ABSTRACT
This study was conducted to assess the capacity of mangroves soils to stock carbon and how degradation can influence its various properties. Transect method was performed. So, two transects of 100 m length and 10 m wide were established according to the degradation level. Total of 18 Soil samples were taken to be described and analysed. The degraded transect (T1) shows a mean carbon stock value of 2102.06 ± 405 Mg.ha-1 while natural (T2) accumulate 2476.6 ± 409 Mg.ha-1. Colour are more light inside the degraded transect (Brown to grayish) than the natural transect (brown to blackish) while spots are more colored in natural transect (gray and yellow) than degraded one (yellow). pH mean value showed that soils of degraded transect was more acidic than those of natural one. Organic matter amount was very high and proves that these soils can be valorized to agricultural activities without previous enrichment. Total Nitrogen was low in the two transects while the available phosphorus values showed that natural transect has more available phosphorus that can be used by the plants than degraded transect. So, degradation would take along reduction of available phosphorus rate in the soils. According to this result showing important different values of carbon stock and soils properties between natural and degraded transect, it is necessary to implement conservation methods in order to stop degradation and enhance capacity of mangroves soils properties.
Key words: Bamusso, degraded transect, natural transect, soils, values.
Along Cameroon’s coast, mangrove swamps are estimated to cover a surface area of roughly 1961.84 km2 (Spalding et al., 2010). They are mainly distributed between two estuaries (Rio del Rey Estuary and Cameroon Estuary) but are equally present in small zones with variable surface areas in the South Region of Cameroon (Rio Ntem Estuary).
Mangrove swamps for some decades now have been recognized as having public interest. Undeniably, their benefits are both economical (Kovacs, 1999) and ecological. Ecotone between land and marine environment, the ecosystem of mangrove swamps is a very unique structural and functional zone (Betoulle, 1998), playing a role in coastal food chains (Dittmar et al., 2001; Laedgaard and Johnson, 2001 ) and constituting an important source of carbon (Twilley et al., 1992; Gattuso et al., 1998). Most recent studies on mangrove swamps have proven the ability of this ecosystem to avert the phenomenon of climate change by sequestrating the carbon of these various components (Trevor et al., 2014; Adame et al, 2013; Donato et al, 2012; Lovelock et al, 2011). Moreover, mangrove swamps play a barrier role against natural disasters. This is the way they safeguard the surrounding populations against damage which can be caused by cyclones, tsunamis and hurricanes (Dahdouh-Guebas, 2006; Alongi, 2008). They also produce goods and services as well as income to the community (Krauss et al., 2008).
Mangrove swamps also constitute an important source of revenue and several other survival and commercial activities such as fishing and wood exploitation are carried out there (Din et al., 2006). However, several studies show that approximately 5 to 85% of the original area of mangrove swamps was predominantly lost during the second half of the 20th century due to degradation and deforestation.
Degradation of ecosystems is qualified as reduction of the quality of factors like forest crown, fauna, soils or carbon stock loss. Associate to the deforestation, degradation constitute about 18% of anthropogenic emission of CO2 through the forest sector (IPCC, 2007). The recent estimation suggest that 9.4% of Amazonian forest at Brazil have been loss and about 16% within Congo Basin forest (Bernou et al., 1999).
The mangrove swamps of Africa endure enormous pressures during the last decades, to the point that in west and central Africa, 20 to 30% of mangrove swamps have vanished in 25 years (UNEP, 2007). This is due to several factors and in particular is urbanization through the development of infrastructures and residences, the exploitation of salt mines and sand, pollution caused by industries, the industrial agrochemical products and the exploitation of oil and gas, the absence of an appropriate legislation, the cutting down of trees for fish smoking (Ajonina and Usongo, 2001; Ajonina et al., 2005), the increase of insidious species and the effects of climate change, amplified by population growth.
Nowadays, Cameroon hydrology and mangrove soils properties are highly disturbed due to vegetation degradation. Deforestation release not only carbon in atmosphere but has also negative effects on biodiversity, soils protection and local climate regulation. More also, use land for urbanization and agriculture activities contribute gradually to release soils and plants carbon stock (Michalak et al., 2011).
In view of the above observations the major objective of the present study is to assess how degradation of vegetation influences carbon stock and soils parameters within Bamusso mangrove's forest.
Study site
The study was carried out in the mangrove forest of the locality of Bamusso (Rio del Rey Estuary). Being the sub-divisional capital, Bamusso is a peninsula located in the Ndian Division of the South West Region. Bordered to the South and the West by the Atlantic Ocean, to the North by Ekondo Titi and to the East by Meme and Fako Divisions (Figure 1), this locality of geographical coordinates latitude 4° 45 ' - 4° 50 ' North and longitude 8° 30 ' - 9° 00 ' East has a vegetation of evergreen forest subjugated by the mangrove swamp and intermittent by a shrubby meadow (Ajonina, 2011). This mangrove forest conquered by the family of Rhizophoraceae is cut apart by a large number of waterways for fishermen, poachers and tourists.
The climate of the region is influenced by the propinquity of the Atlantic Ocean and Mount Cameroon on one hand, and on the other hand, by the Intertropical Convergence Zone where the anticyclone of the Azores of the Northern hemisphere and that of Saint Helena coming from the South converge (Din, 2001). This climate fits into the equatorial domain of littoral type or "Cameroonian" which is characterized by two seasons with a long rainy season (from March to November) that can totally expunge the dry season always interspersed with rains. Bamusso shows annual average precipitation of the order of 3800 mm. The sociological component is made up of non-native and natives. While the natives consider mangrove swamps as sacred sites dedicated to diverse rites, the non-natives use them for construction, fish smoking and to a lesser extent for farming.
Methods
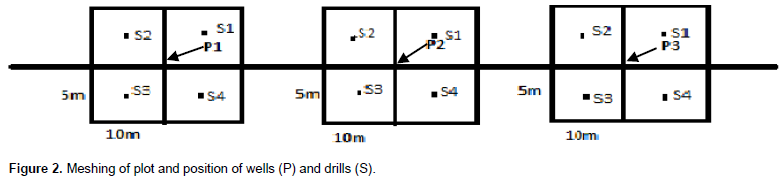
The sampling method is that of transects (Imbert, 1985). Two transects of 100 m length and 10 m wide giving a surface area of 1000 m2 each were established one named transect 1 or T1 inside degraded mangrove forest and another (transect 2 or T2) in natural or non disturbed mangrove forest. They were 5 km apart with base lines directed WSW-ENE and perpendicular to the main channel. Each transect was split into three plots of 20 m x 10 m and each division was further subdivided into four meshes of 10 m x 5 m using systematic approach (Ndema et al., 2008). One manual drill was established in the middle of each mesh and one well (30 cm diameter and one meter depth) in the middle of each plot. Totally, three wells and twelve manual drills (four per plot) were established within each transect (Figure 2). Morphological characteristics of soils were determinate during digging process of drills and wells by observation of soils cuttings and monoliths (Figure 3A and B). So, colour of horizon was determined by using Munsell soils colour chart, structure by soil’s aggregate observation, texture by hand wetted soils touching and organic elements as dead leaf, roots and spots by direct observation.

Analysis of soils samples
Soil samples were taken from six different wells (three per transect) at three depths intervals (0 - 30, 30 - 50 and 50-100 cm). Eighteen composite samples prepared from 0 – 30 cm (six samples), 30 - 50cm (six samples) and 50-100 cm (six samples) depths were analyzed in the laboratory. The parameters analysed included (i) particle size distribution by the pipetting method of Robinson (Schalk, 1988), (ii) bulk density by using a peat auger consisting of a semi-cylindrical chamber as described by Yoro and Godo (1990), (iii) soil pH was measured by reading method through field multi parameters conductive meter (Anderson, 1999), (iv) organic carbon was measured by the procedure of Walkley and Black (1934), (v) exchangeable potassium, sodium, calcium and magnesium of the soil using the procedure of Jackson (1958), (vi) total nitrogen content by the method of Kjeldahl (Bremner, 1996), (vii) available phosphorus by the method of proportioning colorimetric starting from the nitrochlorhydric solution of ashes (Stuffins, 1967), (viii) cationic exchange capacity (CEC) by colorimetric using spectrophotometer (Jackson, 1965) while carbon stock was determinate by the formula C (kg/m2) = C (mg.g-1 Sol)*Da*e (Da=Bulk density, e=thickness of horizon).
Data analyzes
The collected data were statistically handled and analyzed by two approaches: the descriptive and the inferential statistics. The descriptive statistical analysis consisted in arranging the data in EXCEL spreadsheet, and in obtaining the results in the form of tables and graphs. The inferential statistical analysis was made by means of the SPSS software package; and the latter served to determine if there is a significant difference between the soils characteristics of the different transects using one ANOVA test.
Effects of degradation on soil carbon stock
The degraded transect (T1) shows a mean carbon stock value of 2102.06 ± 405 Mg.ha-1 while natural or non disturbed transect (T2) accumulate 2476.6 ± 409 Mg.ha-1 (Table 1). Following depth, in surface (0-30 cm), the carbon stock is 2006.8 ± 199.07 Mg.ha-1 inside degraded transect (T1) while it is 2308.6 ± 503.9 Mg.ha-1 in natural transect (T2). In middle depth, (30-50 cm), the degraded transect shows value of 1375.4 ± 105.71 Mg.ha-1 against 1346.6 ± 139.74 Mg.ha-1 for the natural transect. The stock of carbon in depth horizons is 2954 ± 944.77 Mg.ha-1 in T1 and 3774.6 ± 528.59 Mg.ha-1 in T2.
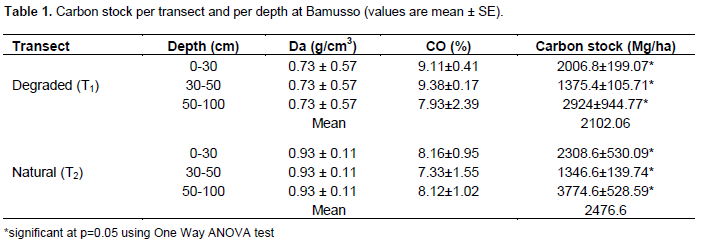
Carbon stock present high value within natural transect than degraded one. So, degradation contribute to reduce carbon stock in the mangroves soils. This carbon is released on atmosphere and enhances climate change phenomena. At Bamusso, degradation would be responsible of release of 374.54 Mg.ha-1 of carbon.
Effects of degradation on morphological properties of soils
The degraded transect showed globally clayey to silty texture, doughy to fibrous structure, brown to grayish color and abundant thin roots, yellows spots as well as decomposed organic matter (Figure 4A, B and C). Within natural transect the texture was clayey to clayey-silty, with a doughy to massive structure, a brown to blackish color and medium roots, yellowish to grayish spots as well as fresh and decomposed organic matter (Figure 5A, B and C). Organic elements are similar between the two transect but the texture is more fine in the natural transect (clayey to clayey-silty) than in the degraded one (clayey to silty). That can be explain by the high microbiological activity that transform macromolecular to fine particles within the natural transect. Also the importance of leaching phenomenon in the degraded transect can explain his less fine texture (Duchaufour, 2001). Color also are more light inside the degraded transect (Brown to grayish) than the natural transect (brown to blackish) while spots are more colored in natural transect (gray and yellow) than degraded one (yellow) as in Abata (1994) study inside the Wouri mangroves. We can conclude that degradation change texture from fine to coarse and color from dark to light. Yellows and grays spot explain the temporary hydromorphy phenomenon of soils within the two transects (Ondo, 2006).
These morphological characteristics are more near to the one described by Baltzer (1995) and Abata (1994) than those of Ondo (2006) (Table 2).
Effects of degradation on physical and chemical characteristics of soils
Particle size shows in mean 45 ± 5.5% of sand, 30 ± 5.5% of silt and 25 ± 0.0001% of clay within degraded transect(T1) against 44 ± 3.3% of sand, 36 ± 0.01% of silt and 20 ± 3.3% of clay in natural transect (T2). pH water means values are 2.25 ± 0.33 in degraded transect and 2.43 ± 0.52 in natural transect. Those of pHKCl are 2.08 ±0.001 in T1 and 2.3 ± 0.52 in T2.
Organic carbon (CO) was 8.81 ± 1.48% inside degraded transect and 7.87 ± 1.22% in natural transect when organic matter (MO) was 15.15 ± 2.26% in T1 and 13.53 ± 2.06% in T2. Total nitrogen (Ntot) was 0.20 ± 0.01% in degraded transect and 0.16 ± 0.01% in natural one. Available phosphorus (Pass) was 13.7 ± 3.57 mg/kg in degraded transect and 17.86 ± 3.57 mg/kg in natural transect. Exchangeable bases were 5.08 ± 2.57 cmol/kg (T1) and 2.26 ± 0.66 cmol/kg (T2) for calcium (Ca) against 4.43 ± 1.81 cmol/kg (T1) and 0.99 ± 0.7 cmol/kg(T2) for magnesium. Cation exchangeable capacity (CEC) was 21.25 ± 7.21 cmol/kg in T1 and 6.66 ± 0.97 cmol/kg in T2 (Table 3).
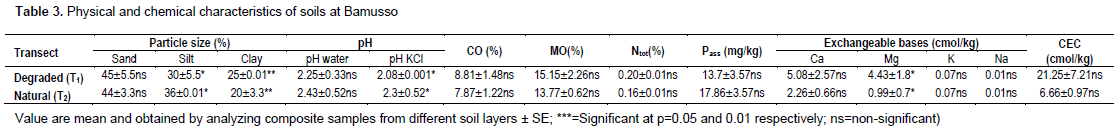
We notice slight increase of sandy particle inside degraded transect (T1). So, degradation of vegetation take along soil's destitution that promote erosion and leaching phenomenon what reduce fine particles within this transect (T1). pH mean value showed that soils of degraded transect was more acid than those of natural one. Degradation contributes to increase acidification of mangroves soils. All the pH values of soils were less than 4 means that they would be qualified as high acid soils (Landon, 1984).
The low value of CO in natural transect (T2) illustrate a higher biologic activity characterized by rapid mineralization of organic carbon in this transect. Degradation would slow down mineralization process of organic carbon. According to Metson (1961), the mangroves soils at Bamusso have a medium carbon rate because the percentage of their organic carbon is between 4 and 10. Organic matter progress to the same direction than organic carbon because the two characteristics are linked by the linear relationship (MO=1.72*CO). Organic matter has a very high rate (Landon, 1984). These soils can be valorized to agricultural activities without previous enrichment because their organic matter rate is widely above to organic matter critical value for agriculture what is 2%.
Total Nitrogen was low (Landon, 1984) in the two transects while the available phosphorus values showed that natural transect has more available phosphorus that can be used by the plants than degraded transect. So, degradation would take along reduction of available phosphorus rate in the soils. Exchangeable bases showed high values of calcium and low values of sodium. Their concentration respect bases leaching order (Etame, 2004) which is: Na<K<Mg<Ca. High level of calcium and magnesium would increase cultural capacities of soils and assimilation of certain elements as phosphorus (Cantin, 2004). Low values of sodium and potassium could be explained by leaching because the two bases are highly soluble. According to Landon (1984), calcium is low to the natural transect while it is medium in degraded transect. Magnesium is medium in natural transect and high in degraded transect. Sodium and potassium have low values in the two transects. Cationic exchangeable capacity showed low values in natural transect and medium one in degraded transects (Table 4).
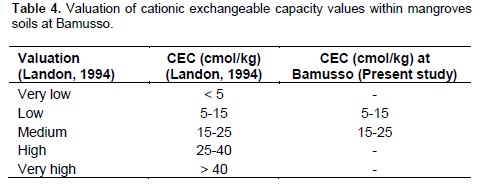
The study of effect of degradation on carbon stock and properties of soils within mangroves forest of Rio Del Rey Estuary (Bamusso) showed that carbon stock of soils samples can be highly different between degraded and natural transect. Also, this stock of carbon are higher than those of another mangroves forest and five times higher than stock of another non mangroves forest.
Total of 18 soils samples are collected inside six wells (three per transect) trough three depth intervals (0-30, 30-50, 50-100 cm) and analyzed. We noticed that degradation of vegetation would reduce fine particles within soils and contribute to their acidification. Degradation would also slow down mineralization process of organic carbon because values of organic carbon are low in natural transect than degraded one. Available phosphorus was more high in natural transect than degraded one while cationic exchangeable capacity was low in natural transect and medium in degraded transect showing that degradation would take along reduction of available phosphorus rate and increase CEC in the mangroves soils.
This study shows globally low value of carbon stock and soils properties inside degraded transect than natural one. It is important to apply conservation measures to stop vegetation's degradation what it is necessary to preserve soils characteristic potential.
Similar studies are to be encouraged in other localities of Rio del Rey as well as other mangrove swamps sites. If these studies could demonstrate the high values of soils carbon stock as it is the case with the present study, the soils of mangrove swamps of Cameroon could play a preeminent role to limit the phenomenon of climate change by sequestrating atmospheric carbon.
The authors have not declared any conflict of interests.
The logistical support provided by the CWCS NGO (Cameroon Wildlife Conservation Society), and the soil laboratory staff of Faculty of Agronomy and Agricultural Sciences (FASA) of University of DSCHANG is highly appreciated.
REFERENCES
|
Abata T (1994). Mangrove of Wouri Estuary (Douala-Cameroon). Study of microtopography and morphological characterization of soils. DIPES II Thesis, University of Yaounde I, 49p + annexe.
|
|
|
|
Adame MF, Kauffman JB, Medina I, Gamboa JN, Torres O (2013). Carbon stocks of tropical coastal wetlands within the Karstic Landscape of the Mexican Caribbean. PLoS ONE 8(2):e56569.
Crossref
|
|
|
|
|
Ajonina GN, Jin E, Mekongo F, Ayissi I, Usongo L (2005). Gender roles and economics of exploitation, processing and marketing of bivalves and impacts on forest resources in the Douala-Edaa Wildlife Reserve, Cameroon. Int. J. Sustainable Dev. World Ecol. 12(2005):161- 172.
Crossref
|
|
|
|
|
Ajonina GN, Usongo L (2001). Preliminary Quantitative impact assessment of wood extraction on the mangroves of Douala-Edéa forest reserve, Cameroun. Trop. Biodiv. 7(2)3:137-149.
|
|
|
|
|
Alongi DM (2008). Mangrove forests: Resilience, protection from tsunamis, and responses to global climate change. Estuarine Coastal Shelf Sci. 76:1-13.
Crossref
|
|
|
|
|
Anderson JM (1999). Tropical soil, biology and fertility hand book methods. 221 p.
|
|
|
|
|
Baltzer F, Rudant JP, Tonye E (1995). Applications of microwaves and C tape teledetection to mangroves cartography of Douala region (Cameroon). Proceedings of the second ERS applications workshop, London, UK.
|
|
|
|
|
Bernou M, Arrouays D, Cerri C, de AlencastroGraça PM, Volkoff B, Trichet J (1999). Assessment of mangroves soils carbon stock in Rondônia (Amazonie Brazilia). Study and Soils Manage. 5:31-42.
|
|
|
|
|
Betoulle JL (1998). Spatio-temporal variation of liter productivity on mangroves of Guyane. Doctorat Thesis. University of Paul Sabatier, Toulouse. 171 p.
|
|
|
|
|
Bremner JM (1996). Nitrogen-total. In: Methods of soil analysis. Part 3. Chemical methods. No.5. (ed. D.L. Sparks), pp. 1085-1121. ASA and SSSA, Madison, WI.
|
|
|
|
|
Cantin J (2004). Effects of mineral fertilizer in the maize farming. Guide manual. 19 p.
|
|
|
|
|
Dahdouh-Guebas F (2006). Mangrove forests and tsunami protection. In : McGraw-Hill (éd). Yearbook of Science & Technology, New York, USA. pp. 187-191.
|
|
|
|
|
Din N, Puig H, Blasco F (2006). Exploitation of woods within Douala mangrove (Cameroon). Ann. Fac. Sci. Univ. Yde I, série Sc. Nat. Vie 36(3):89-103.
|
|
|
|
|
Dittmar T, Lra RJ, Kattner G (2001). River or mangrove? Tracing major organic matter sources in tropical Brazilian coastal waters. Marine Chemistry 73:253-271.
Crossref
|
|
|
|
|
Donato DC, Kauffman JB (2012). Protocols for the measurement, monitoring and reporting of structure, biomass and carbon stocks in mangrove forests. Working Paper 86. CIFOR, Bogor, Indonesia.
|
|
|
|
|
Duchaufour P (2001). Introduction to the soils sciences : Soils, vegetations, environment, Dunod, Paris. 331 p.
|
|
|
|
|
Etame J (2004). Nephelinit rock weathering in the Etinde mount (Cameroon). Thesis, Univ Yde I, 221 p.
|
|
|
|
|
Gattuso JP, Frankignoulle M, Wollast R (1998). Carbon and carbonate metabolism in coastal aquatic ecosystems. Annu. Rev 12:405-433.
Crossref
|
|
|
|
|
Imbert D (1985). Spatio-temporal organisation of vegetal communities within the Great Cul-de- sac marin mangroves (Guadeloupe). University of Montpellier II, Thesis, 132 p + annexes.
|
|
|
|
|
IPCC (2007). Climate Change 2007 R Mitigation of Climate Change - Contribution of Working Group III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. In. Cambridge University Press, Cambridge, Schlensinger 851 p.
|
|
|
|
|
Jackson MI (1965). Chemicals soils Analysis methods applied to exchangeable bases and cationic exchange capacity. Prentice Hall Inc. 112 p.
|
|
|
|
|
Jackson MI (1958). Soils chemical analysis. Prentice Hall Inc. 121 p.
|
|
|
|
|
Kovacs JM (1999). Assessing mangrove use at the local scale. Landscape and Urban Planning 43:207-208.
Crossref
|
|
|
|
|
Krauss KW, Lovelock CE, McKee KL, Lopez-Hoffman L, Ewe SM, Sousa WP (2008). Environmental drivers in mangrove establishment and early development. Aquat. Bot. 89(2): 105-127.
Crossref
|
|
|
|
|
Laegdsgaard P, Johnson C (2001). Why do juvenile fish utilise mangrove habitats? J. Exp. Marine Biol. Ecol. 257:229-253.
Crossref
|
|
|
|
|
Landon JR (1984). Rangement of soils parameters values. Oxon, UK: Booker Tate Limited; Harlow, Essex, UK: Longman, 131 p.
|
|