ABSTRACT
A speciation study of selected heavy metals in soil and stream sediments around a hospital waste dumpsite was investigated. Sequential extraction procedure was used to fractionate the metals into 4 fractions: Exchangeable and bound to carbonate, oxyhydroxide of Fe and Mn, organic matter and the residual fraction. Speciation analysis of Fe, Mn, Zn, Cu, Cd and Pb in soil revealed that a major portion of the metals were associated with the residual fraction. The exchangeable and carbonate fraction was the most important fraction for Mn(Dry/Wet) and Zn(Dry/Wet) with an average of (27%, 35.6%) Mn and (20.8%, 27.3% ) Zn. Cd, Fe and Cu were associated with the oxyhydroxide of Fe and Mn fraction with an average of (61%, 44.2%) Cd(Dry/Wet), (41%, 21%) Fe(Dry/Wet) and (25.3%, 22.5%) Cu(Dry/Wet). A significant amount of Pb(Dry/Wet) (10.7%, 21.8%) was bound to organic fraction during dry and wet season respectively. In sediments, Mn(Dry/Wet) occurred mainly in exchangeable and carbonate fraction (45.2%, 29.3%), Fe(Dry/Wet) and Cd(Dry/Wet) in the oxyhydroxide of Fe and Mn fraction were (41%, 31.2%) and (26.6%, 93.6%) respectively and the amount of Pb(Dry/Wet) bound to the organic fraction was (86.9%, 27.3%). A significant amount of Cu(Dry/Wet) (20.8%, 29.4%) was bound to the organic fraction and Zn(Dry/Wet) (35.5%, 73%) was retained in the residual fraction. In soil and sediments, Mn was potentially more bioavailable. However, the level of lead in organic matter fraction of soil suggest that it may be remobilized under oxidizing condition, hence, bioavailable.
Keywords: Speciation, hospital waste, heavy metal, sediments, soil.
Hospital wastes are waste generated within the hospital environment which comprises of pharmaceutical, radioactive, general, sharp, laboratory waste and radioactive materials capable of causing infectious diseases resulting from patient investigation, treatment and diagnosis (Akter, 2000; Abor and Bower, 2008). Waste produced in the hospital and other health care facilities in developing countries have raised serious concerns due to the inappropriate treatment and final disposal practices accorded to them. In Nigeria, more disposed in open land area (Vivan et al., 2011). Open dumping of hospital waste is an important cause of natural environmental degradation and constitute a health hazard due to the release of heavy metals, chemical solvents, preservatives and infectious substances into the underlying soil and possibility of leachate entering an acquifer or nearby surface water (Akter, 2000).
Heavy metals are metals and metalloids with atomic density of 4 g/cm3, greater than that of water (Hutton and Symon, 1986, Garbarino et al., 1995). Heavy metals are also called trace elements because of their presence in trace amount in various environmental matrices. The essential heavy metals such as Cu, Fe, Cr, Se, Zn, Mn and Ni exert biochemical and physiological functions in plants and animals. They are important constituents of several key enzymes and play important roles in various oxidation-reduction reactions (WHO, 1996). However, toxic heavy metals are arsenic, cadmium, chromium, lead and mercury. Because of their high degree of toxicity, these five elements rank among the priority metals that are of great public health significance. They are all systemic toxicants that are known to induce multiple organ damage, even at lower levels of exposure. According to United State Environmental Protection Agency (USEPA) (2002), these metals are also classified as either “known” or “probable” human carcinogens based on epidemiological and experimental studies showing an association between exposure and cancer incidence in humans and animals. The bioavailability of heavy metals may be influenced by physical factors such as temperature, phase association, adsorption and sequestration. It is also affected by chemical factors that influence speciation at thermodynamic equilibrium, complexation kinetics, lipid solubility and water partition coefficients. Biological factors such as species characteristics, trophic interactions and biochemical/ physiological adaptation, also play an important role (Verkleji, 1993).
Over the years, heavy metals have been a threat to the environment as they can bioaccumulate to toxic level within an ecosystem and endanger its health. When agricultural lands are polluted with heavy metals, they are assimilated by plants (Trueby, 2003); animals feed on contaminated plants and accumulate the metals to toxic level which move quickly through the food chain, affecting the health of animals and humans (Peplow, 1999). Today, it is generally recognized that measurements of total metal concentration has the potential of grossly overestimating the risk (Ogunfowokan et al., 2009).
Despite high total concentration of metals in certain soil and sediments, the metals are not readily available for incorporation in the biota, hence associated environmental effects may be low (Marco et al., 2005).
Therefore, to estimate the effects and potential risks associated with elevated elemental concentrations that result from natural weathering or anthropogenic activities, information on the physicochemical forms: the mobility, pathway and bioavailability is determined by selective chemical speciation in soil and sediments (Kot and Namiesnik, 2000; Davis et al., 1994). Chemical speciation can be broadly defined as the identification and quantification of the different defined species, forms or phases in which an element occurs (Ure et al., 1993; Hu et al., 2003). Investigating the distribution and speciation of heavy metals in soil will not only provide information on the degree of pollution but the actual environmental impact as regards metal bioavailability and their origin.
Previous work done in the study area by Olabanji et al. (2015) on total metal concentration of the soil showed that the hospital waste dumpsite greatly contribute to the increase in concentration of elements in the soil. The pH shows that characteristics of a slightly acidic soil may enhance metal dissolution for soil uptake. Therefore, surface runoff from the hospital dumpsite and erosion from surrounding land areas may eventually increase the level of metals and other pollutants in the stream water and sediments. Hence, this study aimed at quantifying the amount of metal ion that are bioavailable in soil and sediments for uptake. Agricultural practices around the dumpsite and the presence of a nearby stream where run-off from the dumpsite is emptied necessitate this study.
General description of the study area
The area designed for this study was OAUTHC located in Ilesa East Local Government, Osun State. The sampling location is Lat 07 361 81911N and Long 004 441 96811 E with elevation of 387 m.
Sample collection and pre-treatment
The land area around the hospital dumpsite as shown in Figure 1 was divided into five segments, soil samples were collected randomly from each sampling point with a pre-cleaned hand driven auger. Sediment samples were collected from a stream located at110 m from the dumpsite with a stainless steel hand trowel. The samples were put in a polyethylene bags, brought back to the laboratory and air dried. The dry samples of soil and sediment were crushed with pre-cleaned porcelain mortar and pestle and passed through 2 mm mesh size sieve and then stored at room temperature prior to analysis (Sharma et al., 2009) (Figure 2).
Sterilization of apparatus
All glassware, polypropylene tubes were washed with liquid detergent, rinsed first with tap water and distilled water, and then soaked in 10% HNO3 (v/v) for 48 h. Then, they were re-washed with liquid detergent and rinsed thoroughly with doubly distilled water. Thereafter, the apparatus were oven dried for 12 h at a temperature of 80°C. All the reagents used were of analytical grade.
Sequential extraction procedure
The selective sequential extraction method used was based on the procedure used by Baffi et al. (1998), with improvements made according to the European Community Bureau of Reference (BCR 701), which examined and finally eliminated irreproducibility sources. The BCR procedure is made up of three steps, which dissolve the following phases of metals, respectively: exchangeable and bound to carbonate, bound to Fe and Mn oxides and hydroxides, bound to organic matter and sulphides (Ure et al., 1993).
Exchangeable and bound to carbonate phase (phase 1) was extracted with 0.11 M acetic acid, while the fraction bound to Fe-Mn oxides (phase 2) with 0.5 M hydroxylamine hydrochloride, was adjusted to pH 2 with nitric acid (65%). The fraction bound to organic matter and sulphides (phase 3) was extracted with 8.8 M hydrogen peroxide. The metal content of the residual phase was obtained from the difference between the total content and the sum of phases 1, 2 and 3, according to Ianni et al. (2001) and Mester et al. (1998). After each extraction, the samples were centrifuged at 4000 rpm for 20 min by placing the samples in Teflon centrifuge tubes, followed by decantation and filtration. Deionized water was used to wash the residues after subsequent extractions in order to ensure selective dissolution and avoid possible interference from interphase mixing between the supernatants. All samples were run in triplicates.
Quality control measures
Blank determination
Together with each determination was a blank prepared to check for background levels of Fe, Mn, Cu, Zn, Cd and Pb in the reagents used for the various leaching procedure. Blank correction was carried out in the result of metal determination.
Table 1a to f present the mean percent distribution of particular speciation forms of the metals in the stream sediments. Manganese in sediments was found mainly in the exchangeable and carbonate fraction, which is the bioavailable form (45.2 and 29.3%) and the residual fraction (27.9 and 54%) during dry and wet seasons, respectively. In a smaller amount, Mn was bound to the hydrated iron and manganese oxide fraction (16.8 and 14.3%); only 8.9 and 2.4% was found in the organic matter fraction during dry and wet seasons, respectively.
Iron in the sediments was mainly found in the hydrated iron and manganese oxide fractions (41 and 31.2%) and the residual fraction (24.4 and 40.9%) during the dry and wet seasons, respectively. The fraction of iron in the exchangeable and carbonate form was smaller (10.1 and 25.8%) and in the organic matter fraction, it was 20.6 and 2.1% during the dry and wet seasons, respectively.
Zinc in the sediments was mostly retained in the residual fraction (35.5 and 73%), lesser amount of zinc was found in the organic matter fraction (39 and 9.8%), exchangeable and carbonate fraction (21 and 5.6%) was found in the hydrated iron and manganese oxide fraction (4.5 and 11.8%) during the dry and wet seasons, respectively. During the wet season, large amount of zinc is occluded in the residual fraction, which may be responsible for low amount found in the other mobile fractions.
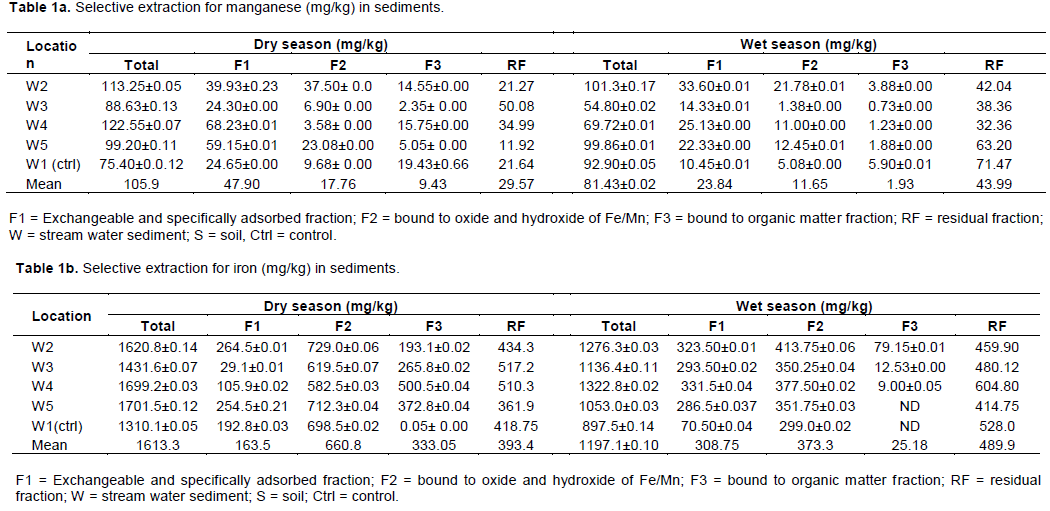
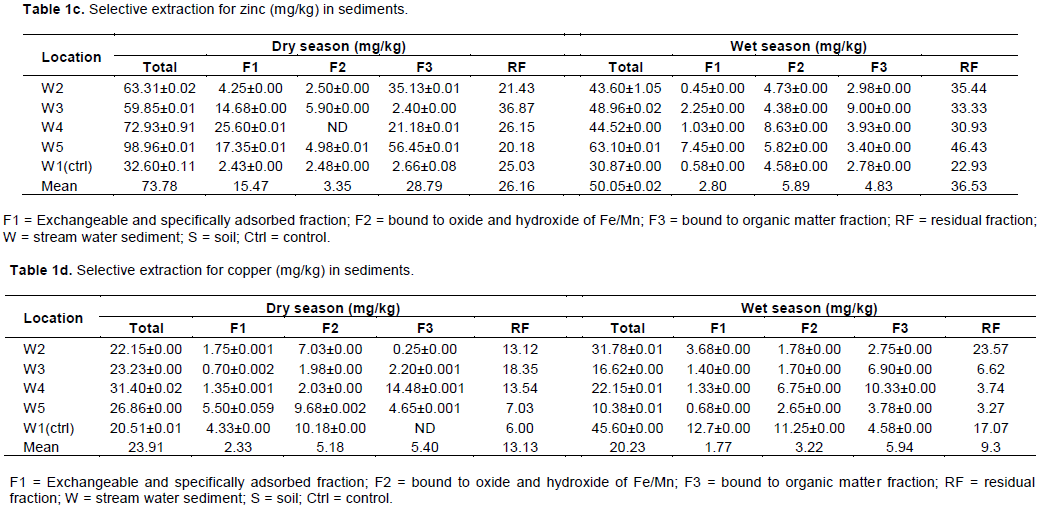
A similar distribution of zinc in the chemical fraction was found in copper, as it occurs in large amount in the residual fraction (50.6 and 46%), lesser amount were bound to the organic matter fraction (20.8 and 29.4%), hydrated iron and manganese oxide fraction (20 and 15.9%) in small amount, and copper was bound to the exchangeable and carbonate fraction (9 and 8.8%) during dry and wet seasons, respectively. Although, a large percentage of copper was retained in the residual fraction, a significant amount was found in z fraction III, the oxidizable fraction. This may be because Cu has high affinity for organic matter, which indicates that Cu is associated with strong organic legends (Qiao et al., 2003).
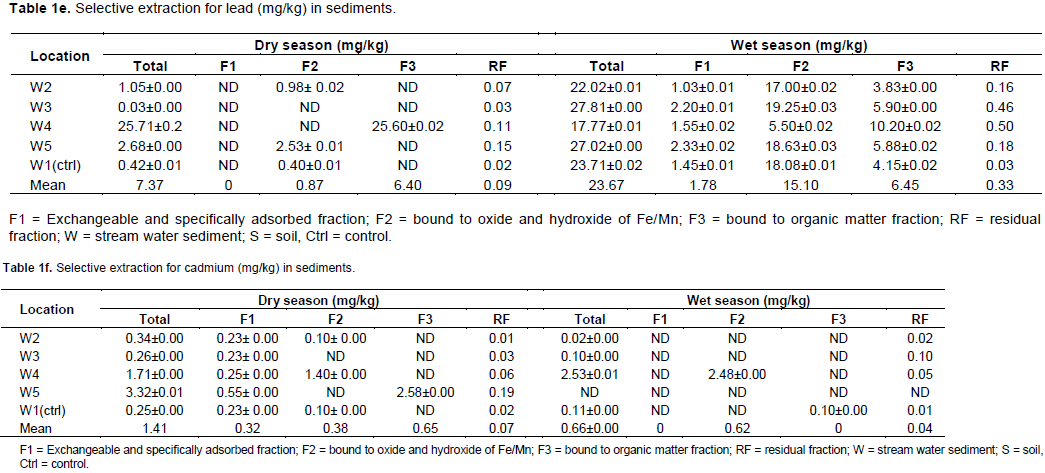
Lead in the sediments was not found in the exchangeable and carbonate fraction, probably, due to minimal concentration of lead extracted in this fraction which is below the detection limit of the instrument. Large amount of Pb was found only in location 3 in the organic matter fraction (86.9%) and in the form bound to hydrated iron and manganese oxide fraction (11.9%) during dry season. However, there is high percentage extraction in all the fractions during the wet season where lead was mainly found in the hydrated iron and manganese oxide fraction (63.8%), and a significant amount of Pb occurred in the organic matter fractions (27.3%) which may be mobilized and released to bulk water under oxidizable condition. Only 7.5% lead was found in the exchangeable and carbonate fraction. Lead in low amounts was determined in the residual fraction (1.22 and 1.4%) during dry and wet season, respectively.
Cadmium in the sediments during dry season occurred mainly in the organic matter fraction (45.8%). Smaller amount was found in the hydrated iron and manganese oxide fraction (26.6%) and in the exchangeable and carbonate fraction (22.4%). During the wet season, a major fraction of cadmium was found in the hydrated iron and manganese oxide form (93.6%). It was below detection limit in the exchangeable and carbonate fractions, and in the organic matter fraction. Like lead, cadmium in low amounts was also found in the residual fraction (5.15 and 6.42%) during the dry and wet seasons, respectively. The low amount of Cd which is below the detection limit in the exchangeable and carbonate fractions and in the organic matter fraction suggests loss of labile metal fraction due to dissolution by acid rain.
The results of sequential extraction of metals in soil are shown in Table 2a to f. In the soil, manganese was sporadically distributed in the hydrated iron and manganese oxide fraction (48.5 and 23.4%), exchangeable and carbonate fraction (27 and 35.6%) and in the residual fraction (23 and 38.6%), and in small amount, manganese was bound to the organic matter fraction (1.4 and 2.4%) during the dry and wet seasons, respectively.
Iron in soil, was mainly found in the hydrated iron and manganese oxide fraction (41 and 20.5%) and in the residual fraction (15.8 and 60.8%) during the dry and wet seasons, respectively. Iron occurred in the organic matter fraction (37.3%) during the dry season only. In smaller amount, it was found in the exchangeable and carbonate fraction (6.2 and 18.8%) during the dry and wet seasons, respectively.
Zinc was mainly retained in the residual fraction (53 and 56.7%), and in smaller amount, it occurred in the exchangeable and carbonate fraction (20.8 and 27%) and hydrated iron and manganese oxide fraction (18.4 and 18%). Only 7.9 and 12.9% of zinc was bound to the organic matter fraction during the dry and wet seasons, respectively.
Copper was mainly found in the residual fraction (54.6 and 48.6%) and in the form bound to hydrated iron and manganese oxide fractions (25.3 and 22.5%) during the dry and wet seasons, respectively. In small amount, copper was bound to organic matter fraction (8.8 and 26%) and in exchangeable and carbonate fraction (5.6 and 2.9%) during the dry and wet seasons, respectively. The high amount of copper in the organic matter fraction may be attributed to the ease of complexation and peptisation products formed between the metal and natural organic matters like humic and fulvic acids. This suggests it high affinity for organic compounds.
Lead in the soil was mostly found in the hydrated iron and manganese oxide fraction (50.9 and 35%) and the residual fraction (37.3 and 43.7%), smaller amount of lead was bound to organic matter (10.7 and 11.7%) during the dry and wet seasons, respectively. 9.6% lead occurred in the exchangeable and carbonate fractions during the wet season, while Pb was below the detection limit during the dry season. As a result, Pb was immobilized in the sediment.
Cadmium in soil was found mostly in the hydrated iron and manganese oxide fraction (61%) and in exchangeable and carbonate fraction (20.5%), and small amount of it was determined in the organic matter fraction (9.4%) and residual fraction (9.1%) during the dry season. In the wet season, cadmium occurred in large amount in the hydrated iron and manganese oxide fraction (54.2%) and the organic matter fraction (33.3%); 12.5% Cd was bound to the residual fraction while it was below detection limit in the exchangeable and carbonate fraction.
The mobile and bioavailable form of metals for uptake in soil and sediments are the exchangeable- carbonate and the organic matter fractions, the release is largely influenced by the pH. Lead and cadmium among other metals determined in this study are toxic metals. The high levels found in the exchangeable carbonate and organic matter fractions which are the bioavailable form of the metals, suggest that the metals could be released and bioaccumulated overtime in the ecosystem. The health hazards of lead include renal failure, impairment of hearing ability, increased frequency of miscarriages and still births, interference with red blood cells and delayed or impaired neurobehavioral development in children (Goyer et al., 1991). Cadmium exposure may lead to chronic kidney stones, severe osteoporosis and osteomalacia with simultaneous severe renal dysfunction (ATSDR, 1999).
Generally, high percentage of metals in the residual and Fe-Mn oxide fractions of the soil and sediment was probably due to the high association or retention ability of the mineral crystal structure, such as with the dendritic silicates and resistant sulphides.
Speciation study of metals has been investigated in soil and sediments. The distribution of the studied metals in the various fractions confirms their differences in bioavailability. There are indications that manganese among other metals occurred mainly in the exchangeable and carbonate forms. Therefore, it can be released to bulk water as a result of changes in ionic composition or decrease in pH of the medium. Iron, cadmium and lead were mostly bound to the hydrated iron and manganese oxide form, organic matter fraction and the residual fraction. Copper in significant amount were found in the organic matter fraction, which indicate the high affinity of copper to form organic compounds. The significant amount of lead and cadmium in the organic matter fraction suggest and that it may be released during decomposition or oxidation of organic matter under oxygen rich condition. Overtime, these metals may be released into the ecosystem, enter the food chain and bioaccumulate to toxic level in man. A similar distribution pattern of the metals in the chemical fraction in soil and sediments indicates that the dumpsite impact the environment negatively. Hence, continuous monitoring of the metals in this study area and similar site is important. Also, proper hospital waste disposal should be enforced for environmental safety.
The authors have not declared any conflict of interests.
REFERENCES
|
Akter N (2000). Medical waste management: A review. Environmental Engineering Program, School of Environment, Resources and Development Asian Institute of Technology, Thailand pp. 1-25.
|
|
|
|
Abor PS, Bouwer A (2008). Medical waste management practices in a Southern African Hospital. International Journal of Health Care Quality Assurance 21(49):356-364.
Crossref
|
|
|
|
|
Baffi F, Ianni C, Soggia F, Magi E (1998). Evaluation of the acetate buffer attack of a sequential extraction scheme for marine particulate metal speciation studies by scanning electron microscopy with energy dispersive x-ray analysis. Analytica Chimica Acta 360:27-34.
Crossref
|
|
|
|
|
Davis A, Ruby MV, Bergstrom PD (1994). Factors controlling lead bioavailability in the Butte Mining District, Montana, USA. Environmental Geochemistry and Health 16(3):147-157.
Crossref
|
|
|
|
|
Garbarino JR, Hayes H, Roth D, Antweider R, Brinton TI, Taylor H (1995). Contaminants in the Mississippi River US Geological survey circular 1133. Virginia USA.
|
|
|
|
|
Hu C, Chao M, Wu K, Chang-Chien G, Lee W, Chang L, Lee WS (2003). Characterization of Multiple Airborne Particulate Metals in the Surrounding of a Waste Incinerator in Taiwan. Atmospheric Environment37:2845-2852.
Crossref
|
|
|
|
|
Hutton M, Symon C (1986). The Quantities of Cadmium, Lead, Mercury and Arsenic Entering the U.K. Environment from Human Activities. Science of the Total Environment 57:129-150.
Crossref
|
|
|
|
|
Ianni C, Ruggieri N, Rivaro P, Frache R (2001). Evaluation and comparison of two selective extraction procedures for heavy metal speciation in sediments. Analytical Sciences. 17:1273-1278.
Crossref
|
|
|
|
|
Kot A, Namiesnik J (2000). The role of speciation in analytical chemistry. TrAC Trends in Analytical Chemistry19:69-79.
Crossref
|
|
|
|
|
Marco R, Serena M, Roberto F, Juan AC (2005). Metal speciation and environmental impact on sandy beaches due to El Salvador copper mine, Chile. Marine Pollution Bulletin 50:62-72.
Crossref
|
|
|
|
|
Mester Z, Cremisini C, Ghiara E, Morabito R (1998). Comparison of Two Sequential Extraction Procedures for Metal Fractionation in Sediment Samples. Analytica Chimica Acta. 359:133-142.
Crossref
|
|
|
|
|
Goyer RA (1991). Toxic effects of metals. In: Cassarett and Doull's Toxicology. The basic science of poisons. 3rd ed. McGraw-Hill, New York.
|
|
|
|
|
Ogunfowokan AO, Oyekunle JAO, Durosinmi LM, Akinjokun AI, Gabriel OD (2009). Speciation study of lead and manganese in roadside dusts from major roads in Ile- Ife, South Western, Nigeria. Chemistry and Ecology 25(6):405-415.
Crossref
|
|
|
|
|
Olabanji IO, Oluyemi EA, Fakoya TO, Eludoyin AO, Makinde WO, (2015). Effects of waste disposal system on metal composition of hospital waste dumpsite in Ilesa, Southwestern Nigeria. Journal of International Environmental Application & Science 10(3):319.
|
|
|
|
|
Peplow D (1999). Environmental Impacts of Mining in Eastern Washington, Centre for Water and Watershed Studies Fact Sheet, University of Washington, Seattle.
|
|
|
|
|
Qiao XL, Juo YM, Christy P, Wong MH (2003). Chemical Speciation and Extractability of Zn, Cu and Cd. In: Two Contrasting Biosolids-Amended Clay Soils. Chemosphere 50:823.
Crossref
|
|
|
|
|
Sharma RK, Madhoolika A, Fiona M (2009). Heavy metals in vegetables collected from production and market sites of a tropical urban area of India. Food and Chemical Toxicology. 47:583-591.
Crossref
|
|
|
|
|
Trueby P (2003). Impact of Heavy Metals on Forest Trees from Mining Areas. In: International Conference on Mining and the Environment in Sudbury, Ontario, Canada.
|
|
|
|
|
Ure AM, Quevauviller PH, Muntau H, Griepink B (1993). Speciation of Heavy Metals in Soils and Sediments. An Account of the Improvement and Harmonization of Extraction Techniques Undertaken under Auspices of the BCR of the Commission of the European Communities. International Journal of Environmental Analytical Chemistry 51:135.
Crossref
|
|
|
|
|
United State Environmental Protection Agency (USEPA) (2002). Supplemental guidance for developing soil screening levels for superfund site. Office of Solid Waste and Emergency Response, Washington, D.C.
|
|
|
|
|
Verkleji JAS (1993). The effects of heavy metals stress on higher plants and their use as biomonitors In Plant: Indicators of Heavy Metals in the Terrestrial Environment. Markert, B., editor. New York pp. 415-424.
|
|
|
|
|
Vivan EL, Blamah NV, Ezemokwe I, Okafor CI (2011). An assessment of hospital waste management in some selected hospitals in Zonkwa District of Zangon- Kataf Local Government Area of Kaduna State. International Journal of Environmental Studies 1(2):23-28.
|
|
|
|
|
World Health Organization (WHO) (1996). World Health Organization. Trace Elements in Human Nutrition and Health. Switzerland, Geneva.
|
|