ABSTRACT
The aim of the present study was to evalute the protective role effect of lycopene and vitamin E on oxidative stress in Oreochromis niloticus exposed to diazinon (DZN). Adult fish were exposed to two sublethal concentrations (0.76 and 2.3 mg/l) of DZN against the ameliorative effect of lycopene (10 mg/kg) and vitamin E (50 mg/kg) for 14 and 28 days. DZN significantly led to a decline in total antioxidant capacity (TAO). However, lipid peroxidation (LPO), DNA fragmentation percentage, super oxide dismutase (SOD) and catalase (CAT) were significantly increased in gills, liver and kidney from the control values. Also, gills showed the highest accumulated DZN residues. Lycopene (LYC) and Vitamin E (VE) supplementation play an appositive role in detoxification of DZN toxicity. The results suggest that DZN can have effect on the antioxidant and oxidative stress biomarkers of fish negatively. Administration of lycopene and vitamin E could not decrease DZN residues in different tissues, but decreases the toxic effect of diazinon, as well as the decrease of LPO and DNA fragmentation near the control values. Also, TAO, CAT and SOD were better than the groups treated with only DZN.
Key words: Fish, residues carotenoide, insecticides, antioxidant, oxidative stress.
The aquatic and terrestrial ecosystems are continuously contaminated with chemical pollutants from industrial, agricultural and domestic activities. Insticides are a major category of toxicants, which have serious toxic impacts on aquatic life and still constitute a significant risk due to their toxicity on non-target organisms including fishes (
Ghazala et al., 2014;
Soloneski and Larramendy, 2012).
Diazinon (DNZ), [O,O-diethyl O-[6-m ethyl-2-(1-methylethyl)- 4-pyrimidinyl] phosphorothioate], an organo-phosphate insecticides is widely used in agriculture and public health (
US, 2006). Few investigations reported the toxic potential effects of diazinon on certain fish (Banaee et al., 2011, 2013; Ibrahim and Banaee, 2014). But its impact on the specific antioxidant and oxidative stress biomarkers is less explored.
It is known that many xenobiotics like insecticides may cause oxidative stress by generating reactive oxygen species (ROS) and alterations in ROS scavenging enzymes (Ibrahim and Harabawy, 2014, Milatovic et al., 2006). Like other organisms, fish have antioxidant defense mechanisms, such as antioxidant enzymes superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPX) and non-enzyme antioxidants (ascorbic acid, thiols, alpha tocopherol) to protect their cells from oxidative damages (Banaee et al., 2013). Superoxide dismutase converts the superoxide radical to peroxide and oxygen (Fridovich, 1978). Catalase targets hydrogen peroxide and quickly converts it to water and oxygen.
Non-enzymatic antioxidants such as vitamins E and Lycopene can act to overcome oxidative stress, as a part of the antioxidant system. Vitamin E, a constituent of plasma membrane, is an effective antioxidant and, as it is present at the site of free radical generation, it may neutralize the toxic effects of ROS (John et al., 2001).
Carotenoids are common highly efficient scavengers of singlet-oxygen and other excited oxygen species. During singlet-oxygen quenching, energy is transferred from single-oxygen to the lycopene molecule, which converts lycopene to the energy-rich triplet state. In contrast, the trapping of other ROS, such as hydroxyl, nitric oxide or peroxynitrite leads to oxidative breakdown of the lycopene molecule. Thus, lycopene may protect against the in vivo oxidation of lipids, proteins and DNA (Stahl and Sies, 2003; Wertz et al., 2004). Recently, lycopene has become a focus of interest because of its highly efficient antioxidant scavenging activity against singlet-oxygen and free radicals. Thus, lycopene may prevent oxidative damage, toxicity, and disease. Lycopene is one of the most effective antioxidants in the carotenoid family (Yonar, 2012; Yonar and Sakin, 2011).
The Nile tilapia Oreochromis niloticus is a widely distributed freshwater fish that can persist in a highly polluted habitat and can be used as a potential bio-indicator for aquatic environmental contaminants including pesticides. The activities and expression levels of antioxidant enzymes and metabolite were used as biomarkers to evaluate the influence of pollution on the biochemical pathway and enzymatic function in fish (Correia et al., 2007; Sun et al., 2006) and for monitoring unacceptable levels of environmental contamination. Therefore, this study was designed to evaluate the time and concentration dependent changes in the activity of antioxidant enzymes (SOD, CAT and Total antioxidant (TAO) activities as well as the concentration of malonaldehyde MDA as a bio-product of lipid peroxidation and DNA fragmentation and diazinon accumulation in gills, kidney and liver of Oreochromis niloticus following diazinon exposure at two sublethal concentrations for 14 and 28 days. Also, this study was designed to evaluate the ability of two antioxidants (Vitamin E and lycopene) to quench diazinon toxicity and accumulation.
Sample collection and chemicals
One hundred and twenty (120) healthy fish of the Nile tilapia, Oreochromis niloticus (157 ± 21.4 g in weight, 22 ± 1.72 cm in length), were caught from the fish farm of faculty of Agriculture, Assiut University, Egypt. The fish were immediately transported to the fish laboratory in the Department of Zoology, Faculty of Science, Assiut University. The experimental fishes were reared in aerated glass tanks (160 L capacity) and acclimatized for two weeks before being used in the experimental study. The experimental fish fed pellets at a rate of 3% of fish body weight twice daily. Faeces and residual food were aspirated regularly. The water temperature, pH and dissolved oxygen concentrations (DO) were measured daily (22.2 ± 1.5°C, 6.9 ± 0.2 pH and 6.5 ± 1.03 mg/1 DO). Light cycle was 12 h light and 12 h dark.
The insecticide Diazinon 9.0% was supplied by Bayer Company for Intermediate Chemicals, Egypt. Lycopene ((EC) No 1272/2008) was purchased from Sigma-Aldrich Chemical (St Louis, MO, USA) and DL-α-tocopherol (VE) acetate were obtained from Merck (Germany).
Experimental design
The fish were weighed, measured and classified randomly into 12 groups (10 fish per each tank) according to doses of DZN, lycopene, vitamin E and their combinations. The diets (maize and soy bean 34% protein, 15 g/kg fish) were pellet after addition of vitamin E and lycopene doses for the treated groups and the addition of suitable amounts of molasses and water. The diets were dried at room temperature and stored in small bags for fish feeding.
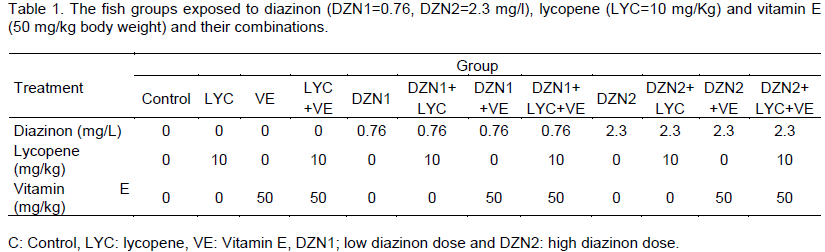
Stock solution (1,000 ppm) of Diazinon (DZN) (0, 0-diethyl-0-[2–isopropyl-6-methyl-4 –pyrimidinyl] phosphorothioate) was prepared and stored in clean glass bottles and diluted to concentrations of 0.76 and 2.3 mg/l in water. Such low sublethal DZN concentrations (1/10 and 3/10 of 96 h LC50) were chosen according to levels monitored by Soyingbe et al. (2012). Diazinon doses were prepared and added constantly to the aquarium for four weeks. The test water was replaced daily with the required amount of stock solution to prevent deterioration of water quality and replenish diazinon levels. Lycopene was added to the diet in concentration (10 mg/kg BW). Dose response of lycopene was described previously by Ural (2013). Also, vitamin E (a-tocopherol) was supplemented in 50 mg/kg BW. Such vitamin E concentration was chosen according to levels monitored by Ortunõ et al. (2001) (Table 1).
Enzyme activity assay
Immediately after the blood samples were collected, the liver, kidney and gill were carefully removed, washed with physiological saline (0.9% NaCl) and stored at -78°C until the biochemical assays. The tissue was homogenized in teflon-glass homogenizer in buffer containing 1.15% KCl at a 1:10 (w/v) ratio to the whole homogenate. The homogenate was centrifuged at 18000 g at 4?C for 30 min before the determination of MDA level and the SOD, CAT and TOA activity.
Lipid peroxidation and total protein
Measurements Total protein contents were determined according to the Biuret method (Gornall et al., 1949) using bovine serum albumin (E. Merck-Darmstadt, Germany) as a standard. Lipid peroxidation (LPO) was determined by the procedure of Utley et al. (1967). The absorbance of each aliquot was measured at 535 nm. The rate of lipid peroxidation was expressed as nmol of thiobarbituric acid reactive substance (TBARS) formed per hour per milligram of protein using a molar extinction coefficient of 1.56 M-1 cm-1 (Buege and Aust, 1978).
DNA fragmentation measurement
DNA Fragmentation was determined by the procedure of Kurita-Ochiai et al. (1999) using spectrophotometer (Micro lab 200 vital scientific Dieren, The Netherlands) at 575 or 600 nm against reagent blank. The percentage of fragmented DNA was estimated by the following formula: % of fragmented DNA = fragmented DNA / (fragmented + intact DNA) × 100.
Total antioxidant (TAO)
The TAO was measured using a colorimetric assay (Randox Laboratories, Crumlin, U.K.). The chromogen ABTS® (2,2´-Azino-di[3-ethylbenzthiazoline sulphonate]) is incubated with a peroxidase and hydrogen peroxide to produce the ABTS radical action. The ABTS radical is detectable due to its blue-green colour that is measured at 600 nm at 37°C. Antioxidants in the sample suppress the formation of the radical action to a degree that is proportional to their concentration. Values are expressed as mmol/L.
Superoxide dismutase (SOD) activity assay
Cellular total SOD activity was measured as described by McCord and Fridovich (1969) with minor modifications. The activity was measured by monitoring the SOD-induced inhibition of cytochrome c reduction by the superoxide radical generated in a xanthine/ xanthine oxidase system. Briefly, the cell pellets were resuspended in cold 50 mM potassium phosphate buffer (pH 7.5) and sonicated as described above. After protein concentration assay, 30 µg of total protein were added to an assay mixture containing 50 mM potassium phosphate buffer (pH 7.5), 0.1 mM EDTA, 0.01 mM cytochrome c, 0.1 mM xanthine and 0.003 units of xanthine oxidase in a final volume of 1 ml. The rate of increase in absorbance was continuously recorded spectrophotometrically at 550 nm at 25°C for 7 min.
Catalase (CAT) activity assay
Catalase activity was measured as described by Aebi (1984). Briefly, the cell pellets were resuspended in cold 100 mM potassium phosphate buffer (pH 7.0) and sonicated with the same procedures as above. After centrifugation, 30 µg of total protein were added to an assay mixture containing 100 mM potassium phosphate buffer (pH 7.0) and 10 mM H2O2 in a final volume of 0.5 ml. The decomposition of H2O2 was followed directly by a decrease in absorbance at 240 nm by spectrophotometer (Micro Lab 200 Vital Scientific). The activity was calculated using the molar extinction coefficient of 0.0436 (mmol-1) -1 cm-1.
Residues analysis
Determination of pesticide residues in water and the fish tissues
Fish tissue and water sampling: Fish samples (0.5-30 g) were taken to determine the residues concentration for tested pesticide in gills, liver and kidney that were immediately removed from the sacrificed fish in each treatment. Water samples (100 ml) were taken to evaluate the persistence of tested pesticide in water.
Extraction and clean up of tested pesticide: Extraction and clean up of diazinon residues from water and fish tissues were carried out according to pesticide analytical manual (Ezemonye et al., 2008).
Determination of diazinon residues by HPLC: The obtained samples were cleaned up and then dissolved in 1 ml methanol, HPLC grade and determined using HPLC instrument with the following condition: a) UV detector, b) C18 column, c) mobile phase was 90% methanol 10% acetonitryl, d) flow rate was 1 ml/min and detection line was 0.005 µg/kg. Duplicate injection (2 µl) of calibration solution and each sample was injected and integrated areas for each peak were recorded and standard peak under ideal condition for diazinon.
Statistical analysis
The results are expressed as the mean ± standard error. The patterns of variation due to diazinon, lycopen and vitamin E doses and their combinations were tested by using two-way, three-way and four-way ANOVA which determined the effects of diazinon, lycopen and vitamin E supplementation as the factors simultaneously tested. The differences between means were done by using The Tukey-HSD test. Range test was used as a post-hoc test to compare between means at P ≤ 0.05 using the SPSS 10.0 computer program (SPSS. 1998). P-Values <0.05 were considered statistically significant.
Fish showed abnormal behavior during the experimental period. At the start of the exposure, fish were alert, lost swimming coordination and buoyancy control with eleva-tion of opercula beat rate, which increased with time. Sometimes, they tried to avoid the toxic water by fast swimming and jumping. In tanks with DZN concen-trations, the fish swam unsteadily with jerky movements and hyperactive excitability. No fish mortality was recorded in aquaria with high dose of DZN.
Lipid peroxidation measurement (malonaldehyde level)
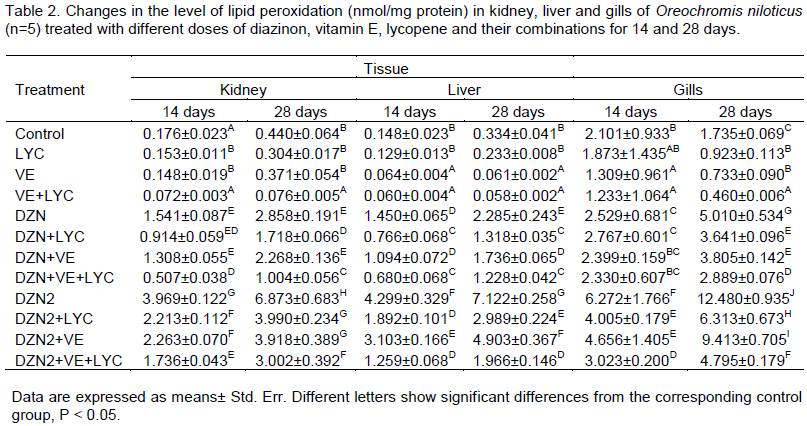
Lipid peroxidation (LPO) results are presented in Table 2. The LPO level was significantly increased in the liver, kidney and gills samples of the DZN groups. The main effects of DZN, LYC, VE and their interactions were significant (P>0.05) in the two periods.
Similarly, the time of exposure main effect was significant (P>0.05), and gills main effect was significant too (P>0.05). The level of LPO was significantly (P>0.01) decreased in liver, kidney and gills of DZN-exposed fishes fed with diets supplemented with vitamin E and/or lycopene.
DNA Fragmentation measurement
The percentages of DNA fragmentation results are presen-ted in Table 3. The main effects of DZN, LYC, VE and their interactions were highly significant (P>0.01) in 14 and 28 days. The time of exposure main effect was significant (P>0.05), while tissues main effect was not significant (P<0.05). Diet supplementation with lycopene and/or vitamin E decreased significantly (P>0.01) the level of DNA fragmentation in different tissues of DZN-exposed fishes.
Total antioxidant (TAO) measurement
The TAO was significantly decreased in the liver, kidney and gills samples of the groups that were exposed to DZN alone. The main effects of DZN, LYC, VE and their interactions were highly significant (P>0.0001) in 14 and 28 days. The time of exposure main effect was significant (P>0.05), and gills main effect was significant too (P<0.05). Diet supplementation with lycopene and/or vitamin E increased significantly (P>0.01) the level TAO in different tissues of DZN-exposed fishes (Table 4).
CAT activity
The tissues' CAT activities showed a statistically significant increase in the groups that were exposed to DZN when compared to the control group tissues (Table 5). The main effects of DZN, LYC, VE and their interactions were significant
(P>0.05) in the two periods. Similarly, the time of exposure main effect was significant (P>0.05), kidney and liver main effects were significant too (P>0.05). The level of CAT was significantly (P>0.01) decreased in liver, kidney and gills of DZN-exposed fishes fed with diets supplemented with vitamin E and/or lycopene.
SOD activity
Results are presented in Table 6. The SOD activity was significantly increased in the liver, kidney and gills of the groups exposed to DZN. The main effects of DZN, LYC, VE and their interactions were significant (P>0.05) in the two periods. Similarly, the time of exposure main effect was significant (P>0.05), gills main effect was significant too (P>0.05). The level of SOD was significantly (P>0.01) decreased in liver, kidney and gills of DZN-exposed fishes fed diets.
Diazinon residues
The accumulation pattern of DZN was gills> kidney> liver. DZN residues results are presented in Table 7. The main effects of DZN were highly significant (P>0.01) in 14 and 28 days. The main effects of LYC, VE and their interactions were insignificant (P>0.05) in the two periods. The time of exposure main effect was significant (P>0.01), and tissues main effect was significant (P<0.05). Diet supplementation with lycopene and/or vitamin E could not decrease the accumulation of DZN in different tissues.
Table 6. Changes in the activitie of Super oxide dismutase (U/mg protein) in kidney, liver and gills of Oreochromis niloticus (n=5) treated with different doses of diazinon, vitamin E, lycopene and their combinations for 14 and 28 days.
In the present work, more or less nervous manifestation of DZN-treated fish in the form of jerky uncoordinated movement, fins stretching and scale loss symptoms was recorded. Changes in color and loss of appetite were also observed for some fishes. Similar behavioral responses determined in this study have been observed with the guppy exposed to pyrethroids (Viran et al., 2003), fresh-water catfish, Heteropneustes fossilis exposed to cypermethrin (Saha and Kaviraj, 2003) and young mirror carp, Cyprinus carpio exposed to deltamethrin (Calta and Ural, 2004). Diet supplementation with LYC and/or VE for 14 and 28 days showed improvement in behavioral changes and the fishes were noticed in better conditions. Similar results for vitamin E and lycopene were observed by Ohaida (2005), Mekkawy et al. (2011, 2013), Soltan et al. (2011) and Ural (2013).
Diazinon insecticides induce oxidative stress leading to generation of free radicals and alteration in antioxidant enzymes or oxygen free radicals scavenging enzyme (El-Shenawy et al., 2010). Lipid peroxidation, an auto-catalytic process caused by free radicals, is considered to be a valuable indicator of oxidative damage in cellular components (Banaee et al., 2014). The present study showed that DZN at both dosages promoted MDA increase in different tissues. This increase in MDA can most likely be ascribed to an excessive production of ROS, which could be related to antioxidant enzyme leakage. Also, the increase in intracellular levels of ROS may lead to lipid peroxidation resulting in an increased permeability of gills, kidney and liver membranes (Banaee et al., 2011). In the present investigation, due to the free radical scavenging properties, significantly increased MDA level partially returned near to the control levels by VE and/or LYC. The level of lipid peroxidation was significantly decreased in different tissues of DZN-exposed fishes fed diets supplemented with vitamin E and/or lycopene. Similar results for vitamin E were observed in different fish (Abdel-Monem et al., 2012; Soltan et al., 2011).
Free radicals that are generated following pesticide exposure may lead to extensive DNA damage (Hatjian et al., 2000). In the present study, administration of DZN resulted in DNA damage of animals exposed for 14 and 28 days. The significantly increased DNA fragmentation percentage was partially returned near to the control levels by VE and/or LYC. Diets with vitamin E and lycopene have a protective effect on DZN-induced DNA and prevent genotoxicity induced by DZN. Yassa et al. (2011) showed that vitamin E had a protective effect against DZN-induced DNA damage and protective effect of vitamin E against genotoxicity of DZN has been reported (Ibrahim and Banaee, 2014). Another possible mechanism of vitamin E might involve selective removal of cells with DNA damage by apoptosis (Singh et al., 2008). Vitamin E allows free radicals to reduce a hydrogen atom from the antioxidant molecule rather than from polyunsaturated fatty acids thus breaking the chain of free radical reactions (Pascoe et al., 1987).
The first line of defense against oxidative stress consists of the antioxidant enzymes TAO, SOD and CAT, which convert superoxide radicals into hydrogen peroxide and then into water and molecular oxygen. A decrease in the activity of these enzymes changes the redox status of the cells. Thus, it is possible that an increase in the activity of these enzymes contributes to the elimination of the ROS induced by pesticide exposure from the cell (Stara et al., 2012). The present study showed a significant decrease in total antioxidant in tissues under investigation after exposure to DZN which could be related to the production of superoxide radicals or to the direct action of pesticides on enzyme synthesis (Bainy et al., 1996). However, Vitamin E and/ or lycopene adminis-tration increased the TAO tissues activity of the DZN-treated fish. The present results showed that lycopene enhanced antioxidant capacity, thus protecting tissues against the DZN-induced damages, as shown by the maintenance of the TAO activity. These results suggest that vitamin E and lycopene have a protective effect on ROS as described in other studies (Mekkawy et al., 2011, 2013).
SOD is a group of metallo-enzymes that plays a crucial antioxidant role and constitutes the primary defense against the toxic effects of superoxide radicals in aerobic organisms. SOD catalysis the transformation of superoxide radicals to H2O2 and O2 and is the first enzyme to cope with oxygen radicals (Kohen and Nyska, 2002). The significant increase in the tissue SOD activities that was observed in this study may be meant to scavenge the overproduction of superoxide anions under the oxidative stress induced by DZN. A similar result of increased SOD activity has been reported in Oreochromis niloticus that were exposed to chlorpyrifos (CPF) (Oruç, 2010). Similarly, Yonar et al. (2012) reported that a significant increase in the SOD enzyme activity occurred in carp tissues following CPF exposure. However, the simultaneous treatments with vitamin E and/or lycopene resulted in a significant decrease in the tissue SOD activities. This decrease in SOD activity can be attributed to the inhibition of superoxide radical formation or the potential free radical scavenging activity of vitamin E and/or lycopene (Ural, 2013).
CAT is an enzyme that is located in the peroxisomes and facilitates the removal of hydrogen peroxide, which is metabolized to molecular oxygen and water (Van der Oost et al., 2003). The present study showed that CAT activity was significantly increased in gills, kidney and liver tissues of Oreochromis niloticus that were exposed to both DZN concentrations. This elevation due to the adaptive response to the generated free radicals, indicating the failure of the total antioxidant defense mechanism to protect the tissues from mechanical damage caused by pesticides, as evidenced by lipid peroxidation. Thus, the superoxide ion generated is dealt with by the enhanced SOD and is converted to H2O2 by CAT. In the present study, the administration of vitamin E and LYC were somewhat effective in restoring the activities of SOD and CAT, which might be due to its ability to scavenge the accumulated free radicals. Total antioxidant, CAT and SOD differed significantly and the effect did not normalize the values, being significance between the control and DZN plus VE and/ or LYC groups.
The present study showed a significant accumulation of DZN in different tissues under investigation. The accumulation pattern of DZN was gills> kidney> liver. Similar results were obtained by Yassa et al. (2011), who found that DZN residues level in gills were higher when comparing to DZN concentration among liver, kidney and muscle tissues. Administration of vitamin E and/or lycopene with DZN insignificantly reduces the residue values in the examined tissues. The increased residual levels in the kidney compared with the liver confirm that the DZN residue was much greater in kidney than that in other organs (Altuntas et al., 2004). The relative high concentration of DZN residue in kidney and gills indicates that these organs play an essential role in the excretion of DZN.
Diazinon toxicity in different tissues could be attributed to the oxidative stress on cells, which was increased leading to the depletion of the antioxidant enzymes that scavenge the toxic superoxide and hydrogen peroxide radicals, leading to an increase of LPO. Nonetheless, lycopene and vitamin E treatments could be useful to decrease DZN toxicity by quenching oxidative stress imposed by DZN. It can be concluded that vitamin E and lycopene, as antioxidants, have protective effects against DZN adverse effects by inactivating (scavenging) free radicals generated following pesticides exposure.
The authors did not declare any conflict of interest.
REFERENCES
|
Abdel-Monem UM, Qar H, Attwa RA (2012). Detoxification of dietary diazinon by clay, vitamin C and vitamin E in rabbits. World App. Sci. J. 19:144-152. |
|
|
Altuntas I, Kilinc I, Orhan H, Demirel R, Koylu H, Delibas N (2004). The effects of diazinon on lipid peroxidation and antioxidant enzymes in erythrocytes in vitro. Hum. Exp. Toxicol. 23:9-13.
Crossref |
|
|
Bainy ACD, Saito E, Carvalho PSM (1996). Oxidative stress in gill, erythrocytes, liver and kidney of Nile tilapia (Oreochromis niloticus) from a polluted site. Aquat Toxicol. 34:151-162.
Crossref |
|
|
Banaee M, Sureda A, Mirvagefei AR, Ahmadi K (2013). Biochemical and histological changes in the liver tissue of Rainbow trout (Oncorhynchus mykiss) exposed to sub-lethal concentrations of diazinon. Fish Physiol. Biochem. 39:489-501.
Crossref |
|
|
Banaee M, Sureda A, Mirvaghefi AR, Ahmadi K (2011). Effects of diazinon on biochemical parameters of blood in rainbow trout (Oncorhynchus mykiss). Pesti. Biochem. Physiol. 99:1-6.
Crossref |
|
|
|
Banaee M, Sureda A, Zohiery F, Nematdoust HB, Garanzini DS (2014). Alterations in biochemical parameters of the freshwater fish, Alburnus mossulensis, exposed to sub-lethal concentrations of Fenpropathrin. Int. J. Aqua Biol: Article in Press. |
|
|
|
Calta M, Ural MO (2004). Acute toxicity of the synthetic pyrethroid deltamethrin to young mirror carp, Cyprinus carpio. Fresenius Environ. Bull. 13:1179-1183. |
|
|
Correia AD, Goncalves R, Scholze M, Ferreira M, Henrigues MA (2007). Biochemical and behavioral responses in gill head seabream (Sperus auratus) to phenanthrene. J. Exper. Marine Boil. Ecol. 347:109-122.
Crossref |
|
|
El-Shenawy NS, El-Salmy F, Al-Eisa RA, El-Ahmary B (2010). Amelioratory effect of vitamin E on organophosphorus insecticide diazinon-induced oxidative stress in mice liver. Pest. Biochem. Physiol. 96:101-107.
Crossref |
|
|
|
Ezemonye LIN, Ikpesu TO, Ilechie I (2008). Distribution of Diazinon in Water, Sediment and Fish from Warri River, Niger Delta Nigeria. Jordan J. Biol. Sci. 1:77-83. |
|
|
Fridovich I. (1978). The biology of oxygen radicals. Sci. World J. 201:875-880.
Crossref |
|
|
Hatjian BA, Mutch E, Williams FM, Blain PG, Edwards JW (2000). Cytogenetic response without changes in peripheral cholinesterase enzymes following exposure to a sheep dip containing diazinon in vivo and in vitro. Mutat. Res. 472:85-92.
Crossref |
|
|
Ibrahim ATA, Banaee M (2014). Ameliorative Effect of Lycopene and Vitamin E on Some Haematological and Biochemical Parameters of Oreochromis niloticus against diazinon toxicity. Adv. Plants Agric. Res. 1:14-23.
Crossref |
|
|
Ibrahim ATA, Harabawy AS (2014). Sublethal toxicity of carbofuran on the African catfish Clarias gariepinus: Hormonal, enzymatic and antioxidant responses. EcotoxicoL. Environ. Safety. 106c:33-39.
Crossref |
|
|
John S, Kale M, Rathore N, Bhatnagar D (2001). Protective effect of vitamin E in dimethoate and malathion induced oxidative stress in rat erythrocytes. J. Nutr. Biochem. 12:500-504.
Crossref |
|
|
Kohen R, Nyska A (2002). Oxidation of biological systems:oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol. Pathol. 30:620-650.
Crossref |
|
|
|
Kurita-Ochiai T, Fukushima K, Ochiai K (1999). Lipopolysaccharide stimulates butyric acid-induced apoptosis in human peripheral blood mononuclear cells. Infect Immun. 67:22-29. |
|
|
Mekkawy IA, Mahmoud U, Wassif E, Naguib M (2011). Effects of cadmium on some haematological and biochemical characteristics of Oreochromis niloticus (Linnaeus, 1758) dietary supplemented with tomato paste and vitamin E. Fish Physiol. and Biochem. 37:71-84.
Crossref |
|
|
|
Mekkawy IAA, Mahmoud UM, Mohammed RH (2013). Protective effects of tomato paste and vitamin E on atrazine-induced hematological and biochemical characteristics of Clarias gariepinus (Burchell,1822). Glob. Adv. Res. J. Environ. Sci. Toxicol. 2:011-021. |
|
|
Milatovic D, Gupta RC, Aschner M (2006). Anticholinesterase toxicity and oxidative stress. Sci. World J. 524:295-310.
Crossref |
|
|
|
Ohaida AMI (2005). The effect of lead and its interaction with supplementation of selenium and vitamin E on the growth performance, biochemical and physiological characteristics, histopathology and cytopathology of Oreochromis niloticus. Ph. D., Assiut Univ, Assiut, Egypt. |
|
|
|
Ortunõ J, Cuesta A, Esteban MA, Meseguer J (2001). Effect of oral administration of high vitamin C and E dosages on the gilthead seabream (Sparus aurata L.) innate immune system. Vet. Immunol. Immunopathol. 97:156-167. |
|
|
Oruç E (2010). Oxidative stress, steroid hormone concentrations and acetylcholinesterase activity in Oreochromis niloticus exposed to chlorpyrifos. Pestic. Biochem. Physiol. 96:160-166.
Crossref |
|
|
Pascoe G, Olafs F, Read D (1987). Vitamin E protection against chemical induced cell injury. Maintenance of cellular protein thiols as a cycloprotective mechanism. Arch. Biochem. Biophys. 256: 150-158.
Crossref |
|
|
Saha S, Kaviraj A (2003). Acute toxicity synthetic pyrethroid cypermethrin freshwater catfish, Heteropneustes fossilis (Block). Int. J. Toxicol. 22 325-328.
Crossref |
|
|
Singh M, Kaur P, Sandhir R, Kiran R (2008). Protective effects of vitamin E against atrazine-induced genotoxicity in rats. Mutat. Res. 654:145-149.
Crossref |
|
|
|
Soltan MA, Saudy AM, Fath El-Bab AF (2011). Rearing of the Nile tilapia (Oreochromis niloticus) on diets containing cotton seed meal enriched with vitamin E. Egypt J. Aquat. Biol. Fish. 15:89-104. |
|
|
Soyingbe AA, Ogunyanwo OO, Hammed TB, Adesope AO (2012). Effects of sublethal concentrations of diazinon on total protein in tilapia fish (Oreochromis niloticus). J. Environ. Sci. Toxicol. Food Technol. 1:22-25.
Crossref |
|
|
|
SPSS (1998). SPSS for Windows. |
|
|
Stahl W, Sies H. (2003). Antioxidant activity of carotenoids. Mol. Aspects Med., 24: 345-351.
Crossref |
|
|
Stara A, Machova J, Velisek J (2012). Effect of chronic exposure to simazine on oxidative stress and antioxidant response in common carp (Cyprinus carpio L.). Environ. Toxicol. Pharmacol. 33:334-343.
Crossref |
|
|
Sun YY, Yu HX, Zhang JF, Yin Y, Shi HH, Wang XR (2006). Bioaccumulation, depuration and oxidative stress in fish Carassius auratus under phenanthrene exposure. Chemosphere. 63:1319-1327.
Crossref |
|
|
Ural MS (2013). Chlorpyrifos-induced changes in oxidant/antioxidant status and haematological parameters of Cyprinus carpio: Ameliorative effect of lycopene. Chemosphere, 90: 2059-2064.
Crossref |
|
|
|
US EPA (2006). Interim Reregistration Eligibility Decision, Diazinon. Pesticides and Toxic Substances (United States Environmental Protection Agency, Office of Prevention: 138 pages. |
|
|
Viran R, Erkoç F, Polat H, Koçak O (2003). Investigation of acute toxicity of deltamethrin on guppies (Poecilia reticulata). Ecotoxicol. Environ. Safe. 55:82-85.
Crossref |
|
|
Wertz K, Siler U, Goralczyk R (2004). Lycopene: modes of action to promote prostate health. Arch Biochem Biophys. 430:127-134.
Crossref |
|
|
|
Yassa VF, Girgis SM, Abumourad IMK (2011). Potential protective effects of vitamin E on diazinon-induced DNA damage and some haematological and biochemical alterations in rats. J. Mediter. Ecol. 11: 31-39. |
|
|
Yonar ME (2012). The effect of lycopene on oxytetracycline-induced oxidative stress and immunosuppression in rainbow trout (Oncorhynchus mykiss W.). Fish Shellfish Immunol. 32:994-1001.
Crossref |
|
|
Yonar ME, Mise YS, Ural MS, Silici S, Düsükcan M (2012). Protective role of propolis in chlorpyrifos-induced changes in the haematological parameters and the oxidative/antioxidative status of Cyprinus carpio. Food Chem. Toxicol. 50:2703-2708.
Crossref |
|
|
Yonar ME, Sakin F (2011). Ameliorative effect of lycopene on antioxidant status in Cyprinus carpio during pyrethroid deltamethrin exposure. Pestic. Biochem. Physiol. 99:226-231.
Crossref |