ABSTRACT
L-Glutaminase is majorly produced by microorganism including bacteria, yeast and fungi. It mainly catalyzes the hydrolysis of gamma-amido bond of L-glutamine. In the present investigation, the potent marine isolate, Bacillus subtilis JK-79 producing L-glutaminase was evaluated for the maximum L-glutaminase production by solid state fermentation (SSF). In this context, different agro-industrial residues reported in literature were tested and among them, wheat bran gave maximum L-glutaminase production (236.67 U/ml) and protein concentration (6.89 mg/ml). Statistical optimization of media components and culture conditions were successfully employed to markedly enhance the L-glutaminase production under SSF by marine B. subtilis JK-79. Optimization was sequentially performed from one factor at a time (OFAT), followed by Plackett and Burman Design (PBD) and response surface methodology (RSM). With the help of PB design, three significant factors such as moisture content, pH and L-glutamine were identified to significantly affect the L-glutaminase production. These three independent variables were then optimized by central composite design (CCD) of RSM. Maximum L-glutaminase production of 672.28 U/ml under flask condition was obtained at the predicted optimal values of moisture content of 62.5% (w/w), pH of 7.1 and glutamine of 2.44% (w/v). The maximum experimental L-glutaminase production was 680.8 U/ml, whereas the predicted value for L-glutaminase production was 672.28 U/ml, indicating a strong agreement between them. Statistical optimization has enhanced the production of the enzyme up to 2.88 fold as compared to the basal wheat bran medium. Thus, application of PBD and RSM for optimization studies proves to be an effective method for improving the L-glutaminase production and also understanding the interaction effects between the factors with minimum number of experiments.
Key words: Bacillus subtilis JK-79, L-glutaminase, Plackett-Burman design, response surface methodology, wheat bran.
Solid state fermentation (SSF) has gained importance in the biotechnology industry due to its potential application in the production of enzymes. SSF has greater advantage as compared to submerged fermentation (SmF) due to the lower capital and operating costs as agro-industrial residues are preferably used as substrates. The low water volume used in SSF also has a large impact on the economy of the process mainly because of the smaller fermenter size, the reduced downstream processing, the reduced stirring and lower sterilization costs (Hölker and Lenz, 2005; Nigam, 2009). SSF is a good alternate to the traditional chemical processes as it has several characteristics that make it eco-friendly, such as lower energy consumption, less waste water generation, use of agro-industrial residues as substrates thus avoiding environmental problems while disposing. The use of agro-industrial residues in SSF processes is of particular interest due to their availability and low cost, besides being an environment friendly alternative for their disposal (Castro and Sato, 2015).
Agro-industrial residues are derived from agricultural activities and these residues are generated in large amounts throughout the year, and are the most abundant renewable resources on earth. They are mainly composed of sugars, fibres, proteins and minerals, which are compounds of industrial interest. The presence of sugars, proteins, minerals and water make the agro-industrial residues suitable substrates for the growth of bacterial strains. If the cultivation conditions are controlled, different products of industrial interest may be produced, avoiding the loss of potential energy sources (Pandey, 2000). Marine bacteria are the best suited for SSF processes due to their unique property to adsorb onto solid particles and can survive in different range of pH and temperature. Hence, there is an increasing interest in marine microorganisms for the production of L-glutaminase under SSF (Unissa et al., 2014). Only scanty reports are available on L-glutaminase production under SSF using agro-industrial residues as substrate. SSF was found to be more suitable than submerged fermentation for L-glutaminase production as 25-fold enhancement was obtained using Pseudomonas flourescens ACMR 171 when wheat bran was used as substrate (Renu, 1991).
L-Glutaminase was produced by yeast Zygosacharomyces rouxii using wheat bran and sesame oil as substrate under SSF. Addition of 10% (w/v) NaCl and seawater to wheat bran and sesame oil enhanced the enzyme production (Kashyap et al., 2002). Beauveria sp., an alkalophilic and salt-tolerant fungus isolated from marine sediment, was used for L-glutaminase production using seawater-based medium supplemented with L-glutamine as substrate (Sabu et al., 2000, 2001). Seawater being a natural reserve for marine organisms can provide them sufficient nutrients when used as supplement in SSF for production of industrially important enzymes. Recent studies by Sayed (2009) showed that wheat bran was the best solid substrate for the production of L-glutaminase by Trichoderma koningii. In the present investigation, the potent marine isolate, Bacillus subtilis JK-79 producing L-glutaminase was evaluated for the maximum production of the enzyme under SSF.
Microorganism and culture maintenance
The isolate, B. subtilis JK-79 (KC492745) used in this study was isolated from marine soil collected from Parangipettai costal area (Lat. 11°.29’ N; Long. 79°.46’ E). The strain was maintained in Zobell’s marine agar slant (Himedia, India) at 4°C and was periodically sub-cultured.
L-Glutaminase production by SSF using agro-industrial residues
Preparation of solid substrate
Different agro-industrial residues (black gram husk, red gram husk, wheat bran, rice husk, green gram husk, coconut oil cake, groundnut oil cake, palm seed powder and sawdust) were collected from local market. Wheat bran and rice husk are widely accepted solid substrate due to their large surface area, high nutrition content and support the growth of different microorganisms. Black gram husk, red gram husk and green gram husk also have high nutrient content and are used in SSF for the production of enzymes. Coconut oil cake and groundnut oil cake were selected for SSF based on their availability, water retention capacity and requirement of reduced pre-treatment procedure. All these substrates were powdered and dried in a hot air oven overnight at 60°C. The solid particles were sieved using standard sieves and the particles 1.4 mm were stored in air-tight containers for further use (Prabhu and Chandrasekaran, 1996). Five grams of the solid substrates were moistened with seawater containing L-glutamine at 1% (w/v) level to obtain 50% moisture content, autoclaved and cooled to room temperature before inoculation.
Inoculation and incubation
The sterilized solid substrate media was inoculated with 2% (v/w) inoculum size of the strain marine, B. subtilis JK-79. The contents were mixed thoroughly and incubated in a slanting position at 37°C for 24 h under 80% relative humidity (Prabhu and Chandrasekaran, 1997).
Enzyme recovery
L-Glutaminase from the fermented solid substrates was extracted with phosphate buffer (0.1 M, pH 7.0) by simple contact method (Renu, 1991; Prabhu and Chandradekaran, 1997).
L-Glutaminase assay and protein estimation
L-Glutaminase was assayed by Imada et al. (1973) method. Protein content in the crude enzyme source was estimated by Lowry et al. (1951) method using bovine serum albumin as the standard and the values were expressed as mg/ml.
Optimization of process parameters by OFAT approach
Among the various agro-industrial residues evaluated, wheat bran gave the maximum L-glutaminase production. Hence, wheat bran was used as the solid substrate for L-glutaminase production. Further, the various process parameters were optimized for maximal L-glutaminase production. The parameters studied include moisture content of the medium, initial pH, particle size of the substrate, incubation temperature, inoculum size, incubation time, amino acid, additional carbon source and nitrogen source in seawater. All the experiments were carried out in triplicates and the mean values were considered.
Effect of particle size of the solid substrate on L-glutaminase production
Impact of substrate particle size on the production of L-glutaminase by the bacteria was evaluated using substrate of different particle size. The solid substrate purchased from the market were of different particle size, hence they were sieved using standard sieves of known mesh size to obtain uniform particle size in the range 0.6, 1.0 and 1.4 mm.
Effect of initial moisture content on L-glutaminase production
The initial moisture content was varied (30 to 80% w/w) to understand the effect of moisture content on the production of L-glutaminase. This was achieved by altering the amount of sea water used for moistening.
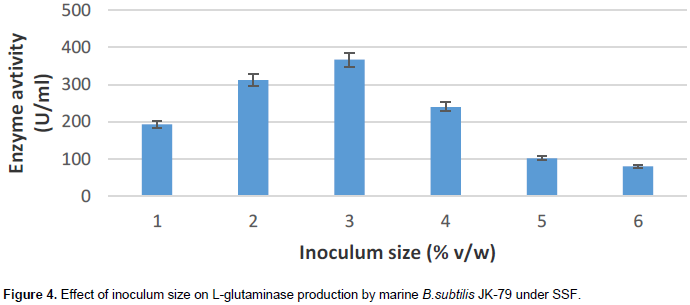
Effect of inoculum size on L-glutaminase production
The effect of inoculum size on L-glutaminase yield by bacteria during SSF were determined by using 24 h old culture (O.D 600nm = 0.8) of increasing size in the range of 1 to 6% v/w
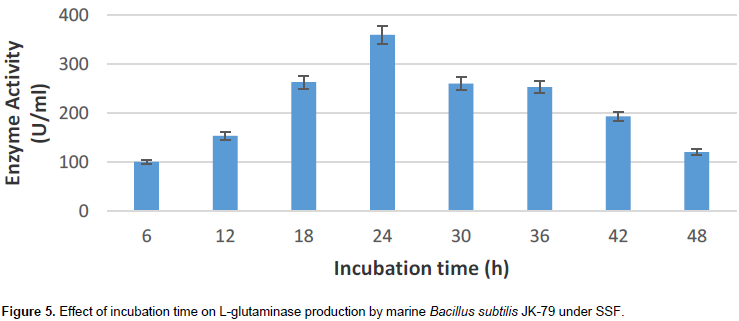
Effect of incubation time on L-glutaminase production
The effect of incubation time on L-glutaminase production by marine B. subtilis JK-79 was analyzed by carrying out the SSF for 48 h.
Effect of initial pH of the medium on L-glutaminase production
The effect of initial pH of the solid substrate medium on L-glutaminase production was determined by adjusting pH of the seawater using 1 N HCl or 1 N NaOH.
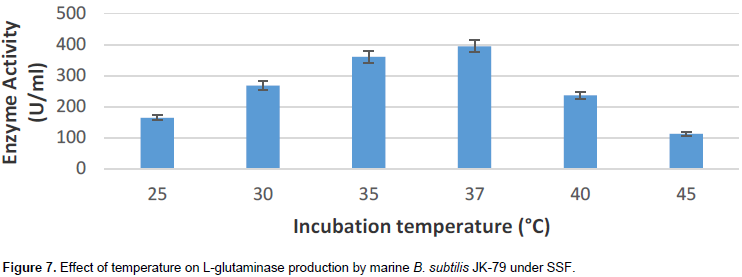
Effect of incubation temperature on L-glutaminase production
The effect of incubation temperature on L-glutaminase production
by marine B. subtilis JK-79 was analyzed by carrying out the SSF at different temperature from 25 to 45°C.
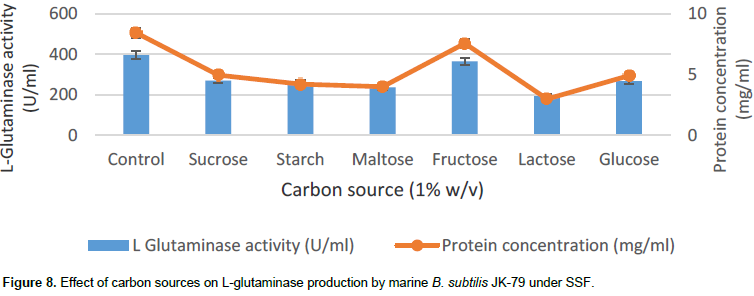
Effect of additional carbon source on L-glutaminase production
The effect of additional carbon sources (sucrose, starch, maltose, fructose, lactose and glucose) on the production of L-glutaminase was evaluated by adding them at 1% (w/v) level, in the SSF medium.
Effect of additional nitrogen source on L-glutaminase production
The effect of additional nitrogen sources (peptone, casein, gelatin, beef extract, tryptone, yeast extract, ammonium chloride, ammonium sulphate, potassium nitrate and sodium nitrate) on the production of L-glutaminase was evaluated by adding them at 1% (w/v) level in the SSF medium.
Effect of amino acids on L-glutaminase production
The effect of amino acids (L-glutamine, phenyl alanine, cysteine, glycine, histidine, methionine and L-aspargine) on the production of L-glutminase was evaluated by adding them at 1% (w/v) level in the SSF medium. Further, the optimum concentration of the amino acid was also determined by incorporating the selected amino acid in the range (1 to 5% w/v). The additional carbon and nitrogen sources did not increase the glutaminase production substantially. Therefore, the optimum fermentation conditions were found to be pH 7, 37°C, incubation period of 24 h, 3% (v/w) of inoculum size, particle size of 1 mm and moisture content of 60%.
Optimization by statistical design
Identifying significant variables by Plackett-Burman design (PBD)
After identifying the variables affecting L-glutaminase production by OFAT approach, the PBD (Plackett and Burman, 1946) was applied to screen the significant factors with respect to their main effects on enzyme production. A total of nine factors such as particle size, moisture content, pH, temperature, inoculum size, incubation time, carbon source, nitrogen source and glutamine concentration were considered for the experimental design. The high and low levels of the different factors are listed in Table 1. The main effect was calculated as the difference between the average of measurements made at the high level (+1) and the average of measurements observed at low level (-1) of each factor (Usha et al., 2011). The factors that have confidence level above 95% were considered the most significant factors that affect the L-glutaminase production.
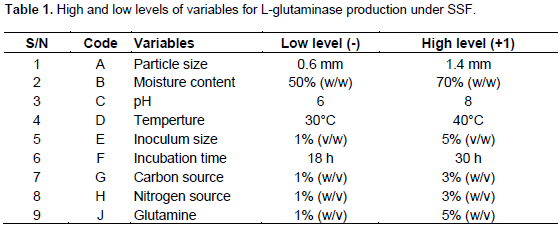
The details of experimental design for screening the different factors are shown in Table 2. The medium was formulated as per the design and the flask culture experiments were performed. All the experiments were performed in triplicates and the average of L-glutaminase production was considered as the response. The PBD is based on the first-order polynomial model:
Y = βo + ∑ βiXi
Where, Y denotes the response (L-glutaminase activity U/ml), βo is model intercept, βi is the factor co-efficient and Xi is the level of independent variable. From the regression analysis, the variables showing p-value below 5% (p<0.05) were considered to have greater impact on L-glutaminase production and used further for CCD analysis.
Response surface methodology
RSM uses statistical experimental designs such as central composite design (CCD), Box-Behnken design etc. to develop empirical models that relate a response and mathematically describes the relationships between the independent and dependent variables of the process under consideration. The RSM has been used to obtain a predicted model for optimizing the fermentation media and/or process parameters, to carry out simulation with model equation and for better understanding of the fermentation process. In the present study, RSM using CCD was adopted for improving the L-glutaminase production under SSF by strain B. subtilis JK-79 using the software Design Expert Release 9 (Stat-Ease INC. Minneapolis MN, U.S.A). A full factorial central composite design was performed. The quadratic regression model can be illustrated as
Y = βo + ∑ βiXi + ∑ βiiXi2 + ∑ βijXiXj
Where, Y is the response (L-glutaminase activity U/ml), βo is the intercept term, βi is the slope or linear effect of input variable Xi, βii is the quadratic effect of input variable Xi and βij is the linear by linear interaction effect between the input variable Xi and Xj. The statistical model was validated with respect to L-glutaminase production under the conditions predicted by the model in shake flask conditions. Samples were withdrawn at the desired intervals and L-glutaminase assay was performed as mentioned earlier.
Production of L-glutaminase using different agro-industrial residues
Selection of suitable agro-industrial residue as solid substrate becomes a pre-requisite for optimizing the process parameters of the SSF fermentation medium. In this context, different agro-industrial residues (black gram husk, green gram husk, red gram husk, wheat bran, rice husk, coconut oil cake, groundnut oil cake, palm seed fibre and saw dust) were evaluated for the production of L-glutaminase by marine Bacillus subtilis JK-79 under SSF. From Figure 1, it is evident that among the various agro-industrial residues, wheat bran gave maximum L-glutaminase production (236.67 U/ml) and protein concentration (6.89 mg/ml). Many authors have reported that wheat bran was found to be most the preferable substrate for L-glutaminase production due to their excellent mechanical properties such as structure retention and lack of particle agglomeration in addition to their good nutritional value (Renu, 1991; Prabhu and Chandrasekaran, 1996; Kashyap et al., 2002; Sayed, 2009; Iyer and Singhal, 2010a,b; Athira et al., 2014). Following wheat bran, green gram husks (200.33 U/ml) and rice husk (183 U/ml) showed maximal L-glutaminase production. The observed variations in L-glutaminase production reveal that the composition of the substrate plays a pivot role in the L-glutaminase production.
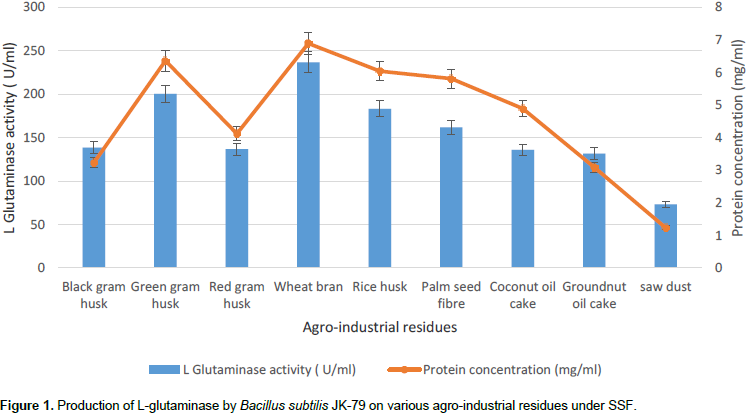
Sathish et al. (2008) has reported that bengal gram husk supported maximal L-glutaminase production (2731 U/gds), followed by the palm seed fibre (1953 U/gds) and wheat bran (1036 U/gds), suggesting the role of agro-industrial residues in the production of L-glutaminase. Similarly, Han et al. (2003) produced L-glutaminase on sufi (soybean cheese) by Actinomucor and Rhizopus oligosporus, while Sabu et al. (2000, 2001) produced L-glutaminase with marine Beauveria sp. BTMFS 10. From the literature reports, it is evident that Bacillus sp. in general has the tendency to utilize wheat bran as the preferred substrate for the production of various types of enzymes. Wheat bran was used as the sole substrate for the production of alkaline protease and α-amylase by Bacillus sp. P-2 (Kaur et al., 2001) and Bacillus sp. AS-1 (Soni et al., 2003), respectively. Baysal et al. (2003) also used wheat bran as substrate for the production of α-amylase from B. subtilis. Kashyap et al. (2003) produced pectinase from Bacillus sp. by using wheat bran along with polygalacturonic acid as the solid substrate. Similarly, Sodhi et al. (2005) has also produced α-amylase from Bacillus sp. PS-7 on a medium containing wheat bran, glycerol and soybean meal. Apart from wheat bran, Bacillus sp. also has tendency to utilize green gram husk as solid substrate which is evident from the report where Prakasham et al. (2006) has produced alkaline protease from Bacillus sp. by using green gram husk as the sole substrate. Since wheat bran showed the maximal L-glutaminase production by marine Bacillus subtilis JK-79, wheat bran was used as substrate for L-glutaminase production under SSF and further studies were carried out.
Optimization of process parameters and media components by OFAT approach
Effect of particle size on L-glutaminase production
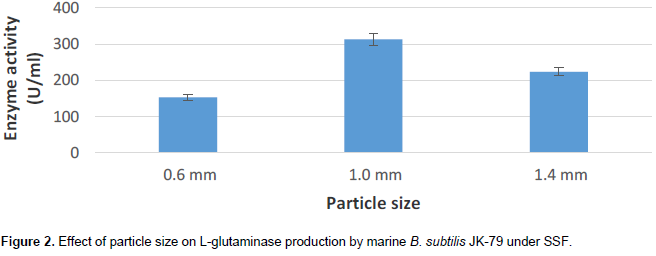
Particle size of substrates is one of the critical factors that influence the fermentation process. Particle size of the substrate is an important factor which affects SSF, as it determines the heat and mass transfer during the process (Pandey et al., 2000). From Figure 2, it is evident that particle size of the range 1.0 mm gave maximal L-glutaminase production (312.89 U/ml) by marine B. subtilis JK-79. The result is in accordance with the literature report of Prabhu and Chandrasekaran (1996). The authors showed maximal L-glutaminase production by Vibrio costicola at 2% (w/v) L-glutamine concentration, initial pH 7.0, 35°C, 60% moisture content, 0.6 to 1.0 mm particle size, and 24 h incubation time using wheat bran and rice husk.
Effect of moisture content of the medium on L-glutaminase production
In SSF, moisture content of the solid medium plays critical role as the microbiological activity on a substrate will eventually increase as the water content increases to the optimal level. Figure 3 reveals that moisture content of the wheat bran plays a significant role in the production of L-glutaminase. It could be seen that maximal L-glutaminase production (326.07 U/ml) was noticed at 60% (w/w) moisture content. Kashyap et al. (2002) and Sabu et al. (2000) has reported L-glutaminase production by Z. rouxii and Beauveria sp. at 64 and 60% initial moisture content, respectively.
Effect of inoculum size on L-glutaminase production
Optimal inoculum size is an essential requirement for maximal L-glutaminase production by the bacteria. From the Figure 4, it is clear that maximal L-glutaminase production by marine Bacillus subtilis JK-79 was obtained at 3% (v/w) inoculum size (367 U/ml). Increase in inoculum size beyond 3% (v/w) resulted in decrease in L-glutaminase production and this may be attributed to nutrient depreciation or accumulation of some toxic substance. However, with lower inoculum size, the decrease in L-glutaminase production could be due to lesser number of cells and hence requires longer time to grow and form the desired product (Sayed, 2009). The result is in accordance with the inoculum size reported by Sayed (2009), whereas Kashyap et al. (2002) reported maximal L-glutaminase production at 2% (v/w) inoculum size with wheat bran and sesamum oil cake.
Effect of incubation time on L-glutaminase production
24 h of cultivation on wheat bran resulted in L- glutaminase synthesis of 360 U/ml (Figure 5) with B. subtilis JK-79. Further increase in incubation time, resulted in a gradual decline in the enzyme production. This is may be because of the inactivation of the enzyme. The result is in accordance with literature reports (Prabhu and Chandrasekaran, 1997; Sabu et al., 2000; Kashyap et al., 2002).
Effect of pH of the medium on L-glutaminase production
Experiments were conducted to optimize the pH of the SSF medium with wheat bran for maximum L-glutaminase yield. The medium with pH 7 supported maximal L-glutaminase production (356.33 U/ml) by B. subtilis JK-79 (Figure 6). This observation is in agreement with the results obtained by marine V. costicola (Prabhu and Chandrasekaran, 1997) and T. koningii (Sayed, 2009).
Effect of incubation temperature on L-glutaminase production
Maximum L-glutaminase production (394 U/ml) was obtained when SSF was carried out at 37°C (Figure 7). The enzyme production decreased above and below 37°C. The result is in good agreement with the optimal conditions reported by Sathish et al. (2008). The author showed 37°C as the optimal temperature for maximal L-glutaminase production by Bacillus sp. Renu (1991) showed that 35°C is the optimal temperature for maximal L-glutaminase production by P. fluorescens ACMR 171 and Vibrio cholera ACMR 347.
Effect of additional carbon source on L-glutaminase production
Incorporation of additional carbon sources at 1% (w/v) level did not show any improvement in the production of L-glutaminase by marine B. subtilis JK-79. This may be attributed to the chemical composition of wheat bran. Among the carbon sources, fructose at 1% (w/v) showed L-glutaminase production of 365 U/ml (Figure 8). However, Sayed (2009), Prabhu and Chandrasekaran (1997) and Kashyap et al. (2002) reported increase in L-glutaminase production by incorporating additional carbon source in wheat bran such as 1% (w/v) glucose, 1% (w/v) maltose and 1% (w/v) glucose, respectively.
Effect of additional nitrogen sources on L-glutaminase production
In the present work, different organic and inorganic nitrogen sources were evaluated (Figure 9). The results show that none of the inorganic nitrogen sources supported significant improvement in the production of L-glutaminase and among the organic nitrogen sources, yeast extract showed marginal increase in the L-glutaminase production. Kashyap et al. (2002), Prabhu and Chandrasekarn (1997) and Sayed (2009) has reported negative impact of additional nitrogen sources on L-glutaminase production by Z. rouxii, V. costicola and T. koningii respectively.
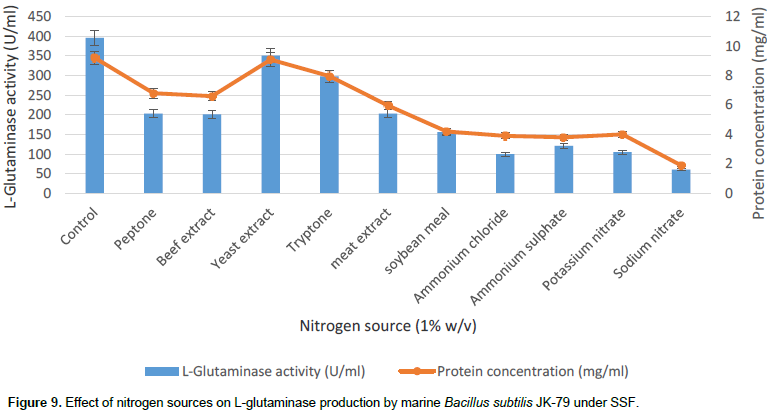
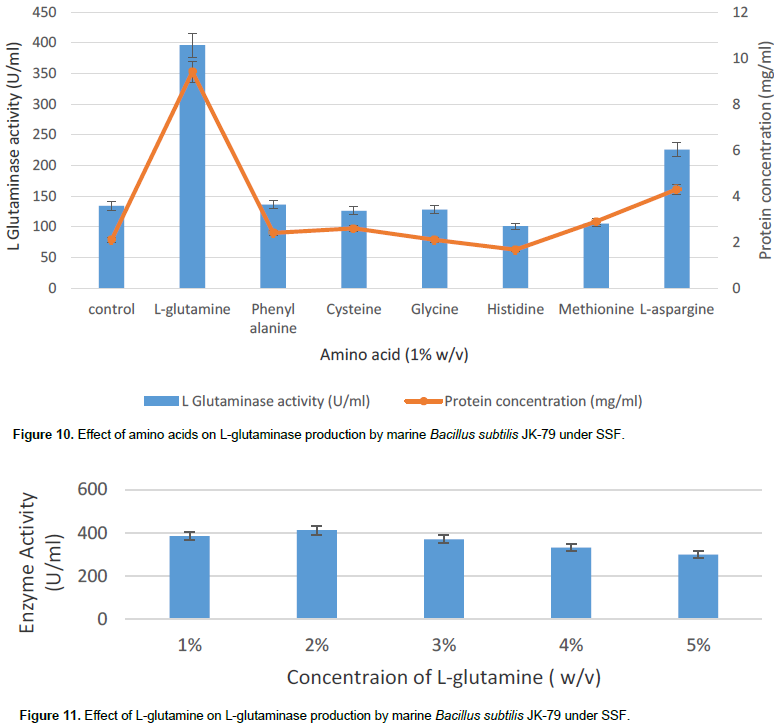
Effect of amino-acids on L-glutaminase production
The effect of different amino acids on the production of L-glutaminase by marine B. subtilis JK-79 was studied by incorporating amino acids in the medium. Among the different amino acids, L-glutamine which is the actual substrate for L-glutaminase showed a significant increase in the production of the enzyme (Figure 10). Thus, L-glutamine serves as an inducer for the production of L-glutaminase. The control medium does not contain glutamine but yet has shown L-glutaminase production. This may be attributed to the presence of L- glutamine amino acid in wheat bran. Further, the optimal concentration of glutamine was evaluated by adding different concentrations of L-glutamine in the range of 1 to 5% w/v. The results indicate that 2% (w/v) of L-glutamine showed maximal production (412 U/ml) of L-glutaminase (Figure 11). The result is in good correlation with the literature reports where Sayed (2009) reported maximal L-glutaminase production at 2% L-glutamine in wheat bran. However, Renu (1991) has reported apart from L-glutamine other amino acids like L-glutamic acid and lysine at 1% (w/v) which was found to induce L-glutaminase production under SSF with V. cholera ACMR 347 and P. fluorescens ACMR 171. Thus, the optimal conditions for the maximal L-glutaminase production by marine B. subtilis JK-9 under SSF was found to be pH 7.0, temperature 37°C, incubation period 24 h with wheat bran as the solid substrate, supplemented with 2% (w/v) glutamine by OFAT approach.
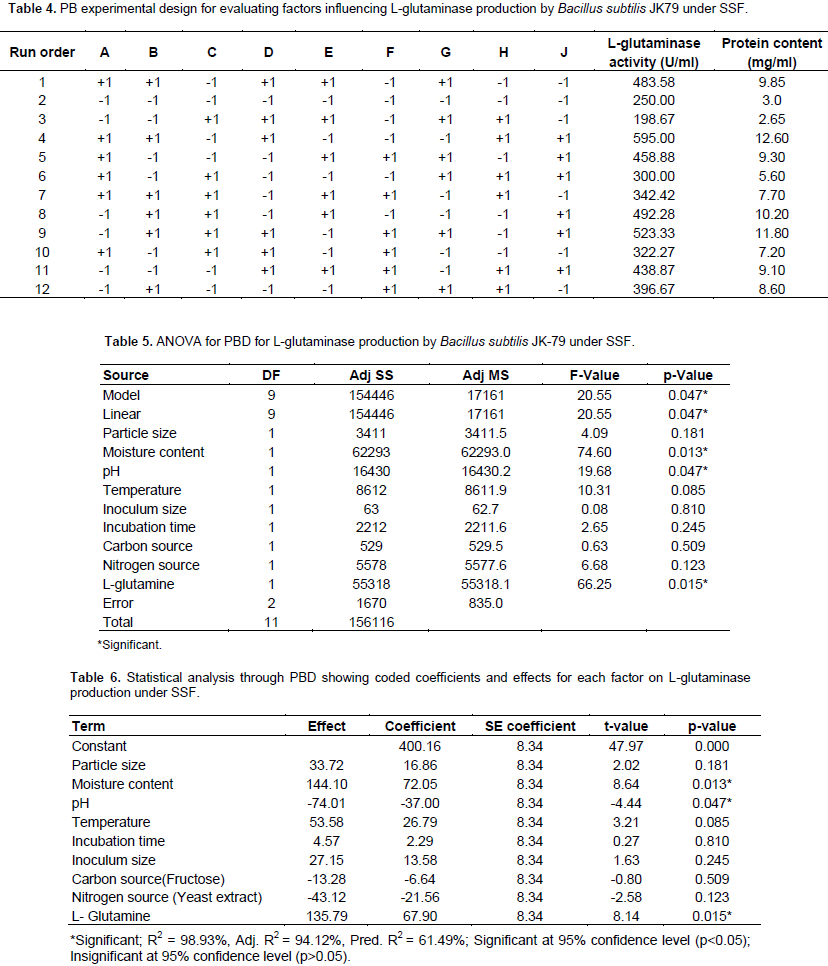
Identification of significant factors using PBD
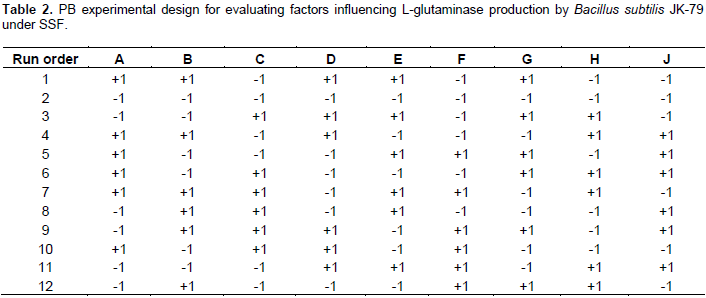
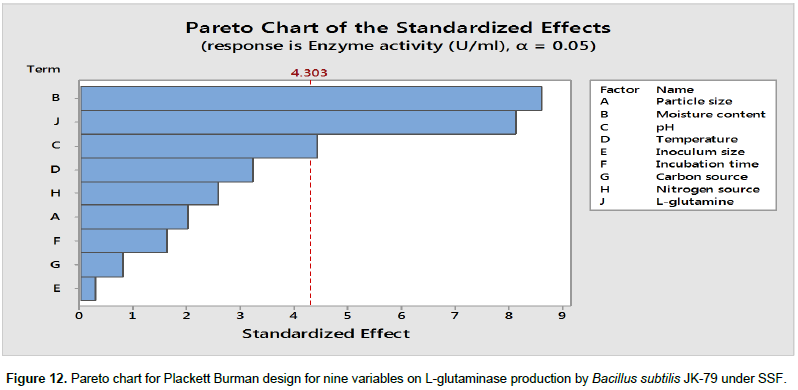
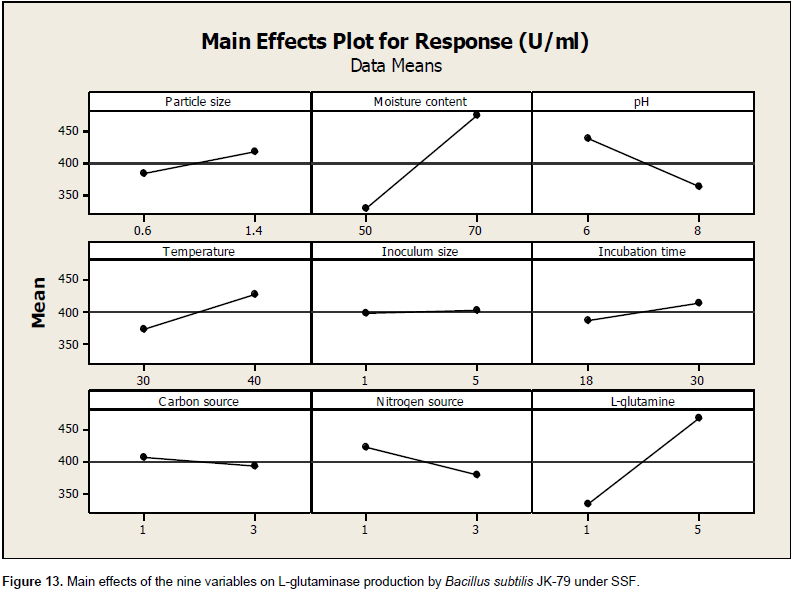
The influence of nine variables namely pH, temperature, moisture content, particle size, inoculum size, incubation time, carbon source, nitrogen source and glutamine on the production of L-glutaminase by the strain, B. subtilis JK-79 was investigated in 12 runs using PBD. Table 4 represents the PBD for the nine variables and the corresponding response for L-glutaminase production (U/ml). Variations ranging from 198.67 to 595.00 U/ml in the production of L-glutaminase was observed by PBD. The Pareto chart (Figure 12) illustrates the order of the significance of the variables affecting L-glutaminase production. On the basis of analysis of variance (ANOVA), and values of coefficient for significance (P<0.05), the factors with high significance were in the order: moisture content, glutamine and pH. Neglecting the insignificant variables, the first order polynomial equation was derived representing L-glutaminase production as a function of independent variables.
Y = -140 + 7.25 Moisture content – 37.0 pH + 67.90 L-glutamine
Where, Y is the response (L-glutaminase production U/ml). The statistical analysis and the ANOVA of the experimental design are shown in Tables 5 and 6, respectively. The significance of each variable was evaluated based on its main effect. The main effect of the factors is shown in Figure 13. Statistical analysis of PBD demonstrated that the model F value of 20.5 is significant and among the significant factors, moisture content and glutamine showed a remarkable effect on the production of L-glutaminase by B. subtilis JK-79. The goodness of fit of the regression model was represented by co-efficient of determination (R2). In the present model, R2 was 98.93%, which indicated that upto 98.93% of the total variability in the response could be explained by the model and only 1.07% variability was not explained. The value of the adjusted determination coefficient (Adj R2 = 0.9412) confirmed the significance of the model as well. L-Glutaminase production, obtained from PBD showed a wide range of variation and this revealed the necessity for further optimization. Therefore, the entire set of insignificant variables was left and further optimization was carried out only with the significant variables.
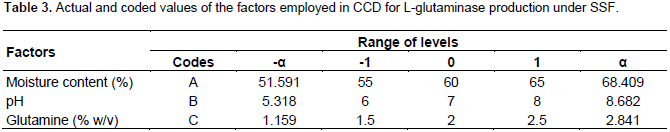
Optimization using central composite design
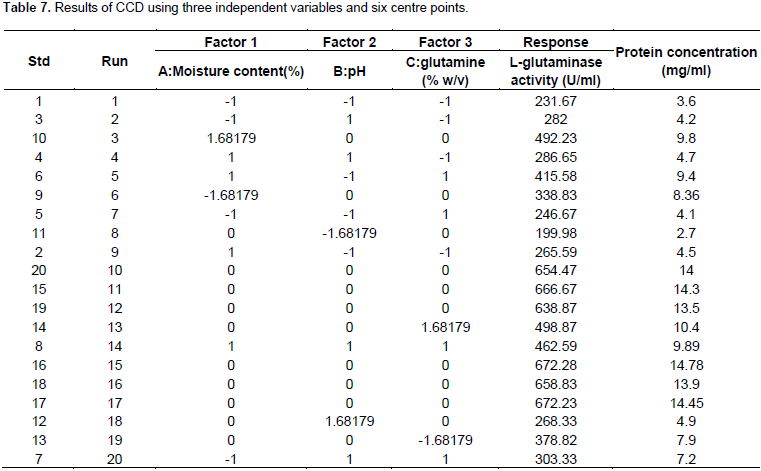
Following the identification of significant variables by PBD, the optimal concentration level of these variables viz glutamine, moisture content and pH were identified by RSM. RSM using CCD, was adopted to understand the interactive effects of these four significant variables. Table 3 illustrates the details of actual and coded values employed in the CCD. The experimental trials were performed based on the CCD (Table 7) and the results obtained were fitted to a second order polynomial equation to explain the dependence of L-glutaminase production with the independent variables.
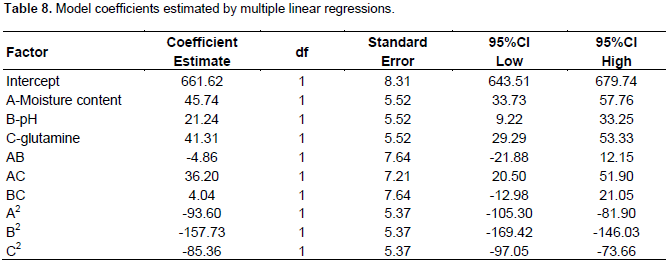
Y = +661.62 + 45.74 * A + 21.24 * B + 41.31 * C - 4.86 * AB + 36.20 * AC + 4.04 * BC – 93.60 * A2 -157.73 * B2 – 85.36* C2
Where, Y is the response of L-glutaminase production, A, B and C are the coded values of moisture content, pH and glutamine, respectively.
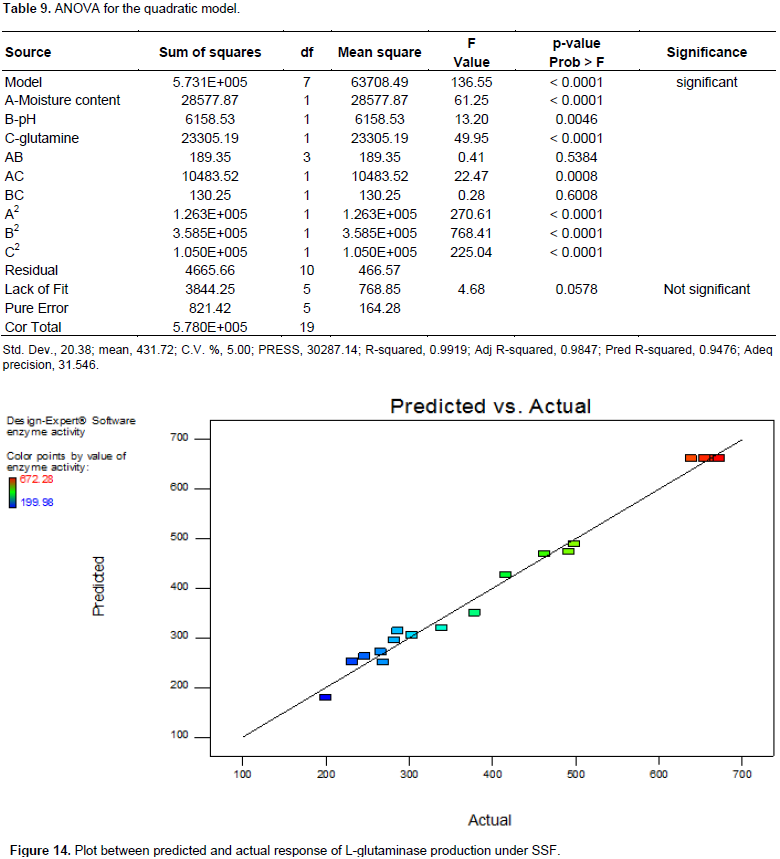
The analysis of variance of the quadratic regression model (Table 9) suggested that the model is very significant which was evident from the Fisher’s F-test (Fmodel = 136.55) and a low probability value (Pmodel <0.0001). The p value for “lack of fit” (0.0578) also indicated that the quadratic model adequately fitted the data. Table 8 gives the model coefficients estimated by regression analysis for each variable and the p values were used a tool to check the significance of each variable. The smaller the magnitude (p< 0.05), the more significant the corresponding co-efficient, while those greater than 0.1000 indicates the model terms were insignificant. In this model, A, B, C, AC, A2, B2 and C2 are significant model terms. The model’s goodness of fit was checked by determination of co-efficient (R2). The R2 was found to be 0.9919, indicating that the model could explain 99.19% variability with the response. The “Adeq Precision” measures the signal (response) to noise (deviation) ratio and for this model, it was found to be 31.546 which indicates an adequate signal. This model can be used to navigate the design space for the response Y. The "Pred R-Squared" of 0.9476 was in reasonable agreement with the "Adj R-Squared" of 0.9847. From Figure 14, it is evident that the actual response was in good agreement with the predicted values.
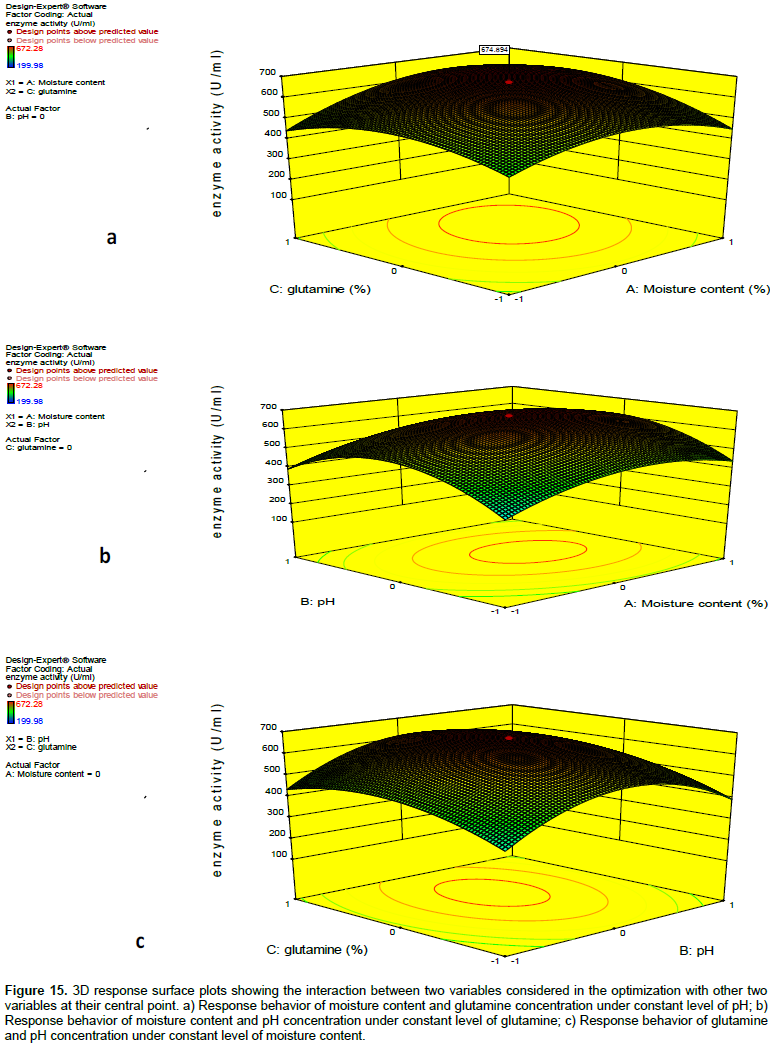
The relationship between the independent variables was assessed by examining the response surfaces. Three dimensional response surfaces were generated by holding one factor constant at a time and plotting the response obtained for varying levels of the other two factors. Figure 15a to c shows the response surface and contour plots generated for the variation in the yields of glutaminase as a function of concentrations of two variables with the other one variable at their central value. From the Figure 15a, it was evident that when the concentration of pH was held at their middle values, the moisture content showed a parabolic response at the different concentrations of glutamine with the highest yield of L-glutaminase obtained in the range of 60 to 65%. Very low and high moisture content was not favourable for L-glutaminase production. Variations in glutamine concentration have also followed a parabolic curve and optimum yield was obtained in the range of 2.0 to 2.25%. In Figure 15b, the response of moisture content was parabolic at different levels of pH and the highest yield of L-glutaminase was obtained in the range of 60 to 65% and similarly, the response of pH was parabolic at different levels of moisture with the highest yield obtained at pH 7. In Figure 15c, the response of pH and glutamine was also parabolic at different levels of glutamine and pH, respectively. The highest yield of L-glutaminase was obtained in the range of 2 to 2.25% at pH 7.
Response optimization
The point prediction tool of design of expert software was used to predict the optimum values of the independent variables. Maximum L-glutaminase production of 672.28 U/ml under flask condition was obtained at the predicted optimal values of moisture content- 62.5% (w/w), pH 7.1 and glutamine 2.44% (w/v). The maximum experimental L-glutaminase production was 680.8 U/ml whereas the predicted value for L-glutaminase production was 672.28 U/ml, indicating a strong agreement between them.
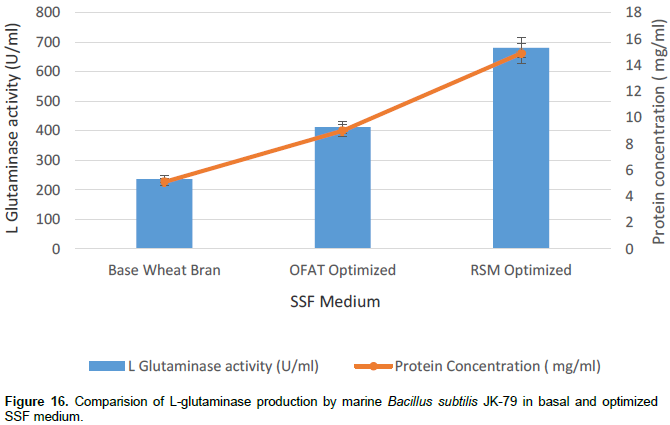
Comparison of L-glutaminase production in basal and optimized medium
The L-glutaminase production under submerged fermentation by marine B. subtilis JK-79 was determined in the unoptimized base medium, that is, wheat bran medium and the optimized OFAT and RSM medium. This was carried out to understand the fold increase in L-glutaminase production in the optimized medium as compared to the unoptimized medium. From the Figure 16, it is evident that about 2.88 fold increase in glutaminase production was obtained by sequentially optimizing the various components of the medium. Several authors have reported an increased fold of L-glutaminase production under SSF by the application of RSM. Sathish and Prakasham (2010) found that a mixed substrate (66:34) of bengalgram husk and wheat bran optimized by simplex centroid design resulted in a significant improvement in the L-glutaminase yield by B. subtilis RSP-GLU. L-glutaminase production by Zygosaccharomyces rouxii under SSF was studied by Iyer and Singhal (2010a, b). The authors employed a CCD to investigate the effect of variables such as moisture content, glucose, corn steep liquor and glutamine on glutaminase production and found a fourfold increase in L-glutaminase production. Nathiya et al. (2011) adopted CCD to obtain the best possible combinations for enhanced production of L-glutaminase by Pencillium brevicompactum. Under the optimal conditions, the experimental yield of L-glutaminase was 136.33 U/mg which is in close agreement with the value predicted by the model. However, Sayed (2009) and Sameera and Raju (2015) employed OFAT alone to attain a 2.2 and 4 fold increase in L-glutaminase production under SSF by T. koningii and Aspergillus wentii MTCC 1901, respectively.
The authors have not declared any conflict of interests.
REFERENCES
|
Ashraf El-sayed SA (2009). L-glutaminase production by Trichoderma koningii under solid state fermentation. Indian J. Microbiol. 49:243-250.
Crossref
|
|
|
|
Athira RN, Elizebeth T, Narendra Sheik Tanweer Ahmed T, Shankar Kumar Gupta, Manoj Chaudary, Siddalingeshwara KG, Pramod T (2014). Investigation on the production of L-glutaminase from Pseudomonas stutzeri strain PIMS6 under solid state fermentation using various agro residues, J. Drug Deliv. Ther. 4:81-85.
|
|
|
|
|
Baysal Z, Uyar F, Autekin C (2003). Production of α-amylase by thermotolerant Baciilus subtilis in the presence of carbon, nitrogen containing compounds and surfactants Ann. Microbiol. 53:323-328.
|
|
|
|
|
Castro de SJR, Sato HH (2015). Enzyme production by solid state fermentation: general aspects and an analysis of the Physico chemical characteristics of substrates for agro-industrial wastes valorization. Waste and Biomass Valorization 6(6):1085-1093.
Crossref
|
|
|
|
|
Chandrasekaran M (1996). Harnessing of marine microorganisms through soild state fermentation', J. Sci. Ind. Res. 55:468-471.
|
|
|
|
|
Dina El-Ghonemy H (2013). Microbial amidases and their industrial applications: A review' J. Med. Microbiol. Diagn. 4:1-6.
|
|
|
|
|
George Box, Donald Behnken (1960). Some new three level designs for the study of quantitative variables. Technometrics. 2:455-475.
Crossref
|
|
|
|
|
Holker V, Lenz J (2005). Solid-state fermentation are there any biotechnological advantage?, Current. Opinin. Microbiol. 8:201-306.
Crossref
|
|
|
|
|
Iyer P, Singhal RS (2010b). Isolation, screening, and selection of an L-glutaminase producer from soil and media optimization using a statistical approach. Biotechnol. Bioproc. Eng. 15:975-983.
Crossref
|
|
|
|
|
Iyer PV, Singhal RS (2010a). Glutaminase production using Zygosaccharomyces rouxii NRRLâ€Y 2547: Effect of aeration, agitation regimes and feeding strategies. Chem. Eng. Technol. 33(1):52-62.
Crossref
|
|
|
|
|
Kashyap DR, Soni SK, Tewani R (2003). Enhanced production of pectinase by Bacillus sp. using solid state fermentation. Bioresour. Technol. 88:251-254.
Crossref
|
|
|
|
|
Kashyap P, Sabu A, Pandey A, Szakacs G, Soccol CR (2002). Extracellular L-glutaminase production by Zygosaccharomyces rouxii under solid-state fermentation. Proc. Biochem. 38:1431-1436.
Crossref
|
|
|
|
|
Kaur Sandeep, Vohra RM, Kappor M, Beg Khalil Q, Hoondal GS (2001). Enhanced production and characterisation of a highly thermostable alkaline protease from Bacillus sp. P-2. World J. Microbiol. Biotechnol. 17:125-129.
Crossref
|
|
|
|
|
Keerthi TR, Suresh PV, Sabu A, Kumar SR, Chandrasekaran M (1999). Extracellular production of L-glutaminase by alkalophilic Beauveria bassiana-BTMF S10 isolated from marine sediment, World. J. Microbiol. Biotechnol. 15:751-752.
Crossref
|
|
|
|
|
Kumar SR, Chandrasekaran M (2003). Continuous production of L-glutaminase by an immobilized marine Pseudomonas sp. BTMS-51 in a packed bed reactor. Process Biochem. 38:1431-1436.
Crossref
|
|
|
|
|
Kumar SR, Sabu A, Keerthi TR, Chandrasekaran M (2001). Production of extracellular L-glutaminase by immobilized marine Pseudomonas sp. BTMS-51. Paper presented at the International Conference on `New Horizons' in Biotechnology, April 18-21, Trivandrum, India, IB-17.
|
|
|
|
|
Lowry OH, Rosebrough NN, Farr AL, Randall RY (1951). Protein measurement with the Folin Phenol reagent, J. Biol. Chem. 193:265-275.
|
|
|
|
|
Nathiya K, Sooraj Nath S, Angayarkanni J, Palaniswamy (2011). Optimised production of L-glutaminase: A tumour inhibitor from Aspergillus flavus cultured on agroindustrial residues, Afr. J. Biotechnol. 10(63):13887-13894.
Crossref
|
|
|
|
|
Noura El-Ahmady El-Naggar, Sara El-Ewasy M, Nancy El-Shweihy M (2014). Microbial L-asparginase as a potential therapeutic agent for the treatment of Acute Lymphoblastic Leukemia: The Pros and Cons. Int. J. Pharm. 1-18.Pandey A (1992). Recent developments in solid state fermentation. Process Biochem. 27:109-117.
|
|
|
|
|
Pandey A (1994). Solid state fermentation: An overview, In solid state fermentation, Wiley Eastern, New Delhi, 3-10.
|
|
|
|
|
Pandey A (2003). Solid-state fermentation. Biochem. Eng. J. 13(2-3):81-84.
Crossref
|
|
|
|
|
Pandey A, Selvakumar P, Soccol CR, Nigam P (1999). Solid state fermentation for the production of industrial enzymes. Curr. Sci. 77(1):149-162.
|
|
|
|
|
Pandey A, Soccol CR, Mitchel D (2000). New developmenta in solid state fermentation: I-bioprocesses and products. Process Biochem. 35(10):1153-1169.
Crossref
|
|
|
|
|
Plackett RL, Burman JP (1946). The design of multifactorial experiments, Biometrika. 33: 305-325.
Crossref
|
|
|
|
|
Prabhu GN, Chandrasekaran M (1995). Polystyrene – an inert carrier for glutaminase production by marine Vibrio costicola under solid state fermentation, World J. Microbiol. Biotechnol. 11: 683-684.
Crossref
|
|
|
|
|
Prabhu GN, Chandrasekaran M (1996). L-Glutaminase production by marine Vibrio costicola under solid state fermentation using different substrates, J. Mar. Biotech. 4:176-179.
|
|
|
|
|
Prabhu GN, Chandrasekaran M (1997). Purification and characterization of an anti-cancer enzyme produced by marine Vibrio Costicola under a novel solid state fermentation process, Process Biochem. 32:285-289.
Crossref
|
|
|
|
|
Prabhu GN, Chandrasekaran M (1999). Impact of process parameters on L-glutaminase production by marine Vibrio costicola in solid state fermentation using polystyrene as an inert support, Braz. Arch. Biol. Technol. 42(3):1-5.
|
|
|
|
|
Prakasham RS, Subba Rao CH, Sarma PN (2006). Green gram husk- an inexpensive substrate for alkaline protease production by Bacillus sp. in solid state fermentation, Bioresource Technology. 97: 1449-1454.
Crossref
|
|
|
|
|
Renu S (1991). L-glutaminase production by marine Bacteria, Ph.D thesis, Cochin University of Science and Technology, Cochin, India.
|
|
|
|
|
Renu S, Chandrasekaran M (1992), Extracellular L-glutaminase production by marine bacteria, Biotechnology Letters. 14:471-474.
Crossref
|
|
|
|
|
Sabu A, Keerthi TA, Kumar SR, Chandrasekaran M (2000), L-glutaminase production by marine Beauveria sp. under solid state fermentation, Process Biochem. 35:705-710.
Crossref
|
|
|
|
|
Sabu A, Keerthi TA, Kumar SR, Chandrasekaran M (2001). Continuous production of extracellular L-glutaminase by Ca-alginate immobilized marine Beauveria bassiana-BTMFS10 in a packed bed reactor, Paper presented at the international conference on 'New Horizons' in Biotechnology, April 18-21, Trivandrum, India, IB-02.
|
|
|
|
|
Sameera V, Jaya RK (2015). Optimization of process parameters for the production of L-glutaminae with mixed substrate solid state fermentation using Aspergillus wentii MTCC 1901. Int. J. Res. Eng. Technol. 4(5):328-333.
Crossref
|
|
|
|
|
Sathish T, Lakshmi GS, Rao Ch.S, Brahmaiah P, Prakasham RS (2008). Mixture design as first step for improved glutaminase production in solid-state fermentation by isolated Bacillus sp. RSP-GLU. Lett. Appl. Microbiol. 47:256-262.
Crossref
|
|
|
|
|
Sathish T, Prakasham RS (2010). Enrichment of glutaminase production by Bacillus subtilis RSPâ€GLU in submerged cultivation based on neural network-genetic algorithm approach. J. Chem. Technol. Biotechnol. 85(1):50-58.
Crossref
|
|
|
|
|
Schmidt FR (2005). Optimization and scale up of industrial fermentation processes, Appl. Microbiol. Biotechnol. 68:425-435.
Crossref
|
|
|
|
|
Singh Yogendra, Srivastava SK (2013). Statistical and evoluntionary optimization for enhanced production of an anti-leukemic enzyme, L-asparginase, in a protease-deficient Bacillus aryabhattai ITBHU02 isolated from the soil contaminated with hospital waste. Ind. J. Exp. Biol. 51:322-335.
|
|
|
|
|
Sodhi KH, Sharma K, Gupta JK, Soni KS (2005). Production of thermostable α-amylase from Bacillus sp. PS-7 by solid state fermentation and its synergistics use in the hydrolysis of malt starch for alcohol production. Process Biochem. 40(2):525-534.
Crossref
|
|
|
|
|
Soni SK, Sodhi HK, Sharma K, Gupta JK (2003). A solid state fermentation based bacterial α-amylase and fungal glucoamyase system and its suitability for the hydrolysis of wheat starch. Process Biochem. 39:185-192.
Crossref
|
|
|
|
|
Subba Rao CH, Sathish T, Brahamaiah P, Kumar TP, Prakasham RS (2008). Development of a mathematical model for Bacillus circulans growth and alkaline protease production kinetics. J. Chem. Techmol. Biotechnol. 84:302-307.
Crossref
|
|
|
|
|
Subba Rao CH, Sathish T, Ravichandra P, Kumar TP, Prakasham RS (2009). Characterization of thermo and detergent stable serine protease from isolated Bacillus circulans and elevation of eco-friendly applications. Process Biochem. 44:262-268.
Crossref
|
|
|
|
|
Unissa R, Sudhakar M, Sunil Kumar Reddy A, Naga Sravanthi K (2014). A review on biochemical and therapeutic aspects of glutaminase. Int. J. Pharm. Sci. Res. 5(11): 4617-34.
|
|