ABSTRACT
Horticulture is one of the fastest growing sectors in Uganda, exporting products worth US$100 million annually. Passion fruit (Passiflora edulis) growing and export is one of the critical contributors to this sector employing over a million farmers. However, a number of biotic and abiotic constraints have initiated widespread enterprise abandonment by farmers. Passiflora improvement efforts by conventional breeding has had limited success calling for research into alternative approaches such as genetic engineering. The study aimed at optimizing existing protocols to develop an efficient and reproducible Agrobacterium mediated transformation system to suit Uganda’s Passiflora cultivars. Agrobacterium tumefaciens strain AGL1 (OD600 of 0.5) harbouring pCAMBIA2301 containing the GUS (uidA) reporter gene was used to infect pre-cultured leaf discs. Leaf discs were then vacuum infiltrated for 1.5 min at 750 mmHg followed by a three day co-cultivation period on MS + acetosyringone (100 µml-1). Putatively transgenic yellow passion fruit shoots were induced on Murashige and Skoog (MS) selection media supplemented with benzylaminopurine (BAP) 8.9 μM, kanamycin (100 mgL-1mgl) and cefotaxime (500 mgL-1). Developed shoots were then transferred to elongation media (MS + 0.44 µM BAP) and later rooted on 5.37 µM naphthaleneacetic acid (NAA). Genetic transformation was monitored using GUS staining. A single independently transformed plant was confirmed by polymerase chain reaction (PCR), translating in a transformation efficiency of 0.456%. A viable in vitro transformation protocol for Uganda’s yellow passion fruit directly from leaf discs was developed using GUS reporter gene. Further investigations are required to improve the reported protocols transformation efficiency.
Key words: Passion fruit, Passiflora edulis, Passiflora improvement, genetic engineering, transformation system.
Against the backdrop of falling and fluctuating prices of traditional export crops (coffee, tea, tobacco, cotton), the Government of Uganda embarked on promotion of the horticultural sector as an alternative source of foreign exchange (National Trade Policy, 2007). Horticulture is now one of the fastest growing sectors in Uganda exporting products worth US$100 million per year mainly to the European Union (Ministry of Agriculture, Animal Industry and Fisheries, 2019). Uganda is currently the second largest producer of fresh fruit and vegetables in sub-Saharan Africa after Nigeria (International Centre for Trade and sustainable Development, 2011).
Passion fruit (Passiflora edulis) growing and export is one of the critical contributors to the horticultural sector employing over a million small holder farmers in addition to the other players in the value chain. Uganda annually earns over US$ 200,000 from passion fruit exports (Agribusiness Development Centre., 2014; Uganda Export Promotion Board., 2016). In addition to its commercial importance, species of Passiflora also have medicinal, nutritional and ornamental value (Manders et al., 1994; Freitas et al., 2007). The demand and potential of passion fruit production is much higher compared to the current production, and this has been attributed to a number of biotic and management-related factors (Ochwo-Ssemakula et al., 2012).
Declining yields from passion fruit farming due to a number of problems ranging from pests, diseases, environmental stress, low yields, have initiated widespread enterprise abandonment by farmers. Diseases are the most significant biotic constraint with viral infections accounting for 40% yield loss and up to 40% reduction in fruit quality in some parts of the country (Ochwo-Ssemakula, 2012; Wangungu et al., 2014). Crop improvement efforts by conventional breeding techniques have had limited success since the process is relatively slow, partially hindered by sexual barriers and high ploidy levels, limited genetic variability and requires many cycles of selfing and backcrossing to eliminate deleterious genes (Varassin et al., 2001; Petri and Burgos, 2005).
These constraints have necessitated research into other alternative approaches for Passiflora improvement such as plant tissue culture and genetic engineering (Drew, 1997). However, there is lack of a reliable, efficient and reproducible transformation system that is compatible with a regeneration method for the successful transformation of Passiflora varieties in Uganda. In addition, the high heterogeneity of genus Passiflora makes genetic transformation very difficult due to a number of physiological and developmental problems. Owing to the increased genetic variability among its species, there was need to optimize existing transformation protocols to suit Uganda’s Passiflora cultivars. The study thus aimed at establishing an efficient and reproducible transformation system for Uganda’s P. edulis f. flavicarpa (yellow passion fruit) using Agrobacterium for routine utilization with an ultimate goal of improving its agronomic value.
Study site, design and source of plant material
The study was conducted at the National Agricultural Research Laboratories (NARL) - Kawanda, an institute under the National Agriculture Research Organization (NARO). The study followed a completely randomized experimental research design. Yellow passion fruit (P. edulis f. flavicarpa) was chosen for the study since it is the most widely cultivated species of Passiflora in tropical countries owing to its tolerance to diseases, larger fruit quality and vigorous vine. High performing P. edulis (yellow) believed to be disease free were originally purchased from the Kawanda Nursery, an entity under NARL with the guidance of the Nursery manager relying on his expertise and documentation. Plants were purposively sampled according to parameters like yield, stature, growth rate, vigour and grown in an insect proof screen house with regular watering.
Immature young leaves were picked from the growing Passiflora for the study. Consent from the Ethics Committee to use the experimental materials in the study was verbal as the study was mainly a proof of concept. The constructs used did not add any known desired trait to the plants but merely a means of assaying the feasibility of transforming Uganda’s P. edulis with no possibility of releasing the genetically modified materials.
Vector and Agrobacterium cells transformation
Commercially available pCAMBIA2301 GUS transformation vector (catalogue number; 101743-168; Marker Gene Technologies, Inc; https://www.markergene.com/pcambia2301 plant-expression-vector.html) was used in the study. Binary vector pCAMBIA2301 (Figure 1) containing the GUS (uidA) reporter gene under constitutive CaMV 35S promoter, nopaline synthase (NOS) terminator, lacZa promoter and a kanamycin selection genes nptII (neomycin phosphotransferase II) for both bacteria and plant selection was used in the study.
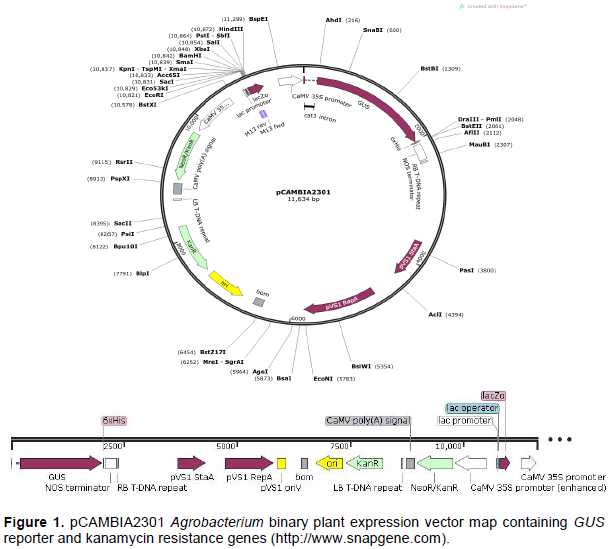
Electrocompetent Escherichia coli cells strain JM109 were prepared using cold sterile double distilled water. Electrocompetent E. coli was then transformed with the plasmid pCAMBIA2301 using an electroporation apparatus set at 2.5 kV, 25 µF and 200 Ohms. Putatively transformed E. coli cells were then plated on Luria Bertani (LB) (10 g Bacto-tryptone, 5 g yeast extract, 10 g sodium chloride and 15 g micro-agar per litre) plates supplemented with kanamycin (100 mgL-1) and incubated overnight at 37°C. The colonies were screened for the presence of the pCAMBIA2301 using a colony polymerase chain reaction (PCR). Electrocompetent AGL1 Agrobacterium tumefaciens cells (without plasmid) were prepared using sterile double distilled water; AGL1 was then transformed with plasmid pCAMBIA2301 by electroporation. Putatively transformed A. tumefaciens were then cultured on LB agar supplemented with Kanamycin ((100 mgL-1), Carbenicillin (250 mgL-1) and rifampicin (50 mgL-1). The plates were incubated at 28°C for 3 days and any colonies that developed were tested for the presence of pCAMBIA2301 using Gus specific primers.
PCR analysis to confirm transformation of E. coli JM109 and Agrobacterium tumefaciens AGL1
The transformation of the respective bacteria with the Ti plasmid was only confirmed by colony PCR using specific Gus primers. The GUS gene (~530 bp) was amplified using the following Gus specific primers (Gus Forward primer 5’-CGACGGCCTGTGGGCATTCA-3’, Gus Reverse primer 5’- TGGTCGTGCACCATCAGCAC-3’). PCR reaction mixture had a total volume of 20 μl for a 1× reaction containing 10 μl Gotaq, 0.4 μl of the forward and reverse Gus primers respectively, 1 μl DNA template (E. coli and Agrobacterium AGL1) and sterile dH2O. The PCR cycling conditions included an initial denaturation step of 94°C for 5 min, followed by 35 cycles of 50 s at 94°C, annealing at 56°C for 1 min and 72°C for 1 min with final extension at 72°C for 7 min. PCR products were electrophoresed on a 1.35% agarose gel at 120 V, 400 mA for 40 min, stained in ethidium bromide and the DNA visualized and photographed under ultraviolet light using Genesnap software (Sambrook et al., 1989).
Preparation of Agrobacterium cultures for transformation of leaf discs
A single clone of AGL1 confirmed by PCR was used to prepare glycerol stocks which were stored at -80°C and routinely used for transfection of yellow passion fruit leaf discs. A week to the actual transformation, AGL1 glycerol stock was streaked onto LB agar plates supplemented with kanamycin (100 mgL-1), carbenicillin (250 mgL-1), rifampicin (25 mgL-1) and grown at 28°C in a BIOCONCEPT digital incubator for 3 days. Four days prior to transformation, a two-day Agrobacterium AGL1 liquid culture was started using a single plate colony in 25 mL LB broth containing kanamycin (100 mgL-1), Carbenicillin (250 mgL-1) and rifampicin (25 mgL-1). Agrobacterium was grown in a MaxQTM bench top orbital incubator shaker at 200 rpm at 28°C in 50-mL falcon tubes. A day to transformation, a fresh culture for infection of the leaf discs was started by inoculating 200 µL of the two day bacterial culture in 100 mL of fresh LB broth supplemented with kanamycin (100 mgL-1), carbenicillin (250 mgL-1) and rifampicin (25 mgL-1) in a 250-mL conical flask. Agrobacterium AGL1 was grown overnight on a MaxQTM bench top orbital incubator shaker at 200 rpm at 28°C.
Preparation of explants for Agrobacterium mediated transformation
Immature leaves excluding buds were excised from the passion fruit plants, rinsed with detergent under running tap water and soaked for 25 min in 2% fungicide (Rovral Aquaflo™, 2mlL-1; Active ingredient; 500 gL-1 Iprodione). Leaves were surface sterilized with 70% (v/v) ethanol for two min before finally being soaked in 2.5% Sodium hypochlorite (commercial bleach) for 10 min in a laminar flow hood (Class II-Type A). At the end of each of the above steps, the leaves were rinsed thrice in sterile distilled water to cleanse them of the previously used solutions. Leaf discs (±1 cm2) were sliced from sterilized leaves and precultured on Murashige and Skoog (1962) medium supplemented with 8.9 µM BAP for 1 day. Murashige and Skoog (MS) media was prepared by dissolving full strength MS basal salts and vitamins, sucrose (30 gL-1), appropriate BAP concentration and pH adjusted to 5.8. Gelrite (2.4 gL-1) was added prior to autoclaving at 121 ºC for 15 minutes.
All biochemicals and media constituents used in the study were tissue culture grade procured from either Sigma-Aldrich (St Louis, Missouri, USA) and Duchefa Biochmie, (RV Haarlem, Netherlands).
Agrobacterium mediated transformation of pre-cultured leaf discs and co-cultivation
On the transformation day, the overnight Agrobacterium culture was harvested by centrifugation at RCF 5,000 x g for 5 min at 4°C using a bench top Thermo Scientific Biofuge PrimoR Heraeus refrigerated centrifuge. The supernatant was discarded and the remaining bacterial cells were re-suspended in 20 mL liquid Murashige and Skoog (MS) media (without gelrite) supplemented with 8.9 µM BAP, 100 μM acetosyringone minus antibiotics for infection and co-cultivation of explants. The bacterial cell suspension was then shaken for 1.5 h at 80 rpm at room temperature on a Stuart Orbital shaker incubator to activate the Agrobacterium. Precultured leaf disc for transformation were heat shocked in plain Passion Fruit Regeneration Media (PFRM) without Agrobacterium AGL1 for 1 min at 45°C in a water bath to prevent apoptosis of injured cells. Leaf discs were then inoculated with A. tumefaciens (OD6000.5) transformed with pCAMBIA2301 for 18-22 min with occasional shaking at 25°C in 50 ml falcon tubes. Explants suspended in Agrobacterium were vacuum infiltrated in falcon tubes for 1.5 min at 750 mmHg using a Biolistic® PDS-1000/He Particle Delivery System. Leaf discs were then blotted dry on sterile blotting paper to remove excess bacteria and transferred onto non selective MS media supplemented with 8.9 µM BAP and 100 µmL-1 acetosyringone for a 3-day co-cultivation period. Cultures were incubated at 22°C in darkness with the adaxial side of the leaves in contact with media. Control leaf discs underwent the same treatment except they were not dipped in A. tumefaciens cell suspension but instead dipped in liquid PFRM supplemented with 100 μM acetosyringone minus antibiotics.
Two transformation experiments were conducted translating into two replicas, in which a total of 266 A. tumefaciens inoculated yellow passion fruit leaf discs were sub-cultured for both replicas while 56 leaf discs were used as the controls. Cultures were incubated in the dark for eight weeks at 26°C and then changed to a 16-h photoperiod (light) at 27 ± 1°C.
Selection, shoot regeneration, elongation and rooting media
After co-cultivation, inoculated discs were washed with 500 mgL-1 cefotaxime and cultured on MS selection media supplemented with BAP 8.9 μM, kanamycin (100 mgL-1) and cefotaxime (500 mgL-1). Shoot regeneration from co-cultivated leaf discs was induced on MS supplemented with 8.9 µM BAP, kanamycin (100 mgL-1) for plant selection and cefotaxime (500 mgL-1) to kill bacteria. Developed shoots were subcultured onto elongation media {MS + 0.44 µM BAP, cefotaxime (300 mgL-1), kanamycin (100 mgL-1)} and cultures maintained at the 16-h photoperiod. Elongated shoots (4 cm and above) were transferred to MS media supplemented with 5.37 µM NAA, cefotaxime (300 mgL-1) and kanamycin (100 mgL-1) for root induction. Control explants were treated in the same way, except that they were neither inoculated nor co-cultivated with A. tumefaciens.
Monitoring, detection and confirmation of transformants
The histochemical assay was done to monitor the efficiency of transformation by screening for expression of b-glucurodinase (GUS gene) activity in putatively transformed yellow passion fruit explants according to protocols by Jefferson et al. (1987). Explants were assayed for expression of the gene ten days after Agrobacterium-mediated transformation following the histochemical procedure below. Explants were incubated at 37°C in a staining buffer with final concentrations of 0.1 mM sodium phosphate, pH 7.0, 10 mM Na-EDTA, 1 mM potassium ferricyanide, 2 mM X-Gluc (5-bromo-4-chloro-3-indolyl-β-D-glucuronide cyclohexylammonium salt) and 0.1% (v/v) Triton X-100 at 37°C for 24 h. Explants were then submerged for 2 days in 90% ethanol to remove chlorophyll in order to bleach explants and enhance visualisation of the blue stain.
The integration of the transgene into the plant genome was detected and confirmed by PCR analysis using Gus specific primers. Total genomic DNA was extracted from fresh leaves of transformed and untransformed (control) plants using the cetyltrimethylammonium bromide (CTAB) method (Chaudhry et al., 1999). Further, concentration of the extracted DNA was determined using the NanoDrop 2000 (Thermo Scientific™) before dilution to 95 ng/µl to run PCR. For PCR analysis of transgenic plants, the GUS gene fragment (~530 bp) was amplified using the above specified Gus primer set. The reaction mixture, with a total volume of 20 μl for a 1× reaction contained 10 μL Gotaq, 0.4 μl volume of the forward and reserve Gus primers, 1 μl (95 ng/20 µl reaction) DNA template and the reaction volume was made up to 20 μL using sterile dH2O. The PCR cycling conditions for GUS included an initial denaturation step of 94°C for 5 min, followed by 35 cycles of 45 s at 94°C, annealing at 56°C for 1 min, elongation at 72°C for 1 min with a final extension at 72°C for 7 min. Plasmid DNA (50 ng/20 µl) was used as a positive control and DNA (95 ng/20 µL) from a control plant as a negative control.
PCR products were electrophoresed on a 1.35% agarose gel at 120V, 400 mA for 40 min, and stained in ethidium bromide. DNA was visualized and photographed using Genesnap software (Sambrook et al., 1989). Data was analyzed using both qualitative and quantitative statistical analysis using Microsoft Excel 2007; descriptive statistical approaches using quantitative measurements was used to analyze and summarize the data in tables using measurements like averages, percentages and frequencies.
There was successful transformation P. edulis f. flavicarpa (yellow) passion fruit using GUS reporter gene via A. tumefaciens mediated transformation.
PCR analysis to confirm presence of pCAMBIA2301 in putatively transformed E.coli JM109 and A. tumefaciens AGL1
Amplification of the predicted size fragments of ~530 corresponding to the amplified internal fragments of the GUS gene for the first and second transformations in both E. coli and A. tumefaciens were positive. The approximated size fragments were observed in most of the colonies tested confirming transformation of bacteria with Ti plasmid (Figure 2). No specific amplification products were observed in case of untransformed E. coli and A. tumefaciens.
GUS histochemical assay
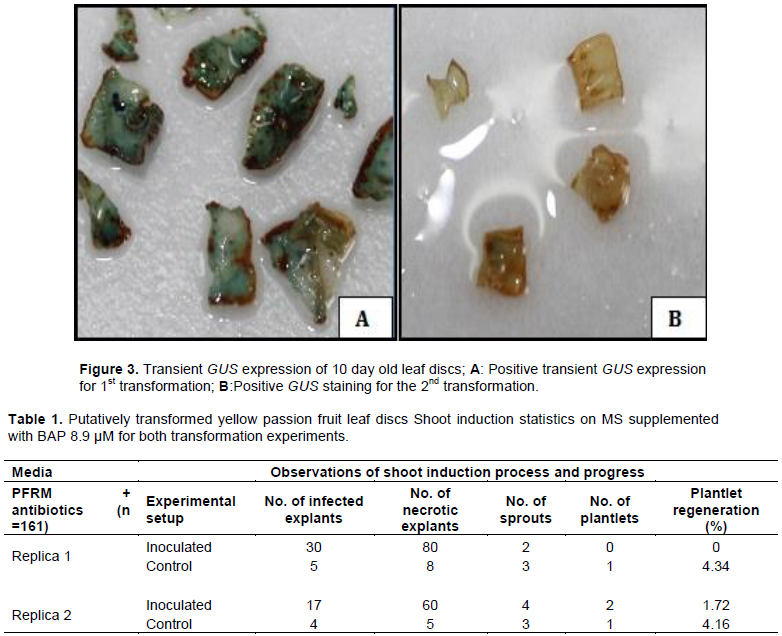
There were clearly visible spatial blue coloration and spots/specks on the leaf discs stained with X-Gluc reagent whereas no blue coloration was observed in the non-transformed control explants (Figure 3). Explants with strong transient GUS expression presented bluer staining and vice versa. Positive Transient GUS staining was an indicator of successful delivery of the uidA gene via A. tumefaciens into yellow passion fruit leaf discs. GUS was an effective reporter of genetic transformation in yellow passion fruit leaf discs. Considering results from GUS staining, transformation efficiency was 70%, that is, 14 of the 20 randomly sampled putatively transformed leaf discs showed some level of transfection (chimeric blue regions).
Regeneration of putatively transformed yellow passion fruit on MS supplemented with BAP 8.9 μM
There was unsuccessful regeneration of putatively transformed plantlets for the first transformation experiment; however two sprouts were produced after approximately 16 weeks (4 months) which later died. Out of the 103 inoculated explants that survived infection, no shoot was produced translating into a regeneration efficiency of 0% for putatively transformed plantlets (Table 1)
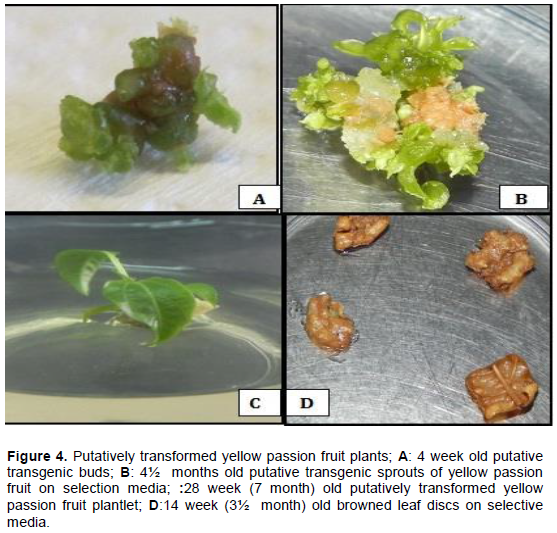
. For the second transformation, there was successful regeneration of putatively transformed plantlets; four shoots were successfully induced on selection media resulting in the development of two rooted plantlets within a period of 4-5 months (Figure 4). Out of the 116 inoculated explants that survived infection, the two regenerated shoots translated into 1.72% (

×100) regeneration efficiency (Table 1)
.
Generally, most of the explants succumbed to kanamycin selection pressure, with the antibiotic lethal and sensitive to the untransformed tissue or partially transformed leaf discs killing them off within 3 months (Figure 4). Leaf discs started drying after 6 weeks on media; they then became brittle followed by large scale necrosis of most of the leaf disc surface within 12 weeks (3 months). Necrosis was probably due to chimerism where one leaf disc was composed of both putatively transformed and untransformed sections. Patches of the leaf disc tissue that were untransformed were selected against the kanamycin while those that were putatively transformed were tolerant to the selective agent. Chimerism was already evidenced by the GUS staining where the blue coloration was not uniformly distributed across the leaf disc surface implying that some cells of the leaf had taken up the plasmid while others had not been transformed (Figure 3).
Plantlet Regeneration % = [Regenerated plantlets/(Number of explants inoculated-Infected explants ) × 100]. For each Replica, a total of 161 explants were used (133 explants inoculated with Agrobacterium and 28 explants as controls), infected explants were those that succumbed to Agrobacterium overgrowth or fungal contamination, Necrotic explants were those that succumbed to kanamycin selection.
PCR analysis of putatively transformed yellow passion fruit plants to confirm integration of pCAMBIA2301
Integration of T-DNA from pCAMBIA2301 was confirmed using PCR in only one of the two plantlets that survived on selection pressure. Amplification of the expected size fragment (~530 bp) using PCR was observed in only one of the two putatively transformed yellow passion fruit plantlets confirming integration of the GUS gene (Figure 5). The other plant did not show DNA amplification of the expected band size suggesting that it was an escape (Figure 5). No amplified product was observed in case of the non-transgenic control plant (negative control).
Considering only the successfully regenerated transgenic plantlet,overall transformation efficiency (Genetic transformation efficiency % = [(Number of plantlets regenerated/ Number of explants inoculated- infected explants) X 100]) for both transformation experiments was 0.456% 1/219×100). Transformation efficiency was higher in the second transformation experiment at 0.970% (1/103×100) compared to 0% in the first transformation experiment.
Introduction of target genes into the genome of elite varieties and regeneration of transgenic plants with high efficiency are the most desirable strategies for production of transgenic plants. The study successfully transformed the commercially important yellow passion fruit variety from leaf discs via Agrobacterium mediated transformation resulting in the production of one transgenic plantlet. This was attributed to the availability of a plant regeneration system and to the fact that explants were susceptible to infection by A. tumefaciens.
The study showed that Agrobacterium mediated genetic transformation of yellow passion fruit is feasible, however the genetic transformation efficiency was very low at 0.456%. Other researchers who have carried out similar studies on the same variety have also reported very low genetic transformation efficiency percentages with Trevisan et al. (2006) reporting genetic transformation efficiencies of 0.11 and 0.21% for two Brazilian yellow passion fruit cultivars IAC-275 and IAC-277 respectively. Monteiro et al. (2011) also reported low genetic transformation efficiencies of 0.67 and 0.19% respectively for the same abovementioned Brazilian yellow passion fruit transgenic lines in a similar study. A related study on P. alata reported a marginally higher transformation efficiency of 0.89% (Correa et al., 2015). The present study revealed slightly higher transformation efficiency compared to previously reported studies probably due to the lower numbers of leaf explants used in the study.
The observed low genetic transformation efficiency could be attributed to a number of possible factors. Studies have reported that transformation efficiency depends upon many factors such as regeneration potential, cultivar, physiological nature of explant, age of explant, pretreatment prior to inoculation, Agrobacterium strain and its density plus antibiotic concentrations used. Studies by Davis et al. (1991) and Madhulatha et al., (2007) reported that co-cultivation time, different growth regulators and their concentrations, transformation conditions all play an important role in the success of transformations.
The recorded low genetic transformation efficiency by the study was majorly due to limitations in plantlet regeneration; there was observed difficulties in promoting shoot elongation and plantlet development. This was further amplified by the low number of initial explants used. Plant regeneration is an integral part of most plant transformation strategies, and can often prove to be the most challenging aspect of a plant transformation protocol. Successful transformation of any plant depends on the development of a quick and efficient regeneration system. An efficient regeneration protocol which is compatible to various transformation techniques is a prerequisite for successful application of genetic engineering (Manders et al., 1994). Trevisan et al., (2006) and Monteiro et al. (2011) to a considerable extent attributed their low genetic transformation efficiencies to limitations in shoot induction and plantlet development. Studies have reported that the basic requirement for a successful gene transfer system for producing transgenic plants is the availability of a target tissue made up of a large number of regenerable cells that are accessible to the gene transfer treatment (Birch, 1997; Cho et al., 2004). These studies continued to emphasize that these cells must retain the capacity to regenerate even after being subjected to varied explant preparation and selection treatments. It was obvious from the study that superior regeneration potential is important for successful passion fruit transformation.
The study observed adverse deleterious effects of kanamycin on morphogenesis and development of transformed leaf discs with large scale browning and necrosis most probably due to the high concentration of kanamycin (100 mgL-1) used. These findings were supported by Holford et al. (1992) and Lin et al. (1995) who noted that some antibiotics have a detrimental effect on plant tissue cultures. Since only a limited number of explant cells are usually transformed after inoculation/co-culture with A. tumefaciens, this leads to chimeric tissue consisting of transformed and untransformed cells. The observed large scale necrosis was probably due to chimerism where the portions of the leaf disc tissue that were untransformed were selected against by the kanamycin creating a cascade effect resulting in the elimination of the entire leaf disc. Developing a selection procedure for non genetically uniform organisms favouring the growth of transformed cells over untransformed cells is extremely critical and difficult (Hanke et al., 2007).
Selection agents like kanamycin significantly decrease the relative density of viable cells by eliminating untransformed cells resulting in severe growth inhibition of the surviving transgenic cells. This was collaborated by Winkler and Quoirin (2002) who reported in a similar study that no yellow passion fruit leaf explant survived on media supplemented with kanamycin (100 mgL-1) with explants becoming chlorotic and later necrotic at the end of thirty days. Cefotaxime was also used in media to kill and prevent bacterial growth yet studies have reported on its negative effect on shoot induction and development (Okkels and Pedersen, 1988; Ling et al., 1998). There was an escape which regenerated on selection media during the study. The regeneration of non transformed plants according to Ghorbel et al. (2000) could be due to inefficient selection where surrounding transformed cells offer protection to non transformed cells from the selection pressure.
Since the explants that were used for the study were picked from the field and thus had to undergo surface sterilization, the harsh sterilization disinfection procedure could have had a negative impact on the regeneration potential of the leaf discs. Oyebanji et al. (2009) stated that it is important to recognize that the sterilization period may differ according to the host species. He continued to argue that the optimal period of disinfection should be determined when working with a new species to eliminate the negative effects of over sterilization which normally results in damage or even death to the leaf tissue.
The element of the appropriate age, physiological condition and genotype of the leaf explants also arose. Leaf explants were picked from fully grown passion fruit plants and not seedlings as recommended by most studies which could have hindered leaf disc transformation and regeneration. Trevisan et al., (2006), in his transformation study collected young leaves from 50 to 75-day old seedlings. Studies have cautioned against the use of older explants since the age of the plantlets is critical for transformation efficiency favouring the use of young healthy green well expanded leaves from the two to three week old in vitro plantlets for genetic transformation (Chabaud et al., 1988; Trevisan et al., 2006).
On the other hand, the efficiency of A. tumefaciens transformation considering the transient expression of b-glucurodinase activity was approximately 70%; this percentage was extremely high compared to the ultimate whole plant transformation efficiency of 0.456%. This showed that transgenic yellow passion fruit regeneration from successfully transformed leaf discs was the limiting factor as discussed above. The observed high transformation efficiency evidenced by GUS staining could be attributed to a number of procedures used in the development of the reported transformation protocol that included a pre-culturing step, vacuum infiltration, optimal cocultivation period and addition of acetosyringone. A number of studies have reported that addition of exogenous acetosyringone increases Agrobacterium T-DNA transfer into host cells (Sheikholeslam and Weeks, 1987; Adachi et al., 2005).
Agrobacterium-mediated transformation assisted by vacuum infiltration was first reported in Arabidopsis by Bechtold et al. (1993) and has since been used in other plants such as soybeans, wheat, rice etc. Vacuum infiltration generates a negative atmospheric pressure that causes the air spaces between the cells in the plant tissue to decrease allowing the infiltration of the infective transformation vector to relocate into the plant tissue.
Co-cultivation of explants with A. tumefaciens for three days allowed bacterial cells to infect and interact with explant cells effecting gene transfer into explant cells. It is essential to establish optimum co-culture conditions of explants and the Agrobacterium to increase frequency of transformation and avoid bacterial overgrowth due to prolonged co-cultivation period (Villemont et al., 1997; Suzuki et al., 2001).
Preculturing explants for one to three days prior to inoculation and co-cultivation with Agrobacterium has been shown to improve genetic transformation frequencies in many plants. Preculturing of explants had a positive effect on the induction of actively dividing competent cells for transformation (Villemont et al., 1997). Suma et al. (2008) reported that preculture of young buds of ginger on callus induction medium for three days increased the transformation frequency almost eight fold compared to explants that did not undergo preculture.
In this study, a viable transformation protocol for Uganda’s yellow passion fruit directly from leaf discs was developed using the GUS reporter gene. Further investigations are needed to decipher the effect of a number of variables in order to improve the protocols transformation efficiency. The method reported here provides new opportunities for the crop improvement of Uganda’s passion fruit with agronomically useful traits.
2,4-D-2,4-dichlorophenoxyacetic acid; GA3-Gibberellic acid; GUS-β-glucuronidase; MS, Murashige and Skoog medium; NAA, naphthaleneacetic acid; PCR, Polymerase chain reaction; T-DNA-Transfer DNA;PFRM, Passion Fruit Regeneration Media; X-Gluc, 5-bromo-4-chloro-3-indolyl-β-D-glucuronic acid, cyclohexyl ammonium salt; BAP, Benzylaminopurine.
The authors have not declared any conflict of interests.
This research was partially funded by Agricultural Technology and Agribusiness Advisory Services (ATAAS). The authors thank Agricultural Technology and Agribusiness Advisory Services (ATAAS) for the financial contribution towards this study and National Agricultural Laboratories NARL, Kawanda for providing laboratory facilities and office space.
REFERENCES
|
Adachi Y, Mori S, Nakano M (2005). Agrobacterium-mediated production of transgenic plants in Trycirtis hirta (Liliaceae). Acta Horticulturae 673:415-419.
Crossref
|
|
|
|
Agribusiness Development Centre (2014). View
|
|
|
|
|
Bechtold N, Ellis J, Pelletier G (1993). In planta Agrobacterium mediated gene transfer by infiltration of adult Arabidopsis thaliana plants. Comptes Rendus de l'Académie des Sciences- Life Sciences 316:1194-1199.
|
|
|
|
|
Birch RG (1997). Plant transformation: problems and strategies for practical application. Annual Review of Plant Physiology and Plant Molecular Biology 48:297-326.
Crossref
|
|
|
|
|
Chabaud M, Passiatore J, Cannon F, Buchanon-Wollaston V (1988). Parameters affecting the frequency of kanamycin resistant alfalfa obtained by Agrobacterium-mediated transformation. Plant Cell Reports 7(7):512-516.
Crossref
|
|
|
|
|
Chaudhry B, Yasmeen A, Husnain T, Riazuddin S (1999). Mini-scale genomic DNA extraction from cotton. Plant Molecular Biology Reporter 17(3):1-7.
Crossref
|
|
|
|
|
Cho J, Yano H, Okamoto D, Kim H, Jung H, Newcomb K, Buchanan B, and Lemaux P (2004). Stable transformation of rice (Oryza sativa L.) via microprojectile bombardment of highly regenerative, green tissues derived from mature seed. Plant Cell Reports 22(7):483-48.
Crossref
|
|
|
|
|
Correa M, Pinto A, Rezende J, Mendes B (2015). Genetic transformation of sweet passion fruit (Passiflora alata) and reactions of the transgenic plants to Cowpea aphid borne mosaic virus. European Journal of Plant Pathology 143(4):813-821.
Crossref
|
|
|
|
|
Davis E, Lineberger R, Miller A (1991). Effects of tomato cultivar, leaf age, and bacterial strain on transformation by Agrobacterium tumefaciens. Plant Cell Tissue and Organ Culture 24(2):115-121.
Crossref
|
|
|
|
|
Drew RA (1997). The Application of Biotechnology to the Conservation and Improvement of Tropical and Subtropical Fruit Species. Seed and Plant Genetic Resources Service. Food and Agriculture Organization of the United Nations, Rome.
|
|
|
|
|
Freitas D, Coelho M, Souza M, Marques A, Ribeiro B (2007). Introduction of the anti-apoptotic baculovirus p35 gene in passion fruit induces herbicide tolerance, reduced bacterial lesions, but does not inhibits passion fruit woodiness disease progress induced by cowpea aphid borne mosaic virus (CABMV). Biotechnology Letters 29(1):79-87
Crossref
|
|
|
|
|
Ghorbel R, Dominguez A, Navarro L, Pena L (2000). High efficiency genetic transformation of sour orange (Citrus aurantium) and production of transgenic trees containing the coat protein gene of citrus tristeza virus. Tree Physiology 20(17):183-1189.
Crossref
|
|
|
|
|
Hanke M, Reidel M, Reim S, Flachowsky H (2007). Analysis of tissue uniformity in transgenic apple plants. Acta Horticulturae 738:301-306.
Crossref
|
|
|
|
|
Holford P, Newbury J (1992). The effects of antibiotics and their breakdown products on the in vitro growth of Antirrhinum majus. Plant Cell Reports 11:93-96.
Crossref
|
|
|
|
|
Internationl Centre for trade and Sustainable development (ICTSD) (2011). View.
|
|
|
|
|
Jefferson RA, Kavanagh TA, Bevan MW (1987). GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO Journal 6(13):3901-3907.
Crossref
|
|
|
|
|
Lin J, Assad-Garcia N, Kuo J (1995). Plant hormone effect of antibiotics on the transformation efficiency of plant tissue by Agrobacterium tumefaciens cells. Plant Science 109(2):171-177.
Crossref
|
|
|
|
|
Ling H, Kriseleit D, Ganal W (1998). Effect of ticarcillin/potassium clavulanate on callus growth and shoot regeneration in Agrobacterium-mediated transformation of tomato (Lycopersicon esculentum Mill.). Plant Cell Reports 17(11):843-847.
Crossref
|
|
|
|
|
Madhulatha P, Pandey R, Hazarika P, Rajam M (2007). High transformation frequency in Agrobacterium-mediated genetic transformation of tomato by using polyamines and maltose in shoot regeneration medium. Physiology and Molecular Biology of Plants 13:191-198.
|
|
|
|
|
Manders G, Otoni W, D'utra Vaz F, Blackhall N, Power J, Davey M (1994). Transformation of passionfruit (Passiflora edulis fv. flavicarpa Degener) using Agrobacterium tumefaciens. Plant Cell Reports 13(12):697-702.
Crossref
|
|
|
|
|
Ministry of Agriculture, Animal Industry and Fisheries (MAAIF) (2019).
View
|
|
|
|
|
Monteiro HA, Jadão A, Mendes BM, Rezende JA, Trevisan F, Piedade S (2011). Genetic transformation of passionflower and evaluation of R1 and R2 generations for resistance to Cowpea aphid borne mosaic virus. Plant Diseases 95(8):1021-1025.
Crossref
|
|
|
|
|
Murashige T, Skoog F (1962). A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physioogia Plantarum 15(3):473-497.
Crossref
|
|
|
|
|
National Trade Policy (2007). Trading Out of Poverty into Wealth and Prosperity. Ministry of Tourism, Trade and Industry. www.mtti.go.ug. Republic of Uganda.
|
|
|
|
|
Ochwo-Ssemakula M, Sengooba T, Hakiza J, Adipala E, Edema R, Winter S (2012). Characterization and distribution of a Potyvirus associated with passion fruit woodiness disease in Uganda. Plant Diseases 96(5):659-665.
Crossref
|
|
|
|
|
Okkels F, Pedersen M (1988). The toxicity to plant tissue and to Agrobacterium tumefaciens of some antibiotics. Acta Horticulturae 225:199-207.
Crossref
|
|
|
|
|
Oyebanji B, Nweke O, Odebunmi O, Galadima N, Afolabi A (2009). Simple, effective and economical explant surface sterilization protocol for cowpea, rice and sorghum Seeds. African Journal of Biotechnology 8(20):5395-5399.
|
|
|
|
|
Petri C, Burgos L (2005). Transformation of fruit trees. Useful breeding tool or continued future prospect? Transgenic Research 14(1):15-26.
Crossref
|
|
|
|
|
Sambrook J, Fritschi EF, Maniatis T (1989). Molecular cloning: A laboratory manual, 2nd Edition, Cold Spring Harbor Laboratory Press, New York.
|
|
|
|
|
Sheikholeslam SN, Weeks DP (1987). Acetosyringone promotes high efficiency transformation of Arabidopsis thaliana explants by Agrobacterium tumefaciens. Plant Molecular Biology 8(4):291-298.
Crossref
|
|
|
|
|
Suma B, Keshavachandran R, Nybe E (2008). Agrobacterium tumefaciens mediated transformation and regeneration of ginger (Zingiber officinale Rosc.). Journal of Tropical Agriculture 46 (1-2):38-44.
|
|
|
|
|
Suzuki S, Supaibulwatana K, Mii M, Nakano M (2001). Production of transgenic plants of Liliaceous ornamental plants Agapanthus praecox ssp. orentalis (Leighton) Leighton via Agrobacterium-mediated transformation of embryogenic calli. Plant Science 161(1):89-97.
Crossref
|
|
|
|
|
Trevisan F, Mendes BM, Maciel SC, Vieira M, Meletti L, Rezende JA (2006). Resistance to Passion fruit woodiness virus in transgenic passionflower expressing the virus coat protein gene. Plant Diseases 90(8):1026-1030.
Crossref
|
|
|
|
|
Uganda Export Promotion Board (2016). Ministry of Trade, Industry and co-operative. Republic of Uganda. http://www.ugandaexportsonline.com.
|
|
|
|
|
Varassin IG, Trigo JR, Sazima M (2001). The role of nectar production, flower pigments and odour in the pollination of four species of Passiflora (Passifloraceae) in south-eastern Brazil. Botanical Journal of the Linnean Society 136(2):139-152.
Crossref
|
|
|
|
|
Villemont E, Dubois F, Sangwan R, Vasseur G (1997). Roles of the host cell cycle in Agrobacterium-mediated transformation of Petunia: evidence of an S-phase control mechanism for T-DNA transfer. Planta 201(2):160-172.
Crossref
|
|
|
|
|
Wangungu CW, Mwangi M, Gathu R, Muasya R (2014). Good orchard maintenance and agronomic practices as working components in management of dieback disease on passion fruit (Passiflora sp.) in Kenya. Annual Research and Review in Biology 4(9):1397-1405.
Crossref
|
|
|
|
|
Winkler LM, Quoirin M (2002). Organogenesis and Genetic Transformation of Yellow Passion Fruit (Passiflora edulis f. flavicarpa Deg.) with the genes CMe-ACO1 and nptII via Agrobacterium tumefaciens. Acta Horticulturae 632:31-40.
Crossref
|
|