ABSTRACT
The performance of a hybrid of Saccharomyces cerevisiae and Saccharomyces paradoxus (Yeast B) compared with an industrial strain of S. cerevisiae (Yeast A) was studied. The two strains of yeasts: Yeast A obtained from the Bioresources Development Centre, National Biotechnology Development Agency Ogbomosho and Yeast B obtained from Scotch Whisky Research Laboratory in Edinburgh, Scotland (Strain 63M) were studied using the SLSF method for bioethanol production at various initial starch concentrations (20, 30 and 50%). The cassava starch used was extracted from Tropical Manihot esculenta (TME 419) cassava strain while the SLSF method for bioethanol production was initiated by adding granular starch hydrolysing enzyme with the yeast strains into the starch solutions at room temperature. The results obtained showed that Yeast B has higher bioethanol yield and tolerance generating 70.34, 87.34 and 120.53 ml/L compared to the Yeast A which produced 63.4, 72.73 and 112.6 ml/L at 20, 30 and 50% starch concentrations respectively. The study suggests that the hybrid strain of S. cerevisiae and S. paradoxus out performs the industrial strain and can favorably substitute or displace the industrial strains for bioethanol production.
Key words: Bioethanol, cassava, fermentation, hybrid, simultaneous liquefaction saccharification and fermentation (SLSF), yeast, Saccharomyces cerevisiae, Saccharomyces paradoxus.
Biofuel has been known to be an excellent replacement for fossil fuels. This is because the latter are by nature non-renewable and have numerous environmental hazards associated with its extraction and utilization (Solomon et al., 2018). Nigeria with a population approximated to be 200 million (National Bureau of Statistics, 2018) has been faced with challenges of sufficiently meeting its ever-increasing energy demands.
With an average daily fuel consumption of about 51 million liters in 2016 to about 54 million litres in the first quarter of 2018 (Petroleum Products Consumption Statistics, National Bureau of Statistics, Qtr 2, 2018), there is absolute need for an alternative fuel to supplement and curtail our over reliance on fossil based energy sources as a Nation. Bioethanol is the most popular alternative fuel that has found applications both as transportation fuel and in industries.
Bioethanol can be produced from various biomass feedstocks such as sugarcane, corn, cassava, etc. Among the feedstock, cassava gives the best starch and has the highest volume per unit of raw materials (Sarocha et al., 2018). The production of bioethanol from cassava has a very high potential in Nigeria because of its ability to give moderate yields even in poor soils. Nigeria has been producing the largest quantities of cassava in the world for the past decades, amounting to about 54 million tons annually (FAO, 2012). The Nigerian Biofuels Policy and Incentives was drafted in 2007 by NNPC with the view of integrating agricultural activities with oil and gas exploration and production (Ben-Iwo et al., 2016).
The conventional method of bioethanol production involves two steps: the hydrolysis of starch which is the conversion of starch to sugar by cooking the starch granules (gelatinization), liquefaction via alpha amylase enzyme and saccharification using amyloglucosidase enzyme. The second step is the fermentation of the sugar produced from hydrolysis using yeast with all processes taking place at known temperatures, pH, concentrations and time. These processes take more time, energy, equipment and labour. In recent times however, the Simultaneous Liquefaction Saccharification and Fermentation (SLSF) process has been employed to produce bioethanol with significant reduction in processing time and energy consumption which have hitherto been major constraints to investors (Chu-Ky et al., 2016) in the ethanol industry. Here, both the starch hydrolysis and fermentation are carried out in one step at room temperature using granular starch hydrolyzing enzymes (Sriroth et al., 2012). This process has been reported to increase the hydrolysis rate and consequently decrease product inhibition in addition to reduction in the number of processing units.
Fermentation process is carried out by yeasts, which are majorly known to rapidly and proficiently convert sugars into ethanol and carbon dioxide (Hossain et al., 2017). Saccharomyces cerevisiae was the first microorganism known to possess the ability to ferment sugars for the production of ethanol and carbon dioxide both aerobically and anaerobically (De Haas and Kreuger, 2010). Currently, most ethanol production systems use strains of S. cerevisiae that are highly adapted to industrial process of converting feedstock to ethanol. These yeast strains combine to efficiently convert sugars into ethanol, and exhibit important industrial characteristics that influence productivity such as low nutrient requirement, ethanol resistance, tolerance to pH, and general robustness (Bharti and Madhulika, 2016). In order to increase the genetic diversities and enhance yeast performance, hybridization mechanisms, which may involve dissimilar strains of same species, several species of same genus or strains of different genera, is often used. The overall advantage is that the new species exhibit better performance than its precursor strains (Steensels et al., 2014).
This work therefore, compares the performances of a hybrid yeast strain of S. cerevisiae and S. paradoxus with that of an industrial yeast strain of. cerevisiae, in terms of bioethanol production and alcohol tolerance. This is with a view to lowering the cost and time of bioethanol production from cassava starch, using SLSF process.
Fresh TME 419 cassava roots and granular starch hydrolysing enzyme (STARGEN 002TM) procured from GENECOR International, Sweden were obtained from the Bioresources Development Centre (BIODEC), National Biotechnology Development Agency (NABDA) in Ogbomoso, Oyo State.
The growth media used for the organisms composed of soluble glucose, ammonium sulphate NH4(SO4)2, Yeast Extract, potassium di-hydrogen phosphate (KH2PO4), magnesium sulphate MgSO4.7H2O. Compositions of the fermentation media used for the bioethanol production were soluble glucose, yeast extract, ammonium sulphate (NH4(SO4)2), potassium di-hydrogen phosphate (KH2PO4), magnesium sulphate (MgSO4.7H2O), copper sulphate (CuSO4. 5H2O), zinc sulphate (ZnSO4), iron sulphate FeSO4, manganese sulphate (MnSO4), with starch concentrations ranging from 1 - 10% used when testing for the effect of different substrate concentrations. Sodium acetate buffer was used to adjust the pH of the fermentation media to 4.5. All chemicals and reagents used in this study were of analytical grade.
The yeast strains used for fermentation were a new hybrid strain of S. cerevisae and S. paradoxus (Strain 63M) collected from Scotch Whisky Research Laboratory at Edinburgh, Scotland (labeled as Yeast B) and an industrial strain of S. cerevisae collected from the NABDA’s BIODEC at Ogbomoso, Oyo State which is being used at the bioethanol plant at the Centre (labeled as Yeast A).
Cassava starch extraction
The method of starch extraction as described by Oyewole and Obieze (1995) was used to extract starch from fresh cassava roots, in this study. Fifty-eight kilograms of fresh TME 419 cassava roots were peeled, washed in water, shredded, sieved and the resultant pulp allowed to sediment for 12 h. The resultant starch was then sun-dried to constant weight.
Yeast activation and sub-culturing
Five millilitre of malt extract broth was dispensed in two McCartney bottles and placed in an autoclave for sterilization at 15 psi, 121°C for 15 min after which the broth was allowed to cool. Thereafter, 0.1 g each of an industrial strain of S. cerevisiae (Yeast A) and the hybrid of S. cerevisiae and S. paradoxus (Yeast B) were inoculated into each of the 5 ml broth that were labeled accordingly and incubated at room temperature (30°C) for 48 h. Each isolate was then sub-cultured into sterile malt extract agar medium and incubated at 30°C for 24 h accordingly to confirm viability. Subsequently, each organism was sub-cultured in agar slant (Malt Extract Agar), appropriately labeled and incubated accordingly. The slant cultures were preserved at 4°C.
Fermentation (SLSF)
Starch concentrations of 20, 30 and 50% were prepared by weighing 20, 30 and 50 g in 100 ml of distilled water inside 250-ml conical flasks. The pH of the starch solution was adjusted to 4.5 using sodium acetate buffer solution. The mixture was stirred thoroughly to ensure that all the dust particles were dissolved. Granular Starch Hydrolyzing Enzyme concentration of 2% w/v and 10 ml of yeast inoculum was then added simultaneously into the prepared starch solution for fermentation reaction to commence. At regular intervals, samples were taken in order to monitor the reaction progress.
Ethanol determination
In analyzing the ethanol produced, 10 ml of the fermenting broth was taken and centrifuged to separate the yeast as sediment; the supernatant was then decanted and the biomass kept for further analyses. Acid dichromate method was used to analyze for the ethanol content of the supernatant. The solution was gently shaken for 1 min and allowed to stand for 2 h at room temperature (Caputie et al., 1986). The formation of a light green coloured reaction product was observed. The absorbance of the solution was read at a wavelength of 576 nm on the Libra S21 UV-visible spectrophotometer (Caputie et al., 1986). The value of absorbance obtained was read off a standard curve prepared with known ethanol concentrations, to obtain the corresponding values of ethanol concentration in the sample.
Glucose determination
Glucose concentration in the supernatant was determined using the DNS method according to Miller (1959). One mililiter of supernatant was mixed with 3 ml of DNS solution in a test tube, the mixture was then placed in boiling water for 15 min after which, the test tube was removed and allowed to cool before measuring the optical density using the Libra S21 Ultra Violet visible spectrophotometer at 540 nm wavelength and the value read off a standard curve previously prepared with known glucose concentrations, to obtain the corresponding value of glucose concentration in the sample.
Ethanol tolerance determination by biomass concentration
Each of the yeasts (the industrial yeast, Yeast A and the hybrid yeast, Yeast B) used in this work was grown in sterile nutrient broth base media with 50 g/l glucose and varying quantities of absolute ethanol added aseptically at concentrations of 5, 10, 15, 20 and 25% in 250-ml conical flasks respectively. Ten ml samples of the nutrient broth from each flask were taken every 4 h from the various flasks. These samples were centrifuged, supernatant decanted and the biomass sediments oven dried to constant weight so as to monitor biomass concentrations. The dry weight was indicative of the growth of yeasts at the various ethanol concentrations.
The performance of the hybrid yeast of S. cerevisiae and S. paradoxus (Yeast B) was studied in the production of bioethanol from cassava starch slurry using SLSF method and compared with that of an industrial yeast strain (Yeast A) which was used as a control strain. From Figures 1 and 2, it can be observed that there was a steady increase in bioethanol concentration as fermentation time increased from 24 to 96 h and as the initial starch concentrations increased from 20 to 50% during the anaerobic fermentation by both yeasts. The results in these Figures 1 and 2 show that after 96 h, the corresponding maximum bioethanol production (in ml per litre starch slurry) at 20, 30 and 50% initial starch concentrations for Yeasts A and B were 73.25, 84.37, 112.61 and 87.81, 96.08 and 120.53 v/v basis respectively, demonstrating the superior ability of Yeast B over Yeast A.
Figure 3 shows the percentage increase in bioethanol concentration after 96 h for both yeasts and it can be observed that bioethanol concentration increased by 56.9 at 20% initial starch concentration, 80.7 at 30% initial starch concentration and 44.7 at 50% initial starch concentration respectively for Yeast A while for Yeast B, ethanol concentration increased by 44.65, 91.86 and 163.64% correspondingly at 20, 30 and 50% initial starch concentrations. The data obtained show that highest rate for bioethanol conversion was attained using 30% starch concentration for Yeast A while only Yeast B performed well at 20% initial starch concentration.
Figures 4 to 6 illustrate the performance of both yeasts at various starch concentrations. It can be observed that at 20% initial starch concentration, Yeast A and the Yeast B had maximum bioethanol concentrations of 73.25 ml/L, increasing from 46.7 and 87.81 ml/L, increasing from 33.31, respectively after 96 h. At 30% initial starch concentration, the performance of the industrial Yeast A and the hybrid Yeast B were compared every 24 h for 96 h. It was observed that hybrid Yeast and the Industrial Yeast attained maximum bioethanol concentrations of 84.37 and 96.08 ml/L respectively. Within the period of fermentation, the concentration increased from 46.7 to 84.37 ml/L for Yeast A while for Yeast B, the bioethanol concentration generated was higher, increasing steadily from 50.08 to 96.08 ml/L during the same period. Using 50% initial starch concentration, a similar comparison of the two yeasts demonstrates that the industrial Yeast A and the hybrid Yeast B attained maximum bioethanol concentrations of 112.61 ml/L increasing from 77.85 and 120.53 ml/L increasing from 83.32 respectively after 96 h.
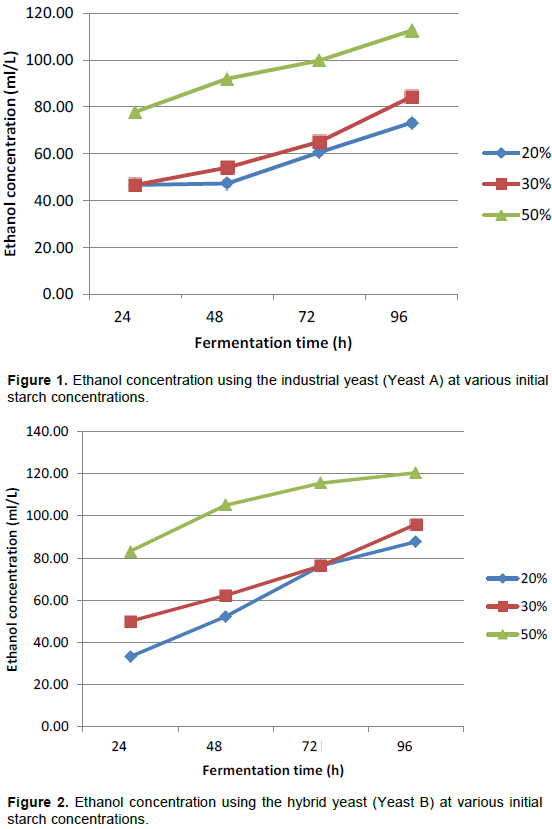
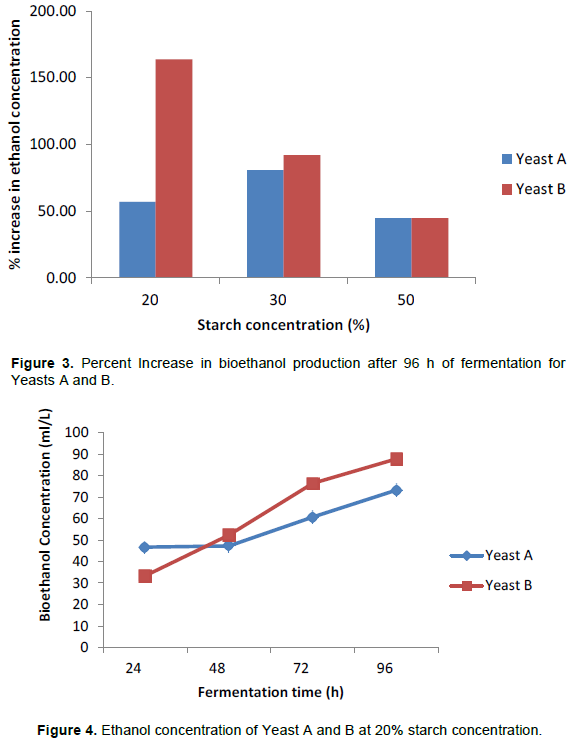
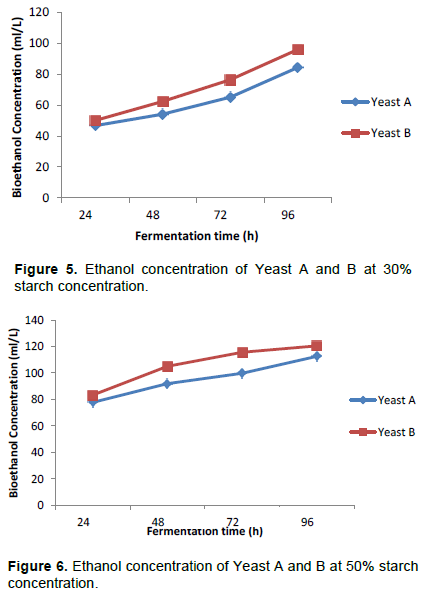
Figure 7 summarizes the comparative performance of the studied yeast strains in the conversion of cassava starch slurry into bioethanol, based on the volume of ethanol produced per liter of starch slurry. In all cases of starch concentrations, it can be observed that the hybrid Yeast Strain B outperformed the industrial Yeast Strain A. For starch concentrations of 20, 30 and 50%, ethanol yield were 73.25, 84.37 and 112.61 ml/L for yeast A as compared with 87.81, 96.08 and 120.53 ml/L for Yeast B. Maximum difference in ethanol yield occurred in the 20% concentration starch fermentation medium.
As illustrated in Figures 8 and 9, glucose concentrations were also observed to decrease for the various starch concentrations as the bioethanol concentration rises and fermentation time increases from 24 to 96 h. This trend was observed for both yeasts, indicating that they both metabolize glucose. Hybrid Yeast Strain B consumed more glucose than the industrial Yeast Strain A respectively at 20 and 30% starch concentration but the reverse was the case for 50% starch concentration where the latter consumed 3.7 moles of glucose as opposed to the 1.85 moles consumed by the hybrid yeast. Even at this, Figure 10 which compares the performance of both yeast strains based on ethanol yield show that the hybrid Yeast Strain B produced 138.06 ml of ethanol per mole glucose consumed as opposed to 117.01 for the Yeast Strain A and 65.06 as opposed to 30.45 ml/mole respectively for 20 and 50% starch concentrations.
Although, for 30% starch concentration, Yeast Strain B consumed 0.7 mole more glucose than Yeast Strain A and produce 112.4 moles less ethanol, its best performance was observed to be at the 50% starch concentration medium where it consumed half glucose consumption of Yeast Strain A and produced more than 200% of its ethanol per mole glucose consumed.
The results obtained from the measurement of biomass concentrations for the determination of ethanol tolerance of both yeasts generally show that biomass concentration of Yeast A and B increased with inoculation time but decreased as ethanol concentration in the inoculum media increased, as described in Figures 11 and 12. Figure 13 compared the performance of both yeasts. For the Yeast Strain A, peak biomass concentration was attained after 16, 12 and 8 h of inoculation respectively in the growth medium that contained 5, 10 and 15, 20, and 25% ethanol concentration while the biomass concentration of Yeast Strain B peaked after 24 h in 5, 10 and 25% ethanol concentration and 16 h in the 20% ethanol concentration. In all cases, Yeast B had higher biomass concentration than Yeast A after 24 h of inoculation, producing four, seven and two-fold more biomass respectively in the 15, 20 and 25% ethanol concentration, than the latter.
For cassava starch concentrations in the range 10 to 100% w/v, Phakping et al. (2014) reported on the effects of uncooked slurry on bioethanol yield using SLSF method. It was reported that higher concentration of cassava starch slurry led to an increase in ethanol concentration as obtained in this study (Figures 1 to 3). From the results displayed in Figure 3, it can be deduced that both yeasts performed optimally at 30% starch concentration giving 80.67 and 91.86% increase in bioethanol production after 96 h. At 50% starch concentration, both yeasts have similar percent increase in ethanol production of around 44.65% even though, Yeast B had higher substrate utilization and produced more bioethanol within the same time frame. Previous reports have been made with regards to very high gravity (VHG) fermentation with substrate concentration of > 30%. Zang et al. (2010) reported higher fermentation efficiency with ethanol concentration of around 18% v/v but also encountered problems of highly viscous starch substrate during fermentation which include handling difficulties, poor solid-liquid separation, incomplete conversion of starch into fermentable sugars, leading to a drastic drop in the efficiency of fermentation (Puligudla et al., 2011). As a result, Sriroth et al. (2010) suggested that the combination of SLSF with VHG (SLSF-VHG) is more efficient and reduces energy consumption by 18.5%. Glucose concentration was observed to decrease as fermentation time increases. This is as a result of increase in the rate of conversion of sugars formed to ethanol, which is higher with the use of the hybrid Yeast B. Biomass concentration also increases rapidly utilizing the nutrients in the fermentation media including the glucose formed for its cell growth (Ajibola et al., 2012).
The hybrid of S. cerevisiae and S. paradoxus performed better than the industrial yeast as higher bioethanol concentration was obtained. The ethanol tolerance of the hybrid yeasts, as indicated by biomass concentration, was higher for Yeast B than that of the industrial yeast A (Figures 11 to 13). Yeast growth is acknowledged to be highly inhibited by ethanol even at relatively low concentrations as it interferes with cell division, decreases cell volume and specific growth rate. At high ethanol concentrations, cell vitality reduces while cell death increases (Siti et al., 2017). Although, fermentation in this study was carried out at room temperature made possible by the use of the granular starch hydrolyzing enzyme in the SLSF process, the hybrid Yeast B performed better than the commonly used industrial Yeast Strain A, producing seven-fold more biomass than Yeast A even at 20% ethanol concentration. Previous work has also suggested that S. paradoxus exhibits its highest growth rate at 37°C or higher and that S. cerevisiae may exhibit its highest growth rate at lower temperatures. Thus, a hybrid of S. paradoxus and S. cerevisiae will likely result into a hybrid strain with a high temperature and ethanol tolerance (Hanyao et al., 2010).
The study revealed that the hybrid strain of S. cerevisiae and S. paradoxus performed better in terms of bioethanol production and ethanol tolerance than the industrial strain of S. cerevisiae. Furthermore, the performance of this hybrid strain should be exploited further in the bioethanol production via the SLSF-VHG process which is believed to be even more energy efficient and produces around 18% v/v ethanol concentration.
The authors have not declared any conflict of interests.
REFERENCES
|
Ajibola FO, Edema MO, Oyewole OB (2012). Enzymatic Production of Ethanol from Cassava Starch Using Two Strains of Saccharomyces cerevisiae. Nigerian Food Journal 30(2):114-121.
Crossref
|
|
|
|
Ben-Iwo J, Manovic V, Longhurst P (2016). Biomass resources and biofuels potential for the production of transportation fuels in Nigeria. Renewable and Sustainable Energy Reviews 63:172-192.
Crossref
|
|
|
|
|
Bharti B, Madhulika C (2016). Bioethanol Production Using Saccharomyces cerevisiae with Different Perspectives: Substrates, Growth Variables, Inhibitor Reduction and Immobilization. Fermentation Technology 5(2):1-4.
Crossref
|
|
|
|
|
Caputie A, Ueda M, Brown T (1986). Spectrophotometeric determination of ethanol in wine. International Journal of Advanced Research 2:888-896.
|
|
|
|
|
Chu-Ky S, Pham TH, Bui KLT, Nguyen TT, Pham KD, Nguyen HDT, Le TM (2016). Simultaneous liquefaction, saccharification and fermentation at very high gravity of rice at pilot scale for potable ethanol production and distillers dried grains composition. Food and Bioproducts Processing 98:79-85.
Crossref
|
|
|
|
|
De Haas I, Kreuger T (2010). The fermentation of different sugars,
View 24 May, 2016.
|
|
|
|
|
FAO (2012). Food production in Nigeria (2017-2019). In FAOSTAT Statistical Database, Statistical Division, (Ed.). Rome: Food and Agriculture Organization of the United Nations.
|
|
|
|
|
Hanyao Z, Aaron S, Richard CG, Matthew RG (2010). Saccharomyces paradoxus and Saccharomyces cerevisiae reside on oak trees in New Zealand: evidence for migration from Europe and interspecies hybrids. FEMS Yeast Research 10(7):941-947.
Crossref
|
|
|
|
|
Hossain N, Zaini JH, Mahlia TMI (2017). A review of bioethanol production from plant-based waste biomass by yeast fermentation. International Journal of Technology 8(1):5-18.
Crossref
|
|
|
|
|
Miller GL (1959). Use of Dinitrosalicylic acid regent for determination of reducing sugar. Analytical Chemistry 31:426- 428.
Crossref
|
|
|
|
|
National Bureau of Statistics, Petroleum Products Consumption Statistics, Qtr 2, 2018.
|
|
|
|
|
Phakping S, Ketudat-Cairns M, Boontawan A (2014). Extractive Fermentation of Ethanol from Fresh Cassava Roots using Vacuum Fractionation Technique. Advanced Materials Research 931-932:1096-1100.
Crossref
|
|
|
|
|
Puligudla P, Smogrovicova D, Obulam VRS, Ko S (2011). Very High Gravity (VHG) Ethanol Brewing and Fementation: A Research Update. Journal of Industrial Microbiology and Biotechnology 38:1133-1144.
Crossref
|
|
|
|
|
Sarocha P, Ankita J, Muhammad B. S, Athapol N, Vijay S (2018). Comparison of Cassava Starch with Corn as a Feedstock for Bioethanol Production. Energies 11:3476-3486.
Crossref
|
|
|
|
|
Siti HMA, Rahmath A, Siti A, Hartinie M, Jualang AG, Ainol AMF, Kenneth FR (2017). Yeasts in sustainable bioethanol production: A review. Biochemistry and Biophysics Reports 10:52-61.
Crossref
|
|
|
|
|
Solomon BO, Aransiola EF, Shittu TD, Adejare YO, Adediran DA, Bamidele CO, Ibrahim Taiwo (2018). The conversion of biomas to biofuel - A Review In Williams AOF, Ogunbayo AO, Abass O. (Eds.), Developments in Biotechnology, Systems, Control and Education: Festschrift in Honour of Professor Rahamon Adisa Bello University of Lagos Press, Lagos.
|
|
|
|
|
Sriroth K, Wanlapatit S, Piyachomkwan K (2012). Cassava Bioethanol, Bioethanol, Prof. Marco Aurelio Pinheiro Lima (Ed.),. InTech Press, Rijeka, Croatia. 290-341.
Crossref
|
|
|
|
|
Steensels J, Snoek T, Meersman E, Nicolino MP, Voordeckers K, Verstrepen KJ (2014). Improving industrial yeast strains: exploiting natural and artificial diversity. Federation of European Microbiological Societies Microbiology Reviews 38(5):947-995.
Crossref
|
|
|
|
|
Zang L, Chen Q, Jin Y, Xue H, Guan J, Wang Z, Zhao H (2010). Energy Saving Direct Ethanol Production from Viscosity Reduction Mash of Sweet Potatoe at Very High Gravity (VHG). Fuel Processing Technology 91(12):1845-1850.
Crossref
|
|