Full Length Research Paper
ABSTRACT
Suaeda salsa is a genus of Salicaria in the Chenopodiaceae family. S. salsa is mainly distributed in coastal areas, growing in saline-alkali soil. Flavonoids are found in Salicyphala salicyphala and have various functions, such as lowering blood glucose, resisting mutagenesis and improving immunity. It was found that flavonoids also had antioxidant effects, and the study of antioxidant activity of flavonoids became the focus. In this paper, the flavonoids were extracted from the halophytic plant S. salsa by 60% ethanol extraction method, and the antioxidant activity was compared with ascorbic acid (VC) solution. To optimize the ethanol extraction process of flavonoids from S. salsa, factors of extraction time, extraction temperature, and liquid-solid ratio on the mass of flavonoids derived from S. salsa were investigated to seek the optimal extraction conditions. The results showed that the optimal extraction conditions were as follows: extraction time 58.63 min, extraction temperature 52.08°C, solid-liquid ratio 1:21.33 g/mL. Under these conditions, the extraction mass of flavonoids reached 6.40 mg/g. When the concentration of flavonoids was 1.5 g/L, the hydroxyl radical removal rate reached the maximum (54.94%). At the same time, the extraction process of flavonoids from Suaeda salicata optimized by response surface analysis is also reasonable and feasible.
Key words: Suaeda salsa, flavonoids, response surface methodology, optimization.
INTRODUCTION
Suaeda salsa is an annual herb, widely distributed in Europe and Asia (Qu et al., 2019). And the same time, it is grown in a lot of areas in China such as Hebei, Hainan, Shandong and so on. According to statistics data, the annual output of Suaeda salsa from the Yellow River delta reaches 3.3×105 t. The seeds of Suaeda salsa containing lots of unsaturated fatty acid have well edible value (Ji, 2015). The saline-alkali land contains a large number of inorganic ions (Ca, Fe, Mg), leading to the content of trace elements in Suaeda salsa being higher than other plants (Zhao et al., 2018). In addition, stems and leaves are also rich in amino acid, flavonoids, vitamins and minerals (Mohammed, 2020). With the expansion of saline-alkali land, the sustainable comprehensive utilization requires more attention. Flavonoids which widely exist in the roots, stems, leaves, flowers, and fruits of higher plants and ferns, is a natural organic substance with a variety of species and complex structure. The oxygen atoms in the first place of the molecules incorporated in a keto carbon base can form a salt with strong acid. Thus, it is also called flavin or flavone due to the yellow hydroxy derivatives including fat flavonoid, flavonols, isoflavones, flavanones, and chalones. Free radicals are the intermediate products of biochemical reactions in the process of human life activities. If the body produces too much or removal too slowly, it will cause damage to cells and organs. It is necessary to search for material with a good capturing ability to hydroxyl radicals. Among the reagents, flavonoids with a good capturing effect from Suaeda salsa are a very effective source (Husain et al., 1987; Oganesyan et al., 2001). The flavonoids promote the healthy functioning of people’s system, such as strong antioxidation, anticancer, antitumor, anti-inflammatory, anti-cardiovascular disease, immune regulation, antibacterial, antiviral, hypoglycemic, and hypolipidemia (Husain et al., 1987; Flowers and Colmer 2008; Yi et al., 2017; Du and Zheng, 2018; Liu et al., 2019; Zhang et al., 2021a).
Response surface methodology is one of the quick and effective methods for acquiring the optimal conditions of experimental results (Bezerra et al., 2008; Xiong et al., 2015; Fabre et al., 2021; Zhang et al., 2021b). This method is used to analyze the planar response curve and apply it to study and solve various technical problems in data processing of nonlinear models. Its main functions include the process design for comprehensive tests, construction of test model, checking the compatibility of factors, and seek for the optimal horizontal combination response conditions. The simple quadratic regression equation with one variable demonstrates the relationship between the independent variable and the response value. During the process, every point of the experiments is conducted independently for continuous data. It is convenient and fast to acquire the horizontal regression fitting response of every related factor. A fast algorithm for contour linear graph and surface of the quasi-plane combination and horizontal response can be realized by the rapid regression of the results of the whole test process. However, the premise of the tool is that the designed experimental sites should include the best experimental conditions. If the experimental points are improperly selected, the optimization of response surface analysis cannot obtain the precise result. Therefore, reasonable experimental factors and levels should be confirmed before optimization.
In this paper, response surface methodology was used for the optimal extraction process of flavonoids from Suaeda salsa, providing the theoretical basis and technical support for further development and utilization of Suaeda salsa from saline-alkali land. Ethanol extraction can effectively avoid impurities such as polysaccharides, tannins, and pectin. The experiment was designed using ethanol as an extraction solvent by Design-Expert software. The oscillating temperature, reaction time, and the solid-liquid ratio were selected as variable and the mass of flavonoids extracted from Suaeda salsa was set as the response value. Then, the relationship between the independent variable and the response value was obtained. Finally, the optimal extraction conditions of flavonoids from Suaeda salsa were determined. Additionally, the capability of the capturing ·OH was determined in the simulated system of hydroxyl radical generated by Fe2+-salicylic acid and H2O2.
MATERIALS AND METHODS
The seeds of Suaeda salsa were washed and dried in a vacuum oven at 60°C. Before screening through a 60 mesh sieve, the dried seed was ground into powder. Anhydrous ethanol was purchased from Beijing Jiaxing Chemical Glass Instrument Industry and Trade Co., Ltd. Petroleum ether was purchased from Shandong Century Tongda Chemical Co., Ltd. Sulfate-iron crystal was obtained from Beijing Fengling Chemical Reagent Technology Co., Ltd. Salicylic acid crystal was purchased from Beijing Northern Tianshand Chemical Reagent Factory. Hydrogen (30%) was provided by Tianjin Pufa Chemical Co., Ltd. Other agents used here are of AR grade as received.
Material pretreatment
After wash and dryness in the shade, seeds of Suaeda salsa were placed in a vacuum oven at 60°C. The dried seeds were pulverized into powder before sifting through a 60 mesh standard sieve. The petroleum ether was added to the powder to immerse and wash with a hot water bath for 1 h repeatedly. When the aforementioned resulting product was filtrated and dried with the addition of water, a natural alkaline S. salsa powder after oxidation skims can be prepared.
Extraction process of flavonoids
In the typical extraction process, 2 g S. salsa powder was transferred in a 50 mL conical flask with a certain solid-liquid ratio. Subsequently, the conical flask was placed in the water bath at a certain temperature and time with stirring speed of 300 r/min. Then, the product was recovered after duplicated suction filtration of the solid-liquid mixture. After three times wash, the combined filtrates were transferred in a 500 mL beaker and concentrated to one-third of their original volume at the temperature of 80°C in a water bath. Next, 4 times volume of water was added to the concentrated filtrate. The mixture was refrigerated and settled at 4°C for 2 h. At the end, the product was centrifuged in the centrifuge at the speed of 3000 r/min for 10 min. The insoluble material was collected in the evaporating dish, and the flavonoid powder was obtained ultimately.
Single factor experiment
According to the alcohol extraction-water precipitation method, flavonoids were separated from the powder of Suaeda salsa using anhydrous ethanol. In every single factor test, two of the variables were fixed and one was changed. Select the factors of extraction temperature, extraction temperature and the solid-liquid ratio as variables to study the optimized extraction process of flavonoids. During the single factor experiment, 10 g Suaeda salsa powder, 200 mL anhydrous ethanol, and 45 min reaction time were selected to investigate the effect of reaction temperature on extraction with the reaction time ranging from 15 to 90 min. Then, 10 g S. salsa powder, 200 mL anhydrous ethanol, and 45 min reaction time were selected to investigate the effect of reaction temperature on extraction with the reaction temperature ranging from 30 to 70°C. Finally, the liquid-solid ratio between the Suaeda salsa powder and anhydrous ethanol was 1:10, 1:15, 1:20, 1:25, 1:30 and 1:35 g/mL when the oscillation temperature was set as 50°C and reaction time was selected as 45 min.
Response surface analysis
According to the single-factor experiment, extraction time, extraction temperature, and solid-liquid ratio three factors three-level experiment were determined. Experiment conditions were designed using the Design-Expert software (Bezerra et al., 2008). Mass of extraction flavonoids was selected as the response value and three factors three levels quadratic regression orthogonal combination test was conducted using the Box-behnken model (Lee et al., 2000; Maiti et al., 2020; Mona et al., 2011). A (extraction time), B (extraction temperature), and C (solid-liquid ratio) were defined as argument (X) and mass of extraction flavonoids was defined as response value (Y). Response surface analysis was performed three times in every group of experiments to acquire the average value.
Capturing effect of flavonoids on hydroxyl radicals
The capturing effect was conducted through a reaction between the hydroxyl group of flavonoid molecules and the hydroxyl radicals. The experiment was performed to construct a model of the reaction system on the basis of Fenton reaction. Though its good activity, the hydroxyl radicals produced by H2O2 and Fe2+ have a short existence time. The addition of salicylic acid which can produce colored substances, can effectively capture the hydroxyl group. The reaction is shown as follows:
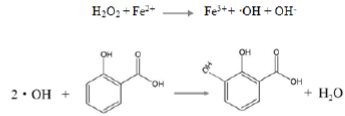
The obtained colored substances have strong adsorption at 510 nm. When another measurable substance that can react with hydroxyl radicals was added to the solution, the color of substances grows weak because they can compete with salicylic acid. In the typical procedure, 1 mL H2O2 (9 mmol·L-1) solution, 1 mL FeSO4 (9 mmol·L-1) solution, 1 mL of salicylic acid-trifluoroethanol solution (9 mmol· L-1) and 1 mL of apparent oid solution were diluted to 10 ml and then added to 3.5 mL cuvette in turn. Finally, the absorption value was measured at 510 nm. Each group of experiments was repeated 3 times, and the average was taken into the following equation.
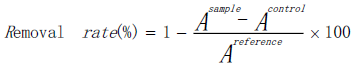
The control group used distilled water replace H2O2 solution and the blank group used distilled water to replace the flavonoid solution. The average value retrieved from the aforementioned repeated experiment (three times), was taken into in the formula. The VC solution was performed as a reference using the same concentration of flavonoid solution.
RESULTS AND DISCUSSION
Single factor experiment
Effects of extraction time on the mass of flavonoids
Extraction time plays an important role in evaluating the performance of separation. The extraction process should be as fast as possible in practical application. The effect of time on the mass of extraction flavonoids was investigated ranging from 15 to 90 min when the temperature of the reaction was 50°C and the solid-liquid ratio was 1:20 g/mL. As displayed in Figure 1, the extracting amount of flavonoids increased with the rise of extraction time from 15 to 45 min and reached the maximum value at 45 min. The mass of flavonoids decreased subsequently when extraction time continued to rise from 45 to 90 min. The mass of flavonoids extracted from Suaeda salsa could not be improved as the extraction time increased by over 45 min. This might be ascribed to the decomposition of flavonoids over time. Therefore, we selected the extraction time of 30, 45, and 60 min near the largest mass of extraction flavonoids to conduct a response surface analysis.
Effect of extraction temperature on the mass of flavonoids
The temperature has a great impact on the extraction capability of flavonoids, which was carried out ranging from 30 to 70°C. Figure 2 shows the effect of temperature on the mass of extraction flavonoid when the time of reaction was 45 min and the solid-liquid ratio was 1:20 g/m. It can be observed that the mass of flavonoids increased with the increase in extraction temperature. When the extraction temperature reaches about 50°C, the maximum mass of flavonoids was obtained from S. salsa. Whereas, if the temperature increased greater than 50°C, the amount of extracted flavonoids decreased slightly which might be caused by destruction of its structure at high temperatures. Thus, the oscillating reaction temperature of the response surface was determined as 40, 50, and 60°C, respectively.
Influence of solid-liquid ratio on the amount of extracted flavonoids
The ratio of solid-liquid takes an important role in the required amount of solvent and the complexity of the extraction process. The effect of the solid-liquid ratio on the mass of flavonoids was displayed in Figure 3. The extraction mass of flavonoids increased with the increase of the solid-liquid ratio from 1:10 to 1:20 g/mL and decreased as solid-liquid ratio rising from 1:20 to 1:35 g/mL. The maximum extraction mass of flavonoids was achieved at 1:20 g/mL. Hence, the solid-liquid ratios of 1:15, 1:20 and 1:25 g/mL were selected for response surface analysis.

Response surface design test results and analysis
Scheme and results of Box-Behnken design
Design-Expert 8.0.6.1 software with the Box-Behnken model was selected to design an experimental simulation condition. The data in Table 1 was adopted to carry out multiple linear regression fitting and a combination of simulation parameters. Second-degree polynomials equation model of extraction time (A), extraction temperature (B), and solid-liquid ratio (C) with regard to total mass of flavonoids during the extraction process were established by means of multiple linear regression fitting. The equation model is as follows:
Y=65.3-0.18A+0.87B+0.95C+0.35AB+0.46AC-0.85BC-1.71A2-2.31B2-2.25C2
Variance analysis of response surface regression model
The validity check of the regression equation is an essential index in practical research work. Effect of different reaction factors on the extraction mass of flavonoids from Suaeda salsa was further studied. The variance analysis of several important factors in the regression equation was performed as listed in Table 2. The regression equation cannot only predict the results but also be used to analyze the actual data.

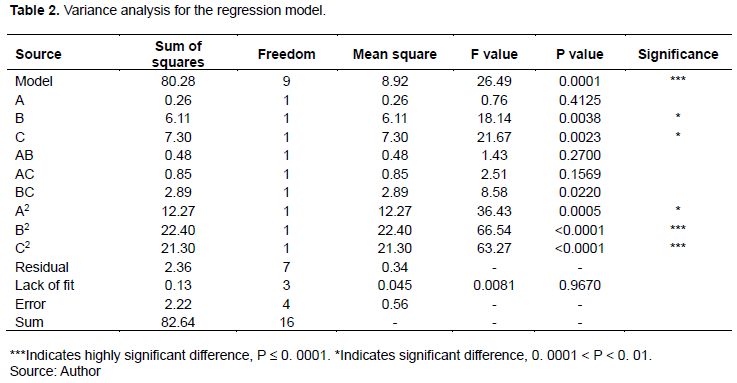
This regression equation can analyze the actual data, as well as the prediction of results. The result of the simulation (F=26.49, P <0.0002) reflects a better-simulated model. In the simulation analysis, the symbol “***” is specially marked, indicating that the simulation is successful and the degree of difference in a response regression model is very significant. A lack of fit (P=0.9670) greater than 0.05 demonstrates that the difference is not obvious. The design and experimental error of the response surface model are small, indicating that the model is very reasonable. In addition, the model variability coefficient C.V. is 7.32%, indicating that the test can be used for statistical analysis. After the significance test of regression equation coefficients, the result shows that P values of B, C, A2, B2, and C2 are less than 0.01, indicating that the extraction temperature, solid-liquid ratio, and binomials of the three factors all have obvious effect. The experimental factors have a nonlinear relationship with the response value because other interaction terms and extraction time significant terms are poor.
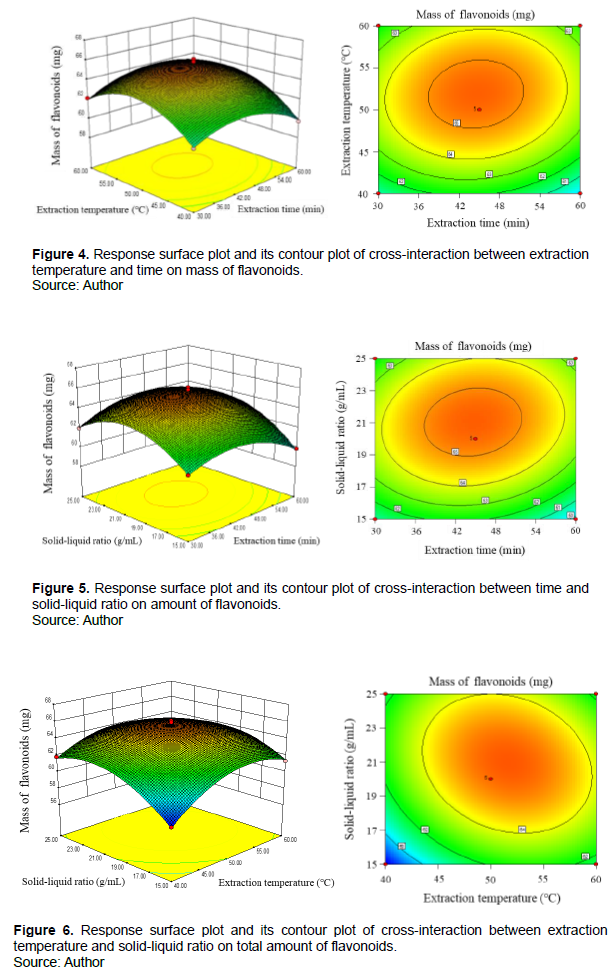
The response surface and contour plot of the regression model clearly shows the influence of three factors on the mass of flavonoids from S. salsa. The steeper the slope of the response surface, the greater influence of response value on the factor of extraction. While the flat curved surface indicates that the response value is insensitive to the factors. Figures 6 to 8 intuitively reflect the influence of various significant influencing factors on the response value. The shape of contour lines can be roughly divided into ellipses and circles. When the contour lines are ellipse, the prediction result is better. The curved surface of extraction temperature and time are steep, indicating that the temperature and time are very important for the extraction of flavonoids.
Figure 4 presents the effect of temperature and time on flavonoid extraction. When time keeps unchanged, the curve will gradually rise with the increase of oscillation temperature. When the temperature of the water bath is kept constant, the curve increases greatly with the increase of oscillation time. The results indicate that the two factors could benefit the extraction of flavonoids. Additionally, the corresponding contour lines are elliptical and dense close to the extraction time in the figure, demonstrating that the oscillation time has a greater impact on the extraction of flavonoids than the oscillation temperature.
The interaction between the oscillation time and the solid-liquid ratio is depicted in Figure 5. The dense lines of oscillation time indicate that the mass of flavonoids is more sensitive than the liquid-solid ratio.
The interaction between the oscillating temperature and the solid-liquid ratio is displayed in Figure 6. The mass profile of flavonoids presents a convex shape with the increase of the oscillation temperature and the solid-liquid ratio. When the oscillation temperature is 50°C and the solid-liquid ratio is 1:21.33 mL/g, the response value reaches the maximum value. On the whole, as for the influence of response value, the factor of time is more sensitive than the others to response value. According to model analysis, the optimal extraction conditions were as follows: A=58.63 min, B=52.08°C, D = 1:21.33 g/mL. The maximum yield of total flavonoids was 6.40 mg· g-1.
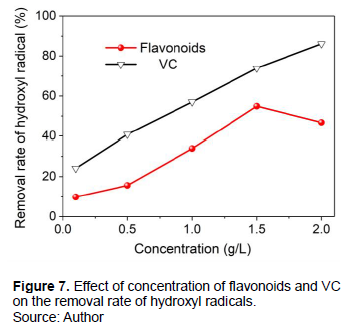
Capturing ability of flavonoids on hydroxyl radicals
It is vital to study capturing ability of flavonoids separated from Suaeda salsa to hydroxyl radicals (Yao et al., 2012; Feng et al., 2015; Zhao et al., 2021). As shown in Figure 7, the removal rate increased from 10 to 54.94% with rising in the concentration of flavonoids, indicating a gradual enhancement in capturing and inhibition of hydroxyl radicals. The higher the concentration, the faster the removal rate. When the concentration increased to 1.5 g/L, the removal rate of hydroxyl radical reached the maximum value of 54.94%. The adsorption capturing capacity subsequently decreased when the concentration of flavonoids continued to rise from 1.5 to 2.0 g/L. The removal rate of flavonoids was weaker than that of VC in general, but the performance was closest at a concentration of 1.5 g/L. From economic considerations, flavonoids have advantage for capturing hydroxyl radicals at the concentration of 1.5 g/L.
CONCLUSION
Suaeda salsa seed powder was used as raw material to study the optimal extraction process of flavonoids by ethanol. Although the test time is longer than the other modern methods, the simple instrument and common reagents make the procedure facile and cost-effective compared to other modern methods. The extraction process of flavonoids from Suaeda salsa is optimized by the response surface analysis on the basis of a test with a single factor. The optimum process conditions: the extraction time is 58.63 min, the extraction temperature is 52.08°C, and the solid-liquid ratio is 1: 21.33 g/mL. Under the optimized conditions, the maximum mass of flavonoids from Suaeda salsa is 6.40 mg/g. It has important significance for the research and development of Suaeda salsa in saline-alkali land.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
ACKNOWLEDGEMENTS
This work is financially supported by the Scientific Foundation of Hebei Normal University of Science and Technology (2019YB009) and the S&T Program of Hebei (20327311D).
REFERENCES
|
Bezerra ma, Santelli RE, Oliveira EP, Villar LS (2008). Response surface methodology (RSM) as a tool for optimization in analytical chemistry. L.A. Escaleira, Talanta 76(5):965. |
|
|
Du H, Zheng H (2018). Comparative Study on Extraction Technology and Activity of Total Flavonoids from Ginseng Flowers. Medicinal Plant 9(6):57. |
|
|
Fabre E, Henriques B, Viana T, Pinto J, Costa M, Ferrrira N, Tavares D, Vale C, Pinheiro-Torres J, Pereira E (2021). Optimization of Nd(III) removal from water by Ulva sp. and Gracilaria sp. through Response Surface Methodology. Journal of Environmental Chemical Engineering 9(5):105946. |
|
|
Feng RZ, Wang Q, Tong WZ (2015). Extraction and antioxidant activity of flavonoids of Morus nigra. International Journal of Clinical and Experimental Medicine 8(12):22328. |
|
|
Flowers Tj, Colmer TD (2008). Salinity tolerance in halophytes. New Phytologist 179(4):945. |
|
|
Husain SR, Cillard J, Cillard P (1987). Hydroxyl radical scavenging activity of flavonoids. Phytochemistry 26(9):2489. |
|
|
Ji J (2015). Research Status on Suaeda heteroptera Kitag. Aquatic Science and Technology 3(2):23. |
|
|
Lee J, Lin Y, Landen WO, Eitenmiler RR (2000). Optimization of an Extraction Procedure for the Quantification of Vitamin E in Tomato and Broccoli using Response Surface Methodology. Journal of Food Composition and Analysis 13(1):45. |
|
|
Liu XX, Liu F, Zhao S, Guo B, Ling PX, Han GY, Cui Z (2019). Purification of an acidic polysaccharide from Suaeda salsa plant and its anti-tumor activity by activating mitochondrial pathway in MCF-7 cells. Carbohydrate Polymers 215:99. |
|
|
Maiti S, Prasad B, Minocha AK (2020). Optimization of copper removal from wastewater by fly ash using central composite design of Response surface methodology. SN Applied Sciences 2(12):2151. |
|
|
Mohammed HA (2020). The Valuable Impacts of Halophytic Genus Suaeda; Nutritional, Chemical, and Biological Values. Journal of Medicinal Chemistry 16:1044. |
|
|
Mona S, Kaushik A, Kaushik CP (2011). Biosorption of chromium(VI) by spent cyanobacterial biomass from a hydrogen fermentor using Box-Behnken model. International Biodeterioration and Biodegradation 65(4):656. |
|
|
Oganesyan ET, Mal'tsev YA, Tvorovskii (2001). Mechanism of Reaction of Flavone Derivatives with Hydroxyl Radical by Semiempirical Methods. Russian Journal of General Chemistry 71(6):939. |
|
|
Qu XJ, Li WT, Zhang LY, Zhang XJ, Fan SJ (2019). Characterization of the complete chloroplast genome of Suaeda salsa (Amaranthaceae/ Chenopodiaceae), an annual succulent halophyte. Mitochondrial DNA B 4(2):2133. |
|
|
Xiong XH, Zhao LP, Chen YM, Ruan QJ, Zhang CM, Hua YF (2015). Effects of alkali treatment and subsequent acidic extraction on the properties of soybean soluble polysaccharides. Food and Bioproducts Processing 94:239. |
|
|
Yao YW, Liu SQ, Guo CM (2012). Function-structural study of anti-LDL-oxidation effects of flavonoid phytochemicals. Journal of Medicinal Plants Research 6(49):5895. |
|
|
Yi LZ, Ma SS, Ren DB (2017). Phytochemistry and bioactivity of Citrus flavonoids: a focus on antioxidant, anti-inflammatory, anticancer and cardiovascular protection activities. Phytochemistry Reviews 16(3):479. |
|
|
Zhang M, Bu T, Liu SL, Kim S (2021b). Optimization of Caffeic Acid Extraction from Dendropanax morbifera Leaves Using Response Surface Methodology and Determination of Polyphenols and Antioxidant Properties. Horticulturae 7(11):491. |
|
|
Zhang YS, Yu WY, Ji RP, Zhao YJ, Feng R, Jia QY, Wu JW (2021a). Dynamic Response of Phragmites australis and Suaeda salsa to Climate Change in the Liaohe Delta Wetland. Journal of Meteorological Research 35(1):157. |
|
|
Zhao YQ, Yang Y, Song YP, Li Q, Song J (2018). Analysis of storage compounds and inorganic ions in dimorphic seeds of euhalophyte Suaeda salsa. Plant Physiology and Biochemistry 130:511. |
|
|
Zhao Z, Wu X, Chen H (2021). Evaluation of a strawberry fermented beverage with potential health benefits. Peer J 9(926):e11974. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0