Full Length Research Paper
ABSTRACT
Chlorophyta hydrodictyon africanum was immobilized on a silica gel matrix to improve its mechanical properties. The algae-silica gel adsorbent was used for batch sorption studies of a cationic dye, methylene blue (MB). Optimum adsorption was obtained with a dosage of 0.8 g bio sorbent. Results from sorption studies show that 124.11 mg·g-1 of MB could be adsorbed at an optimum pH of 8 and immobilization of 300 mg per gram silica. Maximum immobilization was 400 mg biomass per gram silica. Sorption capacity increased with an increase in initial dye concentration and reached equilibrium within 30 min. Three models were used to simulate kinetic data and the pseudo–second order model gave a better fit with R2 greater than 0.98 in all cases. Equilibrium studies revealed that the adsorption of MB followed Freundlich isotherm (R2=1.00).
Key words: Adsorbent, algae, Langmuir model, Freundlich isotherm.
INTRODUCTION
MATERIALS AND METHODS
RESULTS AND DISCUSSION
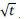 A plot of intra-pa
A plot of intra-pa
CONCLUSION
CONFLICT OF INTERESTS
The authors did not declare any conflict of interest.
REFERENCES
|
Ahmaruzzaman M (2009). Role of fly ash in the removal of organic pollutants from wastewater. Energy Fuels 23:1494-1511. |
|
|
Akar ST, Gorgulu A, Kaynak Z, Anilan B, Akar T (2009). Biosorption of Reactive Blue 49 dye under batch and continuous mode using a mixed biosorbent of macro-fungus Agaricus bisporus and Thuja orientalis cones. Chem. Eng. J. 148:26-34. |
|
|
Barka N, Abdennouri M, El Makhfouk M (2011). Removal of methylene blue and Eriochrome black T from aqueous solutions by biosorption on scolymus Hispanics L.: Kinetics, equilibrium and thermodynamics. J. Taiwan Inst. Chem. Eng. 42:320-326. |
|
|
Caparkaya D, Cavas L (2008). Biosorption of methylene blue by brown alga cystoseira barbatula Kützing. Acta Chim. Slov. 55: 547-553. |
|
|
Daneshvar E, Kousha M, Sohrabi MS, Khataee CA (2012). Biosorption of three acid dyes by the brown macroalga Stoechospermum marginatum: Isotherm, Kinetic and Thermodynamic Studies. Chem. Eng. J. 195-196: 297-306 |
|
|
de-Bashan LE, Bashan Y (2010). Immobilized microalgae for removing pollutants: a review of practical aspects. Bioresour. Technol. 101:1611-1627. |
|
|
DoÄŸar C, Gürses A, Acikyildiz M, Ó¦zkan E (2010). Thermodynamics and kinetic studies of a basic dye from aqueous solution using green algae Ulotrix sp. Colloids Surf. B: 76:279-285. |
|
|
El Jamal MM, Ncibi MC (2012). Biosorption of methylene blue by chaetophora elegans algae: Kinetics, equilibrium and thermodynamic studies. Acta Chim. Slov. 59: 24-31 |
|
|
El Sikaily A, Khaled A, El Nemr A, Abdelwahab O (2006). Removal of methylene blue from aqueous solutions by green alga Ulva lactuca. Chem. Ecol. 22(2):149-157. |
|
|
El-Batal AI, Hashem AM, Hassan MS, Helal AH (2012). Removal of dyes from textile wastewater using treated Aspergillus tamari biomass in batch and column reactor. World Appl. Sci. J. 19(9):1305-1310. |
|
|
Fernandes ME, Nunell GV, Bonelli PR, Cukierman AI (2012). Batch and dynamic biosorption of basic dyes from binary solutions by alkaline-treated. Bioresour. Technol. 106: 55-62. |
|
|
Gong R, Jin Y, Chen J, Hu Y, Sun J (2007). Removal of basic dyes from aqueous solution by sorption on phosphoric acid modified rice straw. Dyes Pigments 73: 332-337. |
|
|
Gupta VK, Suchas (2009). Application of low-cost adsorbents for dye removal – A review. J. Environ. Manage. 90: 2313-2342. |
|
|
Hammud HH, Fayouni L, Holail H, Mostafa ESME (2011). Biosorption studies of methylene blue by Mediterranean alga Carolina and its chemically modified forms. Linear and non-linear models' prediction based on statistical error calculation. Int. J. Chem. 4: 146-163. |
|
|
Iqbal M, Saeed A (2007). Biosorption of reactive dye by loofa sponge-immobilized fungal biomass of Phanerochaete chrysosporium. Process Biochem. 42:1160-1164. |
|
|
Kanchana S, Jeyanthi J, Kathiravan R, Suganya K (2014). Biosorption of heavy metals using algae: A Review. Int. J. Pharm. Med. Biol. Sci. 3(2):1-9. |
|
|
Khataee AR, Vafaei F, Jannatkhah M (2013). Biosorption of three textile dyes from contaminated water by filamentous green algae spirogyra Sp.: Kinetic, Isotherm and thermodynamic Studies. Int. Biodeter. Biodegr. 83: 33-40. |
|
|
Kyzas GZ, Fu J, Matis KA (2013). The change from past to future in treatment of dyeing wastewaters, Materials 6: 5131-5158. |
|
|
Mane VS, Mall ID, Srivastava VC (2007). Kinetic and equilibrium studies for the adsorptive removal of brilliant green dye from aqueous solution by rice husk ash. J. Environ. Manage. 84:390-400. |
|
|
Moreno-Garrido I (2008). Microalgae immobilization: Current techniques and uses. Bioresour. Technol. 99: 3949-3964. |
|
|
Namasivayam C, Dinesh KM, Selvi K, Ashraffanissa BR, Vanathi T, Yanuna RT (2001). Waste coir pitch – a potential biomass for the treatment of dye wastewaters. Biomass Bioenergy 21(6):477-483. |
|
|
Padmesh TVN, Vijayaraghavan K, Sekaran G, Velan M (2005). Batch and column studies on biosorption of acid dyes on freshwater macroalga Azolla filiculoides. J. Hazard. Mater. B 125:121-125. |
|
|
Qui H, Lv L, Pan B, Zhang Q, Zhang W, Zhang Q (2009). Critical review in adsorption kinetic models. J. Zhejiang Univ. Sci. A 10(5):716-724. |
|
|
Rubin E, Rodriguez P, Herrero R, Cremades J (2005). Removal of methylene blue from aqueous solutions using as biosorbent Sargassum muticum: an invasive macroalga in Europe. J. Chem. Technol. Biotechnol. 80:291-298. |
|
|
Srinivasan A, Viraraghavan T (2010). Decoloration of dye wastewater by biosorbents: a Review. J. Environ. Manage. 91: 1915-1929. |
|
|
Wang BE, Hu YY, Xie L., Peng K (2008). Biosorption behavior of azo dyes by inactive CMC immobilized Aspergilus fumigatus bead. Bioresour. Technol. 99: 794-800. |
|
|
Waranusantigul P., Potethitiyook P, Karautrachue M., Upatham E S (2003). Kinetics of basic dye (methylene blue) biosorption by giant duckweed (Spirodela polyrrhiza). Environ. Pollut. 125: 385-392. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0