bacteria isolated from the soil and water samples were Gram positive rods. These microorganisms included B. megaterium, L. fusifomis, B. aryabhattai and Bacillus spp. The absence of Gram negative bacteria in the environment is supported by Edwards et al. (1999), who reported less than 50% of Gram negative bacteria out of the total viable population in an acidic mine environment. This result is also in accordance with Fagade et al. (2010), where Gram positive bacteria were those mainly isolated.
Molecular characterization of organisms showed all isolates were Bacillus spp. and included B. cereus NK1, Lysinibacillus spp. TAI-282, L. fusifomis, B. aryabhattai PM1, B. megaterium and Bacillus spp. The results obtained were similar to those reported by Mohamed and Farag (2015) who carried out 16S rDNA gene sequencing on isolates and identified B. fusiformis, two species of Lysinibacillus, and three species B. cereus.
Bioremediation was carried out using the seven selected Bacillus spp. identified, majority from water samples for five days. There was a significant drop in the level of iron (Fe) present in the water samples.
Result of elemental and mineral analysis showed that the iron content of sample from Primary crushing area one (PC1) which was high with value of 179.738±0.091 mg/l. Nickel from Primary crushing area two has a mean value of 0.150±0.003 mg/l. Beneficiation area had the lowest initial mean values for all selected elements and minerals, calcium 6.300±0.003 mg/l, iron 0.204±0.002 mg/l, arsenic 0.007±0.000 mg/l, manganese 0.053±0.002 mg/l, magnesium 1.501±0.006 mg/l, lead 0.010±0.000 mg/l and nickel 0.014±0.007 mg/l. This result is close to a study carried out by Kakulu and Mathews-Amune (2012) where they recorded low mean values for metallic contents while working on heavy metal pollution from Itakpe mine Kogi State. The recorded mean values were 0.16±0.02 for cadmium, 0.15±0.03 for copper, 0.04±0.02 for magnesium, 0.11±0.02 for nickel, 0.07±0.01 for lead and 0.04±0.03 mg/ml for zinc, respectively.
Bacillus cereus NK1 used in bioremediation of effluents from Primary crushing area one (PC1) and Primary crushing area two (PC2) reduced the arsenic concentration from 0.060±0.001 to 0.021±0.003 mg/l and 0.107±0.001 to 0.009±0.001 mg/l, respectively. In a study carried out by Mohamed and Farag (2015) on arsenic removal from aqueous solutions using different Bacillus and Lysinibacillus spp, a reduction in arsenic concentration from 0.50 to 0.01 mg/l was reported using B. cereus EA5. This is similar to the report of this present study. Also, B. megaterium were reported to remove arsenic through adsorption (Miyatake and Hayashi, 2009).
Bacillus spp. used in bioremediation of effluent from primary crushing area two (PC2) reduced manganese from 7.139a±0.001 to 0.126b±0.004 mg/l. A study on isotherm equilibria of manganese biosorption in drinking water treatment by locally isolated Bacillus spp. and sewage activated sludge carried out by Hasan et al. (2012), produced similar result. Here, they used Bacillus spp. as biosorbent for manganese with initial metal ion concentration of 25 to 300 mg/l and achieved maximum biosorption capacity of 43.5 mg/g indicating the ability of Bacillus spp. to absorb and utilize manganese.
Bacillus spp. reduced the concentration of lead present in sample from Iron ore crushing area from initial concentration of 0.487±0.002 mg/l. This result is related to a study on bioremediation of heavy metals from sewage discharge canal bank by Guo et al. (2010) where they reported reduction in lead concentration after 24 h from 825±25 to 200±80 mg/l. The investigation showed the multi-metal resistance and hormesis of endophytic bacterium (EB) L14 exhibited excellent adaptation abilities for practical in-situ bioremediation of heavy metals by Bacillus spp. L14.
Bioremediation of water samples from Itakpe iron mine site using Bacillus spp. from this environment identified through molecular analysis was effective. These organisms were two Bacillus spp., L. fusifomis, B. aryabhattai PM1, Lysinibacillus spp. TAI-282, B. cereus NK1 and B. megaterium. These indigenous microorganisms can be used to reduce heavy metals concentrations in soil and water thereby bringing about reduction of contaminants within the mining site and effluents that will be discharged into water bodies and leached into the environment.
The authors have not declared any conflict of interests.
REFERENCES
|
American Public Health Association (APHA) (1998). Standard methods for examination of water and wastewater. American Public Health Association, American Water Works Association and Water Pollution Control Federation. 20th edition. Washington DC. USA, pp. 5-17.
|
|
|
|
Applied Biosystems (2010). BigDye Terminator v3.1 Cycle Sequencing Kit Protocol. Available [Online] at
View
|
|
|
|
|
Bergey DH, John GH (2000). Bergey's Manual of Determinative Bacteriology. Philadelphia. Lippincoft Williams and Wilkins.
|
|
|
|
|
Donkor ES, Dayie NTKD, Adiku TK (2014). Bioinformatics with basic local alignment search too (BLAST) and fast alignment (FASTA). Journal of Bioinformatics and Sequence Analysis 6(1):1-6.
Crossref
|
|
|
|
|
Edwards KJ, Gihring MT, Banfield FJ (1999). Seasonal variations in microbial population and environmental conditions in an extreme acid mine drainage environment. Journal of Applied Environmental and Biological Sciences 65(8):3627-3632.
Crossref
|
|
|
|
|
Fagade OE, Okunlola OI, Ogunjobi AA. Oyelade AA (2011). Bacteria isolated from mine effluent of National Iron Ore Mining Project, Itakpe-Okene and bioremediation potential of selected species. New York Science Journal 4(5):54-58.
|
|
|
|
|
Guo H, Shenglian L, Liang C, Xiao X, Qiang X, Wanzhi W, Guangming Z, Chengbin L, Yong W, Jueliang C, Yejuan H (2010). Bioremediation of heavy metals by growing hyperaccumulator endophytic bacterium Bacillus sp. L14. Bioresource Technology 101(22):8599-8605.
Crossref
|
|
|
|
|
Hammer M, Hammer M (2004). Water and wastewater technology.Fifth edition. Pearson Education. New Jersey pp. 308-310.
|
|
|
|
|
Hasan HA, Abdullah SRS, Kofli NT, Kamarudin SK (2012). Isotherm equilibria of Mn+2 biosorption in drinking water treatment by locally isolated Bacillus species and sewage activated sludge. Journal of Environmental Management 111:34-43.
Crossref
|
|
|
|
|
Ifeanyi JN, Okengwu K, Adesoji A (2013). Predicting the concentration characteristics of Itakpe iron ore for cut-off grade estimation. Journal of Applied Sciences and Environmental Management 17(2):315-319.
Crossref
|
|
|
|
|
Jamal A, Yadav HL, Pandey SS (2015). Heavy metals from acid mine drainage in coal mines- a case study. European Journal of Advances in Engineering and Technology 2(8):16-20.
|
|
|
|
|
Jiang W, Xu H (2017). Treatment and recycling of the process Water in iron ore flotation of Yuanjiacun iron mine. Journal of Chemistry 1-8.
Crossref
|
|
|
|
|
Kakulu S, Matthews-Amune OC (2012). Heavy metal pollution around Itakpe mine, Kogi State, Nigeria. International Journal of Physical Sciences 7(28):5062-5068.
Crossref
|
|
|
|
|
Kumar S, Stecher G, Tamura K (2016). MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Molecular Biology and Evolution 33(7):1870-1874.
Crossref
|
|
|
|
|
Miyatake M, Hayashi S (2009). Characteristics of arsenic removal from aqueous solution by Bacillus megaterium strain UM-123. Journal of Environmental Biotechnology 9(2):123-129.
|
|
|
|
|
Mohamed EAH, Farag AG (2015). Arsenic removal from aqueous solutions by different Bacillus and Lysinibacillus species. Bioremediation Journal 19(4):269-276.
Crossref
|
|
|
|
|
Morris RC (2012). Genesis of iron ore in banded iron-formation by supergene and super gene metamorphic processes a conceptual model. Hand book of Strata-bound and stratiform ore deposits 13:73-235.
Crossref
|
|
|
|
|
Murugalatha NK, Sonam S, Anoop B, Vinay C, Naveen CB (2018). Isolation and characterization of bacterial isolates from agriculture field soil of Roorkee region. Journal of Pharmacognosy and Phytochemistry SP5:108-110.
|
|
|
|
|
Nafanda WD (2005). Implications of abattoir waste on the environment and public health in Ibadan and Yola. Nigerian Journal of Animal Science 75:1541-1635.
|
|
|
|
|
Ozaki T, Kimura T, Ohnuki T, Yoshida Z, Francis A (2003). Association mechanisms of Europium (III) and Curium (III) with Chlorella vulgaris. Environmental Toxicology and Chemistry 22(11):2800-2805.
Crossref
|
|
|
|
|
Saitou N, Nei M (1987). The neighbor-joining method: A new method for reconstructing phylogenetic trees. Molecular Biology and Evolution 4(4):406-425.
|
|
|
|
|
Sardrood BP, Goltapeh EM, Varma A (2013). An Introduction to Bioremediation, Department of Plant Pathology, Ramin Agricultural and Natural Resources University, Mollasani, Ahwaz, Iran.
Crossref
|
|
|
|
|
Shakeri A, Moore F (2010). The impart of industrial complex on freshly deposited sediments: Chener Rahdar river case study, Shiraz, Iran. Environmental Monitoring and Assessment 169(1):321-334.
Crossref
|
|
|
|
|
Thompson PJ, Stevenson KE (1984). Mesophilic sporeforming aerobes. In: Speck, M ed. Compedium of methods for the microbiological examination of foods. Americam Public Health Association, Washington, USA pp. 211-220.
|
|
|
|
|
Vinodhini R, Narayanan M (2008). Bioaccumulation of heavy metals in organs of fresh water fish Cyprinus carpio (common carp). International Journal of Environmental Science and Technology 5(2):179-182.
Crossref
|
|