ABSTRACT
Domestic wastewater from ten (10) different residential halls in the Federal University of Technology, Akure were collected and analyzed by considering microbiological and physiochemical characteristics and their degradation with time. Pour plating technique was used for the microbiological analysis, physico-chemical parameters were assayed using the American Public Health Association methods, while degradation was non-synthetic. The rates of degradation, changes in physicochemical parameters as well as the microbial composition were studied using standard methods. The result showed that all the samples were heavily populated with microorganisms, having microbial load of 1.86 x 107 cfu/ml. The coliform was highest in sample from Akindeko hostel with a microbial load of 1.85 x 107 cfu/ml. A total of sixteen bacterial isolates were identified among which are Proteus vulgaris, Shigella dysenteriae, Serratia marcescens and Clostridium botulinum. Eight fungi were isolated with Aspergillus flavus predominating. The pH values were all alkaline ranging from 7.10 to 9.20. The dissolved oxygen decreased with increased days of degradation. Conductivity of the wastewater also increased with days of degradation while the total dissolved solid decreased with increased days of degradation. Mineral analysis showed decrease in all the samples with increase in days of degradation. The studied wastewaters are therefore toxic and should not be discharged into water bodies without adequate treatment and certification of their safety level microbiologically.
Key words: Akure, residential halls, wastewater, physico-chemical, degradation, microbiological.
Steady growth in the number of students admitted into the Federal University of Technology, Akure (FUTA) (Adebisi et al., 2015) subsequently indicates steady increase in generation of domestic wastewater from each of the residences accommodating the students. The wastewaters generated from these residences are dis-charged without treatment directly into the environment. Municipal wastewater contains a variety of inorganic substances from domestic and industrial sources which include a number of potentially toxic elements such as arsenic, cadmium, chromium, copper, lead, mercury and zinc (Mara, 2003). High levels of biochemical oxygen demand (BOD) and a reduction in dissolved oxygen which is as a result of biodegradable organic matter in receiving waters is detrimental to aquatic life. This is due to high competition for oxygen within the ecosystem (Ogbomida et al., 2016). Nutrients (nitrogen and phosphorus) enrichment in receiving sensitive bodies of water can cause eutrophication by stimulating the growth of algae (called an algal bloom) (Ogbomida et al., 2016). Blooming and finally collapse of algae may lead to hypoxia/anoxia and hence mass mortality of benthic invertebrates and fish over large areas (Wu, 1999; Foroughi et al., 2010) due to aquatic dissolved oxygen depletion. In advance, biodegradability tests need to be carried out in laboratory; this is to verify possibility of treating the wastewater biologically before it is released back to a body of water. This study aims at assessing the effect of the degradation process on microbiological and physiochemical parameters of domestic wastewater generated in University residential areas.
Sample collection
The domestic wastewater samples were collected from ten (10) different residential halls within and outside the Federal University of Technology, Akure. The halls include Akindeko, Abiola, Jibowu, Annex, and Postgraduate hostels and the senior staff and junior staff quarters. Wastewater was collected in sterile 500 ml sample bottles according to standard methods of Cheesbrough (2006) for microbiological analysis. Two litres of domestic wastewater samples were also collected in clean sterile plastic containers and transported for physico-chemical analysis. The water samples were collected with the bottles facing upward and underneath stream towards the flow of water to avoid contamination (Cheesbrough, 2006). The collection was made in the morning hours when more wastewaters are usually generated and transported immediately to the laboratory within 4 to 6 h after collection for analysis. These samples were used for day 0; before commencement of degradation.
Degradation of wastewater samples
Five litres of domestic wastewater were collected from different locations in clean sterile containers. These were subjected to natural degradation for 32 days during which physico-chemical parameters and microbial isolation were carried out every 7 days.
Preparation of culture media
The following media was used for this study: nutrient agar and MacConkey agar. The agars were prepared according to manufacturer’s instructions.
Enumeration of microorganisms from sample sources
Serial dilution of each of the collected wastewater samples was carried out to a dilution factor of 104 and 0.1 ml aliquot was pipetted into sterile Petri dishes. Sterile agars were aseptically poured into inoculated Petri dishes. The plates were incubated in an inverted position at 37°C for 24 h, while plates for the isolation of fecal coliforms were incubated at 44°C for 24 h. The control of each batch of the test medium was confirmed by incubating one un-inoculated plate along with the inoculated plates. The coliforms and total mesophilic bacteria counts were enumerated on MacConkey and Nutrient agar, respectively.
Isolation and identification of isolates
Representative colonies of bacteria were picked from various plates after incubation. Pure cultures of isolates were obtained with the aid of streaking discrete and different morphological typed colonies on freshly prepared nutrient agar plates. The agar plates were duly incubated. The resulting distinct colonies were used for succeeding characterization tests. Bacterial isolates were identified in accordance with the schemes of the Bergey’s Manual of Deter-minative Bacteriology (Holt et al., 1994). The identified bacteria were maintained on nutrient agar respectively, slanted at 4°C in refrigerator for subsequent use.
Determination of parameters of wastewater
Physico-chemical parameters of wastewater samples such as the dissolved oxygen (DO), pH, total suspended solids, total dissolved solids, temperature, conductivity, turbidity, biochemical oxygen demand (BOD) were determined using the methods of Ademoroti (1996) and APHA (1998).
Data analysis
Variations in day intervals in relation to the physicochemical and microbial conditions were statistically measured. Data obtained were analyzed by one way analysis of variance (ANOVA) and means were compared by Duncan multiple range test (DMRT) using SPSS 18.0 version. Differences were considered significant at P ≤ 0.05.
Isolation and identification of microorganisms
The results of the bacterial load of isolated micro-organisms from domestic wastewater are shown in Figure 1. All the samples were heavily populated on day one, with the highest value recorded in Jibowu hall wastewater having 18.6 × 106 cfu/ml and the least was found in Annex Hall wastewater with 12.0 × 106 cfu/ml. However, there was reduction in the microbial load alongside the days of degradation with the least found in Akindeko hall with 1.8 × 106 cfu/ml on day 32. Figure 1 also shows the result of the coliform count isolated from the domestic wastewater.
This follows similar trend as the microbial load isolated, with the highest isolation found in Akindeko hall wastewater with 18.5 x 106 cfu/ml and the lowest in Jibowu hall wastewater with 8.3 x 106 cfu/ml on day 1, reduction was noticed with the day of degradation with the least count in Akindeko hall wastewater on day 32 with 1.3 x 106 cfu/ml.
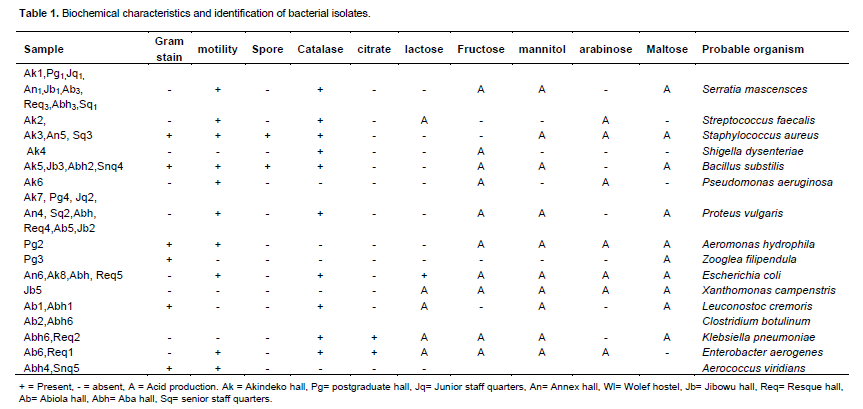
The identified bacteria as shown in Table 1 include Staphylococcus aureus, Serratia marcenscens, Proteus vulgaris, Shigella dysenteriae, Bacillus substilis, Escherichia coli, Streptococcus faecalis, Klebsiella pneumonia, Pseudomonas aeruginosa, Aeromonas hydrophila, Xanthomonas campenstris, Zooglea filipendula, Leuconostoc cremoris, Clostridium botulinum, Enterobacter aerogenes and Aerococcus viridians.
The trend of microbial load corroborates with Okpokwasili et al. (2005) that observed that human pathogens in water decreases with increase in the days of degradation. The decrease in the microbial load of the wastewater is perhaps associated with organisms making use of the organic materials present in the water for their biological activities. According to Michael (2013), decrease in nitrogen and phosphorus levels as well as decrease in organic materials in water led to decrease in microbial load of organisms in that water. According to Willey et al. (2006), these organisms are found as normal flora of soil, water and certain foods and may therefore be found where foods are decaying. According to Nester et al. (2004), the presence of this organism often found in wastewater makes wastewater unsafe for animal consumption.
Physico-chemical and metal parameters of wastewater samples
There was an increase in the temperature of the domestic wastewater subjected to degradation (Figure 3 down right). At day 0, low temperature was recorded with the highest temperature in postgraduate hostel wastewater and the least temperature was observed in Resque hostel wastewater. An increase was also noted with days of degradation with the highest temperature on day (32), the highest temperature for day (32) was found in junior staff quarters wastewater (29.5°C), while the least temperature was recorded in Aba hostel wastewater (28.2°C).
The pH (Figure 3 down left) shows increase with days of degradation. At day 0, senior staff quarters and Akindeko hostel samples had the least and highest pH of 7.10 and 7.78, respectively while at day 32, values increased to 9.20 (postgraduate hostel wastewater). The total suspended solids results (Figure 3 top left) shows that the wastewaters were highly polluted on day 0 due to high value recorded. Reductions were observed with degradation days where the highest at day 32 was 7.15 mg/l from postgraduate hostel.
From Figure 2 top left, there was slight decrease in dissolved oxygen (DO) of the wastewaters samples with degradation days, high values were recorded at day 0, during the course of degradation, there was slight reduction to day 32 with postgraduate hostel wastewater having the highest value of 4.11 mg/l and Resque hostel wastewater had the least value of 1.67. The highest biochemical oxygen demand in Figure 2 down left was on day (0) with the value of 4.96 mg/l in Akindeko wastewater and the least value of 2.69 mg/l was recorded in Resque hostel wastewater. During degradation, there was reduction in the BOD with the highest on day 32 observed in Jibowu hostel wastewater (2.20 mg/l) and the least value of 0.44 mg/l was recorded in Resque wastewater. The conductivity of the wastewaters in Figure 2 top right increased with days of degradation with the highest conductivity recorded on day 32 with 763 mg/l and the least value of 318 mg/l was found in Annex hostel wastewater.
The total dissolved solid of the samples increased gradually with degradation in Figure 2 top left, the highest value was recorded on day 32 with the value of 813 mg/l in Annex hostel wastewater and the least value on the same day was 405 mg/l in Resque hostel wastewater. The mineral analysis (Figures 4 to 5) showed decreased values in all the samples with degradation days. The result of the physico-chemical properties of wastewater subjected to degradation showed that pH plays a major role on the rate of degradation. According to Adams and Moss (1999), degradation rate increases with increase in pH level of waste and wastewater.
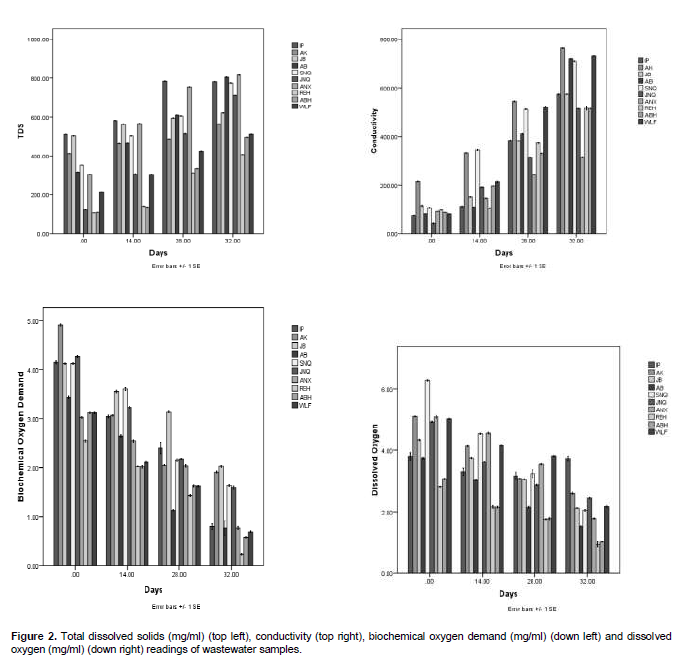
The results obtained in this work also corresponds to that of Willey et al. (2006) which stated that the rate of degradation increases with increase in pH. The increasein temperature is an indication that degrading activities is on the increase. According to Nester et al. (2004), increase in temperature of degrading liquid is usually due to microbial activities on the substrate and particles present in waste or fluid on which they feed. The total suspended solids in the wastewater showed that as degradation progresses, there is a reduction in the total suspended solids. This may be due to the fact that the microorganisms present in the wastewater may feed on these suspended solids for their survival. According to Robert et al. (2006), suspended solids which include food particles provide the bulk of food on which the microorganisms present in such water samples feed.
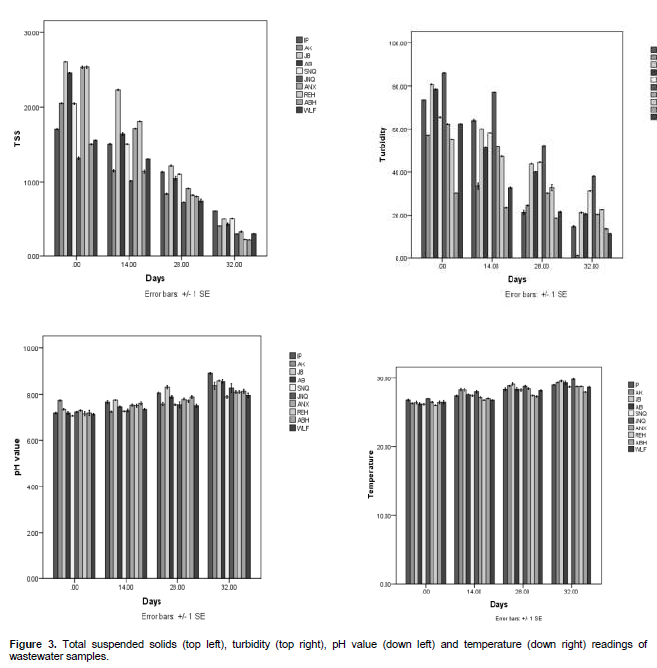
Dissolved oxygen and biochemical oxygen demand both decreases respectively with increase rate of degradation. Oxygen in the wastewater would have been used up by the microorganisms degrading the wastes in the wastewater as the days increased. Okoh et al. (2007) obtained similar result in which the oxygen demand and biochemical oxygen demand reduced as degradation progressed. The wastewater conductivity on the other hand increased with increase in days of degradation. According to Wasserman et al. (2006), increased conductivity is as a result of breakdown of solid mineral particles that may be in the water. Also, Vilia-Elena (2006) reported that increased conductivity in water may be due to microbial activity on the solid waste particles being activity on the solid waste particles broken down by the microorganisms present in such water.
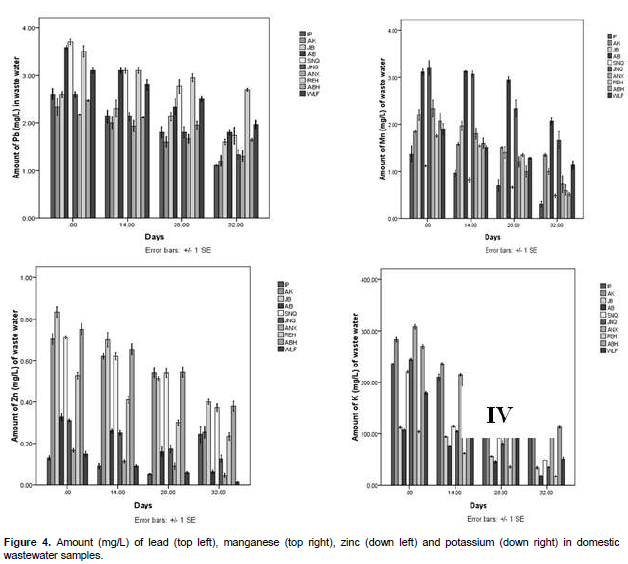
Therefore, increased conductivity in the result obtained could be due to either one of the two reasons or a combination of both reasons. Okoh et al. (2007) also reported the two reasons to be responsible for the increase in the total dissolved solid of wastewater. Therefore, increased total dissolved solid obtained in this work is in agreement with Okoh et al. (2007). Igbinosa and Okoh (2009) emphasized the utilization of major minerals in water by organisms for metabolic activities as being responsible for decrease in minerals during degradation of wastewater. This is also in agreement with the result obtained in this work. Degradation has significant effect on both microbial and physico-chemical parameters of wastewater; there is also obvious impact on the mineral analysis of wastewater.
Wastewater effluents are major contributors to a variety of water pollution problems. The discharge of these wastewaters into water bodies without proper treatment has impact on the water quality. Incorporation of low technology management practices such as primary settling should be carried out to reduce the period of delayed degradation. This study emphasizes the information that treatment of wastewater will reduce the microbial content of the wastewater hence mitigate associated diseases from these microorganisms.
The physico-chemical and mineral analysis before and after degradation are also an indication that these parameters can be altered to suite safety. To achieve unpolluted wastewater discharge into receiving water bodies, there is the need for careful planning, adequate and suitable treatment, regular monitoring and appropriate legislation. This will enhance science-based decisions and ensure the sustainability of the environment and the health of plants and animals.
The authors have not declared any conflict of interests.
REFERENCES
|
Adams MR, Moss MO (1999). Food Microbiology. New Age International, New Delhi, 4th ed., pp. 188-206.
|
|
|
|
Adebisi OS, Ezeokoli NB, Oletubo AA, Alade TJ (2015). Rental Analysis of Residential Properties in Close Proximity to the Federal University of Technology, Akure, Niger. J. Econ Sust. Dev. 6(10):140-147.
|
|
|
|
|
Ademoroti CMA (1996) Standard method for water and effluent analysis, March prints and consultancy, Foludex press Ltd, Ibadan, pp. 182-186.
|
|
|
|
|
American Public Health Association (APHA) (1998). Standard Methods of Examination of Water and Wastewater, 20th Ed., APHA, Washington D.C. pp. 1-140.
|
|
|
|
|
Cheesbrough M (2006). District Laboratory Practice in Tropical countries, part 2. Cambridge University Press pp. 758-759.
Crossref
|
|
|
|
|
Foroughi M, Najafi P, Toghiani A, Honarjoo N (2010). Analysis of pollution removal from wastewater by Ceratophyllum demersum. Afr. J. Biotechnol. 9(14):2125-2128.
|
|
|
|
|
Holt JG, Krieg N, Sneath PHA, William ST (1994). Bergey's Manual of Determinative Bacteriology. 9th Ed., Williams and Wilkins Co. Baltimore, USA. pp. 20-24.
|
|
|
|
|
Igbinosa BO, Okoh AI (2009). Impact of discharge wastewater effluent on the physico – chemical qualities of a receiving watershed in a typical rural community. Int. J. Environ. Sci. Technol. 6(2):1735-1472.
Crossref
|
|
|
|
|
Mara DD (2003). 1944-Domestic wastewater treatment in developing countries, Earthscan, UK, pp. 1-6.
|
|
|
|
|
Michael JP (2013). Microbiology of water supplies, wastewater and other aquatic environment. Encyclopedia Britannica, Inc.
|
|
|
|
|
Nester EW, Anderson DG, Roberts CE, Pearsall NN, Nester MT (2004). Microbiology; A human Perspective. 4th ed., McGraw Hill, New York, USA. P 17.
|
|
|
|
|
Ogbomida ET, Kubeyinje B, Ezemon LI (2016). Evaluation of bacterial profile and biodegradation potential of abattoir wastewater. Afr. J. Environ. Sci. Technol. 10(2):50-57.
Crossref
|
|
|
|
|
Okoh AI, Odjadjare EE, Igbinosa EO, Osode AN (2007). Wastewater treatment plants as a source of microbial pathogens in the receiving watershed. Afr. J. Biotechnol. 6:25.
|
|
|
|
|
Okpokwasili GC, Nweke CO (2005). Microbial growth and substrate utilization kinetics. Afr. J. Biotechnol. 5(4):305-317.
|
|
|
|
|
Robert MG, Elizabeth MM, Ian T (2006). Microbiology. Pearson Benjamin Cummings, San Francisco. pp. 758-759.
|
|
|
|
|
Vilia-Elena S (2006). Parkinson's disease and exposure to manganese during welding. Tech J Welding Allied Process, 7th ed, 2(5):106-11.
|
|
|
|
|
Wasserman GA, Liu X, Parvez F (2006). Water manganese exposure and children's intellectual function in Araihazar, Bangladesh. Environ. Health Perspect. 114:124-129.
|
|
|
|
|
Willey JM, Sherwood LM, Woolverton CJ (2008). Prescott, Harley, and Klein's Microbiology, NY, McGraw Hill, P 1096.
|
|
|
|
|
Wu RSS (1999). Eutrophication, water borne pathogens and xenobiotic compounds: Environmental risks and challenges. Mar. Pollut. Bull. 39(1-12):11-22.
|
|