ABSTRACT
Despite their great interest for the integrated management of water resources, information on the ecology of aquatic oligochaetes is still sketchy in Cameroon. The present study aims at contributing to the knowledge on the distribution, microhabitat and life history of Naididae tubificids taxa in some eight water bodies of the city of Yaoundé. A total of 132 samples were analysed and the morphospecies Branchiura spp. and Limnodrilus spp. were identified. The most abundant species were Branchiura spp. with 2035 individuals versus 880 Limnodrilus spp. Both of them demonstrate low seasonal variations. It appeared that, these annelids are more abundant on clay-rich soils than on sand and the herbarium. Assessment of the organic pollution index indicates an organic pollution of the sampled waters ranging from moderate to high (3.67-2). The redundancy canonical analysis shows that Branchiura spp. are more present in saline waters revealing high organic pollution factors variables. During the study period, some 10 individuals of Limnodrilus spp. presented a shrunken tail. That reveals a strong environmental pressure due to the action of predators or the presence of heavy metals in the aquatic system evaluated. All these characteristics indicate a high pollution and predation pressure in the milieu.
Key words: Annelids oligochaete, ecology, bottom nature, polluted water.
In the city of Yaoundé, which is the political capital of Cameroon, surface water is used for the production of drinking water, irrigation of crops in urban agriculture, fish farming activities (aquaculture), recreation, and the list is very extensive. Nevertheless, due to uncontrolled urbanization, these waters are subject to very high pollution (Kemka et al., 2004; Zébazé et al., 2006; Ebang et al., 2012; Ajeagah et al., 2014; Kapso et al., 2018; Ngong et al., 2019). In fact, since the population grows faster than the basic public services, a drastic lack of sanitary infrastructures is noticed. Consequently, almost 82.8% of the untreated wastewater is directly discharged into the environment (Ngambi, 2015) and this alters the quality of the natural resource.
For a good monitoring of the surface water bodies, aquatic oligochaetes can be an important ecological tool. The application of Naididae, especially those belonging to the group of tubificids as bioindicators of water quality, is quite common in ecological studies (Vivien et al., 2011; Rodriguez and Renoldson, 2011; Vivien et al 2020). Naididae tubificids constitute a well-diversified group. It includes 6 subfamilies namely Limnodriloidinae, Phallodrilinae, Rhyacodrilinae, Rhyacodriloidinae, Telmatodrilinae and Tubificinae (Martin et al., 2008). All of them are endobenthic worms (Martin and Ait, 2012).
Due to their high resistance to organic pollution and their bioaccumulative capacity, most of them adapt to harsh environmental conditions (Schenková and Helešic, 2006). Besides being excellent indicators of water quality, Naididae tubificids are also highly involved in the flux and the availability of organic matter and pollutants in the water body. For example, they play both a direct role in the elimination of heavy metals via their own metabolism (biodegradation, bioaccumulation, detoxification), and an indirect role (as the result of their bioturbation activities) through the modification of the physico-chemical conditions and the stimulation of sulfo-reducing and metallo-reducing bacteria (Lagauzère, 2008). However, despite their great interest for the integrated management of water resources, information on their ecology is still sketchy in Cameroon. Although several studies have been carried out on the group of macroinvertebrates (Foto, 2012; Ajeagah et al., 2013; Tchakonté, 2016; Ajeagah et al., 2018; Foto et al., 2019, 2021), the only recorded article dealing with the fauna of freshwater oligochaetes in Cameroon is that of Dahl (1957) on the banks of the Nyong in the localities of Mpoume, Makak and Nenyanga. So this study aims at examining the spatio-temporal distribution of these organisms and evaluating the environmental parameters of the sampling sites in order to assess the quality of water.
Sites and period of study
This study was conducted in the city of Yaoundé, that is located in the forest region of the southern plateau of Cameroon, between longitudes 11°20’ and 11°40’, latitudes 3°30’ and 3°58’. This town is subject to an equatorial climate that is characterized by four seasons: the short rainy season (SRS) from March to June, the short dry season (SDS) from July to August the long rainy season (LRS) from September to November, and the long dry season (LDS) from late December to February (Sighomnou, 2004). This climate combined with the relief characterized by hills, valleys, the high density of the human population (Ngambi, 2015) and the extensive hydrographic network, favours urban agriculture in the city of Yaoundé.
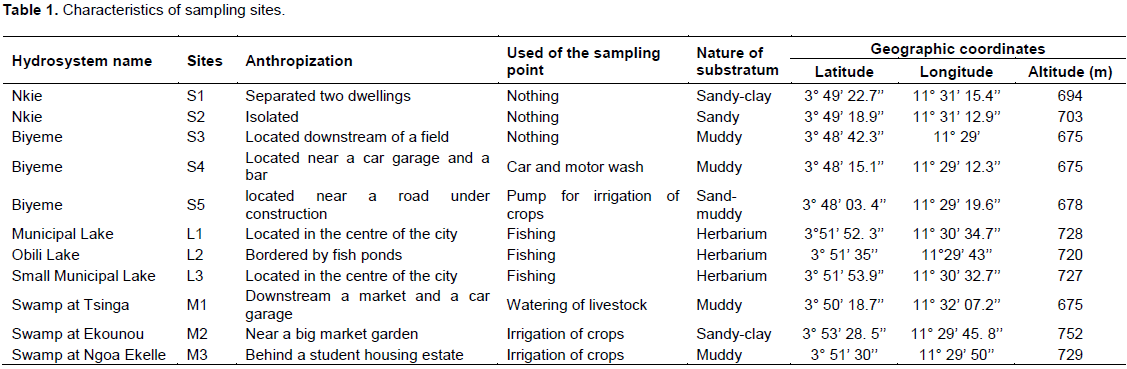
This research was carried out from March 2016 to February 2017. The environmental parameters were measured following a monthly sampling frequency. Since metals are non-biodegradable and persist in the environment for a long time (Briffa et al., 2020), the heavy metals (Cd, Pb, Hg, Zn, Cr, Fe) were measured only once. Physico-chemical and biological data collected were assessed on seasonal basis (SRS, SDS, LRS, LDS). The sampling sites chosen (Figure 1) were taken into account on the different types of hydrosystems that can be found in the city. Five sampling sites were chosen on two streams (S1 and S2 on the Nkie stream, S3, S4 and S5 on the Biyeme stream). Three sampling points were chosen on three lakes (L1 on the Municipal lake, L2 on the Obili lake and L3 on the small Municipal lake). Three other sampling points were chosen on three different marshy areas (M1 at Tsinga, M2 at Ekounou and M3 at Ngoa Ekelle). Some characteristics of these points are given in Table 1.

Physico-chemical analysis
The water temperature, potential of Hydrogen (pH), colour, suspended solids (SS), turbidity, alkalinity, salinity, electric conductivity, heavy metals, dissolved carbon dioxide (CO2), dissolved oxygen (O2), nitrogen compounds (NH4+, NO2-, NO3-) and orthophosphate (PO43-) were measured according to the protocol of Rodier et al. (2009) and APHA (1990). The organic pollution index (Leclercq, 2001) was calculated in order to give a synthetic account of the degree of pollution of the water sampled by using the three basic parameters required in organic pollution evaluation, namely: ammoniacal nitrogen (NH4+), nitrite (NO2-) and orthophosphate ions (PO43-). This index allows to define 5 ecological quality classes of water that are: Zero pollution (4.6 ≤ IPO ≤ 5), low pollution (4.0 ≤ IPO ≤ 4.5), moderate pollution (3.0 ≤ IPO ≤ 3.9), high pollution (2.0 ≤ IPO ≤ 2.9), very high pollution (1 ≤ IPO ≤ 1.9) (Leclercq, 2001).
Biological analysis
The sampling of annelids was carried out by means of a net which is 30 cm × 30 cm dimension that is fixed to a handle and equipped with a conical thread of 100 μm ? opening of mesh and 50 cm deep (Martin and Ait, 2012). The multihabitat approach proposed by Stark et al. (2001) and adapted from Barbour et al. (1999), which consists of a total of 20 shots, equivalent to an approximate surface area of 3 m2 in a station of 100 m long was applied. After sampling, organisms were fixed in 10% formalin for a maximum of 24 h; they were then rinsed and stored in 70% alcohol-contained in pill bottles (Morgan and Morgan, 1990; Martin and Ait, 2012). In the laboratory, each oligochaete specimen was placed in a drop of glycerine which allows, because of its lightening power, to observe the external and internal structures of the organism under an optical microscope equipped with a camera. The identification was carried out using the keys proposed by Brinkhurst (1985) and Martin and Ait (2012). Mature individuals were distinguished from immature by their larger size and the presence of a clitellum. The species richness (S) of each community and the abundance of each population were sought.
Statistical analysis
The software Microsoft Office Excel 2007, SPSS 20. 0 and XLSTAT 11.0 made it possible to: (i) establish the descriptive statistics of individuals and sites (summaries tables, redundancy canonical analysis); (ii) measure the robustness of the spatio-temporal distribution of biological variables using the Kruskal-Wallis test associated with the Mann-Whitney "U" test. (iii) calculate the Spearman "r" coefficient between the abundance of organisms and the characteristics of the biotope. The differences observed are significant at 1 and 5% safety thresholds.
Spatio-temporal variations of physico-chemical parameters of water
The physico-chemical results of our sampling points are presented in Table 2 (central values of physico-chemical parameters), Table 3 (seasonal values of physico-chemical parameters) and Table 4 (values of heavy metals in each site). As lakes and swamps are stagnant waters, the velocity of water flow has only been assessed in the streams. The highest flow was recorded at S5 and the lowest at S2 (p < 0.05). The highest temperature values were recorded in lakes and the lowest in streams. However, within each type of ecosystem the temperature variations were not significant (p > 0.05). There was no significant difference for suspended solids, colour turbidity, pH and carbon dioxide contain within or between ecosystems. However swamps more often had relatively higher values. Sampled water, with values ranging from 7.14±0.83 to 7.83±048 were slightly basic while relatively higher values of CO2 were recorded in swampy areas. Electrical conductivity, total dissolved solid, and alkalinity followed almost the same spatial and temporal variations. Mean values of these parameters were significantly lower in lakes than in swamps and stream sites (p < 0.05). In stream sites, the lower values were obtained at stations S1 and S2. In lakes, values obtained at L3 were significantly lower than those obtained at L2 and L1 (p < 0.05). In swamps the lower values of that parameters were recorded at M2 (p < 0.05).
Values of salinity were more important in streams and marshes than in lakes (p < 0.05). Within the stream ecosystem, the salinity of water at S3 was significantly higher than at S1 and S2. In the other types of ecosystems, the salinity of water did not vary significantly between the different sampling sites. Values of orthophosphates, nitrites, nitrates and ammonia nitrogen did not significantly differ from one station to another within each ecosystem. However, nitrates were significantly higher in swamps than in streams and lakes. The mean values of dissolved oxygen did not vary significantly within and between ecosystems, nor the organic pollution index (OPI) values which indicated an organic pollution of the sampled waters essentially ranging from moderate (3.06±0.75 at S2 and 3.00±0.86 at S1) to high (from 2.86±0.73 to 2.42±0.38 in the rest of sampling sites). Values of the physico-chemical parameters, except for OPI, were comparable between the seasons (p > 0.05). In streams and lakes, the highest values of OPI were obtained during the SRS and the lowest values during the LDS (p < 0.05). In swampy areas, the highest values of OPI were obtained during the SDS. These were significantly higher than those obtained during the LRS and LDS (Table 3).
The highest values of heavy metals were registered in swamps (Cd: 9.51±8.35 μg/L, Pb: 1.02±1.63 μg/L, Hg:7.35±12.60 μg/L, Zn: 1.77±2.98 μg/L, Cr :0.37±0.57 μg/L, Fe: 124.85±171.75 μg/L). Streams were the least rich in Cd (0.43±0.64 μg/L), Pb (0.09±0.08 μg/L), and Hg (0.06±0.08 μg/L), while the lakes were the least rich in Zn (0.82±0.20 μg/L), Cr (0.05±0.04) and Fe (14.88±16.08 μg/L) (Table 4).
Spatial distribution of Naididae
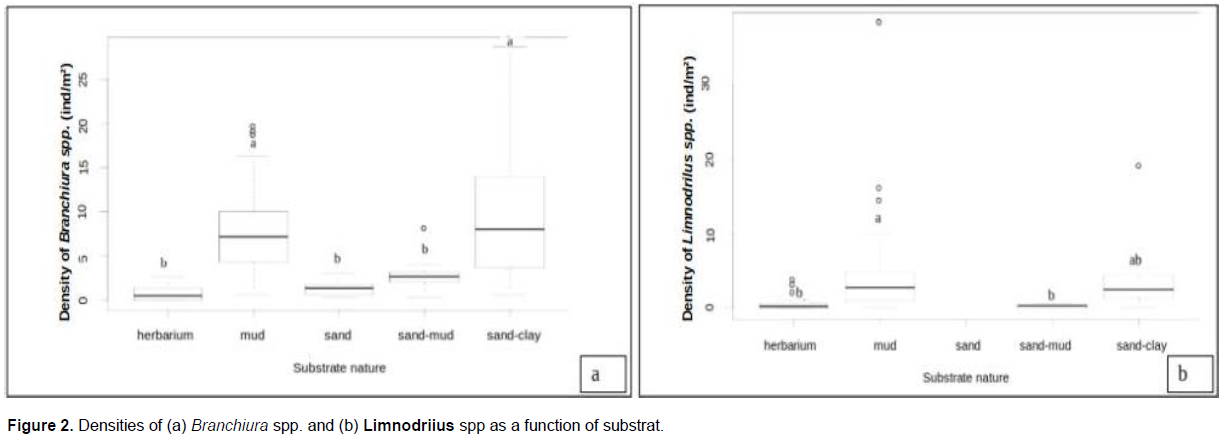
A total of 2035 Branchiura spp. individuals (1789 adult and 246 immature) and 880 Limnodrilus spp. (678 mature and 202 immature) were identified (Table 5). About 10 individuals of Limnodrilus spp. presented shrunken tail. Branchiura spp. as well as Limnodrilus spp. abundance varied significantly from one ecosystem to another following the profile: swampy areas (30±20 Branchiura spp., 14±21 Limnodrilus spp.) > streams (15±11 Branchiura spp., 6±9 Limnodrilus spp.) > lakes (2±2 Branchiura spp., 2±2 Limnodrilus spp.). No organism was collected at L3. Concerning the distribution of Branchiura spp. in streams, with a mean abundance of 31±7 individuals, S3 was the most populated site (p < 0.05). Limnodrilus spp were not collected at S2. At the other stream stations, their abundance was lower in S5 (1±1 individual).
In lake systems, no significant difference was revealed in spatial distribution of taxa (p > 0.05). In swampy areas, the abundance of Branchiura spp. was significantly higher at M2 (45±15 individuals) and that of Limodrilus spp. at M3 (26±31 indiiduals). Branchiura spp. were overall more abundant than Limnodrilus spp. in streams and marshes (p < 0.05) and not different in lakes (p > 0.05). In streams and marshes, Branchiura spp. were always the more abundant taxa in all sites except at S1 and M3 where the both taxa were equally abundant.
Seasonal distribution of Naididae
Table 6 shows no significant seasonal variation of the abundance of Branchiura spp. and Limodrilus spp. within each ecosystem (p > 0.05). More than 87% of Branchiura spp. specimens and 75% of Limodrilus spp. specimens were mature during all seasons.
Density of the populations
With a median density of 8 individuals/m² (Figure 2a), Branchiura spp. were significantly more abundant in the mud and sand/clay substrates than in the other ones (herbal, sandy and sand/mud), where the density of these oligochaetes fluctuated between 1 to 3 individuals/m². Similarly, the density of Limnodrilus spp. (Figure 2b) was significantly higher in the mud (0-39 individuals/m²) as well as in the sand/clay substrates (0-19 individual/m²). This taxa was not found in the sand while in the sand-mud substrate, it could reach 1 individual/m². In herbarium, the density of this taxa fluctuated between 0 to 4 individuals/m².
Influence of environmental parameters on the dynamics of organisms
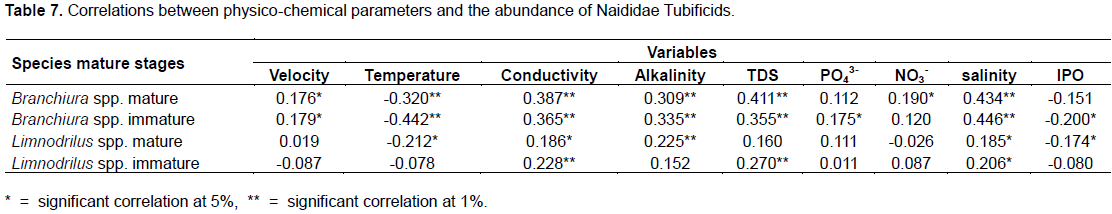
The water temperature and the organic pollution index were negatively and often significantly correlated with the abundance of all the maturity stages of both taxa. All the other parameters (electrical conductivity, alkalinity, total dissolved solids, salinity, orthophosphate and water velocity) were positively and often significantly correlated to the abundance of the mature stages of these oligochaetes. Nitrate was positively and only significantly correlated to mature Branchiura spp. and negatively but not significantly correlated to mature stage of Limnodrilus spp. (Table 7).
The canonical redundancy analysis (Figure 3) presents the environmental variables that described the distribution of Naididae tubificids in our sampling sites. This canonical redundancy analysis explains 58.5% of species-environment correlation with a variance of 70.2%. The distribution of species along axis 1 are explained by the electrical conductivity, the total dissolved solids, the salinity, the dissolved carbon dioxide, the alkalinity, the nitrates, the temperature and the Organic Pollution Index. Axis 2 is significantly correlated to suspended solids, orthophosphates, nitrates, salinity, dissolved carbon dioxide and the organic pollution index. Thus, conditions of high electrical conductivity, salinity, total dissolved solids content, alkalinity, nitrates and low temperature values appeared favourable to the development Branchiura spp. while Limnodrilus spp. prefered environments that were rich in dissolved carbon dioxide and fairly poor in suspended solids, orthophosphates, nitrates and ammonium ions.
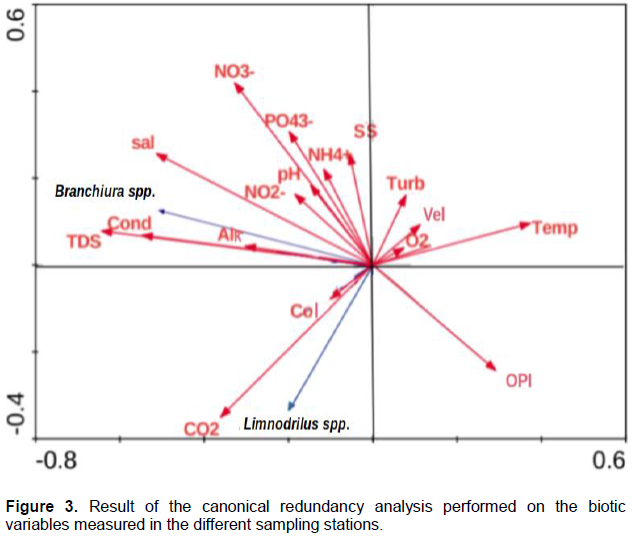
Oligochetes belonging to the genera of Branchiura (Beddard, 1892) and that of Limnodrilus (Claparède, 1862) were collected in the different sampling sites during the study period. Among these genera only the first one has already been identified in the surface water in Cameroon, precisely in urban streams of Douala (Tchakonte, 2016). None of these has been identified in poor anthropized streams of the center and the west regions of Cameroon (Nyamsi, 2018; Kengne, 2018). Naididae tubificids are for the most characteristic of well mineralized and hypoxic milieu (Martin and Ait, 2012; Schenková and Helešic, 2006). Species belonging to the genus Branchiura have developed gills to cope with these harsh conditions (Martin and Ait, 2012). Limodrilus hoffmeisteri also possesses a hemolymph that is rich in erythrocruorine which can address easily low oxygen conditions (Martin and Ait, 2012). Therefore the high abundance of Branchiura spp. and Limodrilus spp. in our milieu indicates the low oxygenation of these environments. This is justified by the non satisfied values of dissolved oxygen in our samples (< 75%, Table 2).
The distribution of tubificids in our study sites is governed by many factors. The significant correlations between the indicators of water organic pollution (electrical conductivity, TDS, Salinity, Nitrates) and oligochaetes abundance (Table 7) indicate that these organisms prefer polluted water. Schenková and Helešic (2006) obtained similar results in a small stream in the Czech Republic, where the abundance of the oligochaetes community was significantly and positively correlated with the electrical conductivity and nitrate. Water temperature was negatively and significantly correlated with the abundance of Branchiura spp. and Limnodrilus spp. This physical parameter varies between 21.9 and 31°C in this tropical area (Table 2). As the Naididae originate from temperate zones (Timm, 1980), we suggest that the above species are sensitive to some degree of heat. In fact, Timm (2020) demonstrates that the reproductive capacity of Oligochaetes decreases at elevated temperatures (25° to 35°C). In the literature, we also notice that the reproduction rate of Branchiura sowerbyi, for example, declines when the water temperature exceeds 25°C (Aston and Miler, 1982; Bonacina et al., 1994).
No specimen of Limnodrilus spp. was collected at S2. The lower pH values obtained at this station (sometimes 5UC) could justify this result. Roff and Kwiatkowski (1977) also observed the absence of L. hoffmeisteri in lakes with a pH below 6.6. As with Ragonha et al. (2013), the substrate composition played an important role in the oligochaetes community structure and distribution in this study.
In the current study, tubificids preferentially colonised muddy and sand-clay substrates (Figure 2). This result does not agree with those of Ragonha et al. (2013) who reported the dominance of tubificids in sand rich organic matter. Schenková et al. (2001) also found more tubificids in fine sand that is rich in organic matter than in other substrates.
There was no significant difference between seasonal distribution of Naididae tubificids identified in the present study (Table 6). Conversely, Nijboer et al. (2004) argued that the seasons mostly influence the hydrographic regime of a given site, with consequences on the development of the vegetation, and the grazing groups of oligochaeta such as Naididae-Naidinae, Pristininae. Schenková and Helešic (2006) also associated the seasonal variation of oligochaetes to the development of the vegetation which favours the installation of Naididae-Naidinae. It is worth to also make clear that Branchiura spp. and Limnodrilus spp. weakly colonised herbarium (Figure 2) Mature individuals of Branchiura spp. as well as of Limnodrilus spp. were collected throughout the year (Table 6). A year-round maturity period had already been reported for Tubifex tubifex and L. hoffmeisteri in rivers in Spain (Martinez-Ansemi, 1990). Contrary to the results obtained by Tchakonte (2016) in the polluted waters of the city of Douala, T. tubifex was not collected in our study stations. According to Zeybek et al. (2018), this specie is very abundant in polluted environments where it often co-occurs with Limnodrilus hoffmeisteri, although T. tubifex is a poor competitor in the environment (Nijboer et al., 2004). It only thrives in extreme environmental conditions that limit the development of other benthic macroinvertebrates (Nijboer et al., 2004). Competitive or predatory pressure is a limiting factor for its presence in aquatic environments (Milbrink, 1983; Zeybek et al., 2018). In our study stations, the observation of a shrunken tail in some oligochaetes, which is a sign of regeneration, could precisely reflect a certain predatory pressure in our environment. In this respect, Kaliszewicz (2003) demonstrated the regeneration of the anterior, the posterior and even both parts of the body in some Naididae such as Stylaria lacustris following their sectioning by insect larvae. The predation pressure could also explain the low densities of oligochaetes in our study area (Figure 2) where some stations even contained fish. The shrunken tail could also be a result of a decontamination strategy developed by these aquatic worms. Bouché et al. (2000) observed the amputation of the tail of T. tubifex following the exposure to concentration of 0.01 to 0.05 mg/l of cadmium. After having accumulated the metal in its tail, this annelid gets rid of it. Since the cadmium concentrations obtained at some stations are at least equal to those reported by Bouché et al. (2000), Limnodrilus spp. collected might have had the same response to this stress. The values of cadmium and mercury found in swampy stations (M2 and M3) are above the water quality standards for crops irrigation and may be harmful to human.
The dominance of the abundance of Branchiura spp. over that of Limnodrilus spp. (Table 5) can on one hand be explained by their better adaptation to pollution. The Canonical Redundancy Analysis (Figure 3) showed a higher link between Branchiura spp. and the indicators of organic pollution. It could also be explained by their possible better (a) adaptation to the predation pressure and (b) annual productivity rate. In fact, studies on Branchiura sowerbyi showed that it possesses sensorineural specializations that maximize the detection of vibrations in the substrate, water movement and contact which make it more successful in escaping predators (Drewes and Zoran, 1989). Raburu et al. (2002) also demonstrated that B. sowerbyi reproduced and developped faster than L. hoffmeisteri.
This study enabled us to isolate and identify, to the genus level, the Naididae tubificids present in 8 water bodies of the city of Yaoundé. These are Branchiura spp. and Limnodrilus spp. The distribution of these organisms in the study sites was governed by the oxygenation of the milieu, its mineralization, the nature of the substrate and the temperature. Branchiura spp. and Limnodrilus spp. are two polluo-resistant species. Their presence in those water bodies, in addition to showing the high pollution level of the water, also shows the high predation pressure in the milieu studied.
The authors have not declared any conflict of interests.
The authors appreciate Dr. Tchakonté Simeon for statistical analysis, Njiawoua Pountigni Eric for accompanying on field and the laboratory of hydrobiology and environment for the physico-chemical and biological assessment, and thank Dr. Patrick Martin for providing valuable guidance, sampling and identification documents of the oligochetes.
REFERENCES
|
Ajeagah GA, Bikitbe JF, Longo F (2013). Qualité bioécologique d'un milieu lacustre hyper-eutrophisé en zone équatoriale (Afrique Centrale) : peuplement de protozoaires ciliés et macroinvertébrés bentho-aquatiques. Afrique Science 09(2):50-66.
|
|
|
|
Ajeagah GA, Kekenou S, Njiawoua Pountigni EN, Foto Menbohan S (2014). Morphologie et abundance des stades de développement d'Ilyocoris cimicoïdes Linné 1758 (Heteroptera: Naucoridae) dans un lac anthropisé en zone tropicale (Cameroun). Journal of Applied Bioscience 79:6926-6937.
Crossref
|
|
|
|
|
Ajeagah GA, Mbainaissem MS, Njiawouo P, Ngakomo RA (2018). Caractérisation physico-chimique et biologique des eaux en zone périurbaine en Afrique équatoriale : cas de Ngoumou au Centre du Cameroun. International Journal of Innovation and Applied Studies 23(1):33-43.
|
|
|
|
|
American Public Health Association (APHA) (1990). Standard Methods for the Examination of Water and Wastewater. 19e edition, Washington DC, USA.
|
|
|
|
|
Aston RJ, Milner AGP (1982). Conditions required for the culture of Branchiura sowerbyi (Oligochaeta: Tubificidae) in activated sludge. Aquaculture 26(1-2):155-160.
Crossref
|
|
|
|
|
Barbour MT, Gerritsen J, Snyder BD, Striblking JB (1999). Rapid biomass assessment protocols for use in stream and Wadcable Rivers: periphyton, benthic macroinvertebrates and fish. 2nd edition, US Environmental Protection Agency, Office of water, Washington, DC, EPA 841-B- 99-002 11 chapters and 4 apendices 664 p.
|
|
|
|
|
Beddard FE (1892). A new branchiate Oligochate (Branchiura sowerbyi). Quaterly Journal of Microscopical Science 33:325-341.
Crossref
|
|
|
|
|
Bonacina C, Bonomi G, Marzuoli D (1994). Quantitative observations on the population ecology of Branchiura sowerbyi (Oligochaeta, Tubificidae). Hydrobiologia 278:267-274.
Crossref
|
|
|
|
|
Bouché ML, Habets F, Biagianti-Risbourg S, Vernet G (2000). Toxic effects and bioaccumulation of cadmium in the aquatic oligochaete Tubifex tubifex. Ecotoxicology Environmental Safety 46(3):246-251.
Crossref
|
|
|
|
|
Briffa J, Sinagrab E, Blundell R (2020). Heavy metal pollution in the environment and their toxicological effect on human. Heliyon 6(9):e04691.
Crossref
|
|
|
|
|
Brinkhurst RO (1985). Guide to the Freshwater Aquatic Microdrile oligochaetes of North America. Fisheries and Aquatic Sciences 268 p.
|
|
|
|
|
Claparède ER (1862). Recherches anatomiques sur les Oligochetes. Mémoires de la société de physique et d'histoire naturelle de Génève 16:217-291.
|
|
|
|
|
Dahl IE (1957). Results from the Danish Expedition to the French Cameroon, 1949-195. Bulletin de l'Institut français d'Afrique noire 1:1154-1172.
|
|
|
|
|
Drewes CD, Zoran MJ (1989). Neurobehavioral specializations for respiratory movements and rapid escape from predators in posterior segments of the tubificid Branchiura sowerbyi. Hydrobiologia 180:65-71.
Crossref
|
|
|
|
|
Ebang MD, Zebaze Togouet SH, Foto Menbohan S, Kemka N, Nola M, Boutin C, Nguetsop VF, Djaouda M, Njiné T (2012). Bio-écologie des diatomées épilithiques de la rivière Mfoundi (Yaoundé, Cameroun): diversité, distribution spatiale et influence des pollutions organiques. Revue des Sciences de l'eau 25(3):203-218.
Crossref
|
|
|
|
|
FAO (2003). L'irrigation avec des eaux usées traitées, manuel d'utilisation. Bureau Régional pour le Proche-orient et Bureau sous-régional pour l'Afrique du Nord 68 p.
|
|
|
|
|
Foto MS (2012). Recherche écologique sur le réseau hydrographique du Mfoundi (Yaoundé): Essai de biotypologie. Thèse de Doctorat d'État. Université de Yaoundé I, Cameroun 179 p.
|
|
|
|
|
Foto MS, Dzavi J, Nzongang KC, Biram À Ngon EB, Ntchantcho R (2019). Impact of the Anthropogenic Activities on the Diversity and Structure of Benthic Macroinvertebrates in Tropical Forest Stream. International Journal of Progressive Sciences and Technologies 15(1):280-292.
Crossref
|
|
|
|
|
Foto MS, Nwaha M, Biram à Ngon BE, Dzavi J, Boudem CR, Sob Nangou PB, Nyame Mbia D (2021). Water quality and benthic macroinvertebrates of tropical forest stream in South-West region, Cameroon. International Journal of Progressive Sciences and Technologies 25(1):183-192.
Crossref
|
|
|
|
|
Kaliszewicz A (2003). Sublethal predation on Stylaria lacustris: a study of regenerative capabilities. Hydrobiologia 501(1):83-92.
Crossref
|
|
|
|
|
Kapso Tchouankep M, Ajeagah GA, Nkeng GE, Ngassam P (2018). Bio-characterization of some free living Amoebae in surface water of the city of Yaounde: relationship to physico-chemical Parameters of the medium. Journal of Applied Biotechnology 6(1):26-41.
Crossref
|
|
|
|
|
Kemka N, Njiné T, Zébazé Togouet SH, Niyitegeka D, Nola M, Monkiedje A, Demanou J, Foto Menbohan S (2004). Phytoplancton du lac municipal de Yaoundé (Cameroun) : succession écologique et structure des peuplements. Revue des Sciences de l'eau 17(3):301-316.
Crossref
|
|
|
|
|
Kengne FJ (2018). Bio-évaluation des cours d'eau de la région Ouest du Cameroun à l'aide des macroinvertébrés benthiques et construction d'un indice multimétrique régional. Thèse de Doctorat Ph.D. Université de Yaoundé I, Cameroun, Université de Lille, France 222 p.
|
|
|
|
|
Lagauzère S (2008). Influence de la bioturbation des macro-invertébrés benthiques sur le comportement biogéochimique de l'uranium au sein des sédiments d'eau douce Sandra. Thèse de Doctorat Ph.D. Université de la Méditerranée Aix-Marseille II, France 310 p.
|
|
|
|
|
Leclercq L (2001). Intérêt et limites des methods d'estimation de la qualité de l'eau. Station scientifique des Hautes-Fagnes, Belgique, Document de travail 44 p.
|
|
|
|
|
Martin P, Boughrous AA (2012). Guide taxonomique des oligochètes dulçaquicoles du Maghreb. Abc Taxa 12:194.
|
|
|
|
|
Martin P, Enrique M, Pinder A, Timm T, Wetzel MJ (2008). Global diversity of oligochaetous clitellates («Oligochaeta»; Clitellata) in freshwater. Hydrobiologia 595:117-127.
Crossref
|
|
|
|
|
Martinez-Ansemil E (1990). Étude biologique et écologique des Oligochètes aquatiques de la rivière Tambre et de ses milieux associés (Galice, Espagne). Annales de Limnologie 26:131-151.
Crossref
|
|
|
|
|
Milbrink G (1983). An improved environmental index based on the relative abundance of oligochaete species. Hydrobiologia 102(2):89-97.
Crossref
|
|
|
|
|
Morgan JE, Morgan AJ (1990). The distribution of cadmium, copper, lead, zinc and calcium in the tissues of the earthworm Lumbricus rebellus sampled from one uncontaminated and four polluted soils. Oecologia 84:559-566.
Crossref
|
|
|
|
|
Ngambi JR (2015). Déchets solides ménagers dans la ville de Yaoundé (Cameroun) De la gestion linéaire vers une économie circulaire. Thèse de doctorat en droit. Université du Maine, France 490 p.
|
|
|
|
|
Ngong AI, Ajeagah GA, Kapso TM, Nguepidjio G, Sotchang MIO, Fouossong BI, Nnah MJP, Kechia KAI, Enoka P (2019). Physicochemical Quality of Water and Influence on the dynamics of bacteria circulating in water points for domestic use in Yaoundé (Cameroon). International Journal of Natural Resource Ecology and Management 4(5):112-119.
Crossref
|
|
|
|
|
Nijboer RC, Wetzel MJ, Piet FM (2004). Diversity and distribution of Tubificidae, Naididae, and Lumbriculidae (Annelida: Oligochaeta) in the Netherlands: an evaluation of twenty years of monitoring data. Hydrobiologia 520:127-141.
Crossref
|
|
|
|
|
Nyamsi TNL (2018). Macroinvertébrés benthiques du réseau hydrographique de la Méfou : habitat, diversité et dynamique des peuplements, évaluation de l'intégrité biologique des cours d'eau. Thèse de Doctorat Ph.D. Université de Yaoundé I, Cameroun 202 P.
|
|
|
|
|
Raburu P, Mavuti KM, Harper DM, Clark FL (2002). Population structure and secondary Productivity of Limnodrilus hoffmeisteri (Claparede) and Branchiura sowerbyi Beddard in profundal zone Lake Naivasha, Kenya. Hydrobiologia 488:153-161.
Crossref
|
|
|
|
|
Ragonha FH, Chiaramonte JB, Fontes Junior HM, Ribeiro da Cunha E, Benedito E, Takeda MA (2013). Spatial distribution of aquatic oligochaeta in Ilha Grande National Park, Brazil. Acta Scientiarum 35(1):63-70.
Crossref
|
|
|
|
|
Rodier J, Legube B, Marlet N, Brunet R (2009). L'analyse de l'eau. 9e édition, Paris: Dunod 1579 P.
|
|
|
|
|
Rodriguez P, Reynoldson TB (2011). The pollution biology of aquatic oligochaetes. New York: Springer 265 p. https://doi: 10.1007/978-94-007-1718-3.
Crossref
|
|
|
|
|
Roff JC, Kwiatkowski RE (1977). Zooplankton and zoo-benthos communities of selected northern Ontario lakes of different acidities. Canadian Journal of Zoology 55:899-911.
Crossref
|
|
|
|
|
Schenková J, Helešic J (2006). Habitat preferences of aquatic Oligochaeta (Annelida) In the Rokytná River, Czech Republic-a small highland stream. Hydrobiologia 564:117-126.
Crossref
|
|
|
|
|
Schenková J, Komárek O, Zahrádková S (2001). Oligochaeta of the Morava and Odra River basins (Czech Republic): species distribution and community composition. Hydrobiologia 463:235-240.
Crossref
|
|
|
|
|
Sighomnou D (2004). Analyse et redéfinition des régimes climatiques et hydrologiques du Cameroun: perspective d'évolution des ressources en eau. Thèse de Doctorat d'état. Université de Yaoundé I, Cameroun 292 p.
|
|
|
|
|
Stark JD, Boothroyd IKG, Harding JS, Maxted JR, Scarsbrook MR (2001). Protocols for Sampling macroinvertabrates in wadeable streams. New Zealand macroinvertabrate working group report, no 1. Prepared for the Ministry of Environment. Sustainable management fund project, 5103, 57 p.
|
|
|
|
|
Tchakonté S (2016). Diversité et structure des peuplements de macroinvertébrés benthiques des cours d'eau urbains et périurbains de Douala (Cameroun). Thèse de Doctorat Ph.D. Université de Yaoundé I, Cameroun 233 p.
|
|
|
|
|
Timm T (1980). Distribution of aquatic oligochaetes. In: Aquatic oligochaeta Biology. Springer, Boston, MA pp. 55-77.
Crossref
|
|
|
|
|
Timm T (2020). Observations on the life cycles of aquatic Oligochaeta in aquaria. Zoosymposia 17:102-120.
Crossref
|
|
|
|
|
Vivien R, Lafont M, Perfetta J (2011). Proposition d'un seuil de toxicité des métaux lourds des sédiments mis en évidence par les vers oligochètes dans quelques cours d'eau. Bulletin de la Société Vaudoise Des Sciences Naturelles 92:153-164.
|
|
|
|
|
Vivien R, Apothéloz-Perret-Gentil L, Pawlowski J, Werner I, Lafont M, Ferrari BJ (2020). High-throughput DNA barcoding of oligochaetes for abundance-based indices to assess the biological quality of sediments in streams and lakes. Scientific reports 10(1):1-8.
Crossref
|
|
|
|
|
Zébazé TSH, Njine T, Kemka N, Niyitegeka D, Nola M, Foto Menbohan S, Djuikom E, Ajeagah G, Dumont HJ (2006). Biodiversity and spatial distribution of Rotifera in a shallow hypereutrophic tropical lake (Cameroun). Journal of the Cameroun Academy of Sciences 6(3):149-165.
|
|
|
|
|
Zeybek M, Ko?al ?S, Y?ld?z S (2018). The aquatic oligochaeta (Annelida) fauna of the Karasu stream. Limnofish 4(1):30-35.
|
|