Review
ABSTRACT
Heavy metals exist in the environment naturally aside those due to anthropogenic impact. These metals are removed from effluents and water using different techniques like adsorption, oxidation/reduction, chemical precipitation, membrane separation, filtration and ion exchange. Biosorption is very effective because it is highly renewed naturally, is cheap, and can remove metals greatly because the pollutant can be recovered either by desorbing or incinerating the biomass. Therefore, this work aims to identify some biochars utilized as adsorbents to remove lead, chromium, mercury and copper in soil and water, according to different researchers. In conclusion adsorption is a very effective method to remove or recover heavy metals from the environment. These biochars can be used in place of commercial activated charcoal because, besides being cheap, they are very effective treatment in removing metal ions based on wastewater discharge standards.
Key words: Biomass, adsorbents, activated carbon, biochar, heavy metals.
INTRODUCTION
Soil and water contamination with significant metals are often attributed to several completely different sources like agricultural, mining activities, industrial and residential activities. A trending environmental downside is contamination of soil and water due to rise of harmful pollutants derived from waste effluents may be. The foremost toxic wastes are significant metals like lead, nickel and others. These might be useful in minute concentrations, but adversely affect aquatic life and human health. The existence of those elements will result in metabolic process issues, immunologic weakness, excretory organ and liver disorders, high blood pressure, genetic mutations, in a worse case death (Zhang et al., 2016). Numerous correction techniques, supported either mobilization or immobilization processes are developed preserving security of human health and also the maintenance of sustainable environment (Souza, 2009). In recent years, it has been found that more soils worldwide are contaminated with toxins, due to waste emissions from various anthropogenic processes and improper use of pesticides and chemicals for agricultural production (Mench et al., 2010). Furthermore, tailings may be characterized by a total absence or low levels of organic matter and macronutrients and will normally have an acidic pH, although some tailings may be alkaline (Krzaklewski and Pietrzykowski 2002; Gbadebo and Ekwue 2014). Aside from that, tailings are also said to be devoid of normal soil structure and support a highly
stressed heterotrophic microbial community (Mendez et al., 2007). Further pollution of soil and tailings can be curbed by deploying pollution by employing environmentally sound technologies as alternatives (Beesley et al., 2011). For instance use of compost as soil amendment has been embraced by rural farmers due to its cost effectiveness (Umeobika and Onmonya, 2020). Many disadvantages of widespread use of chemical fertilizers include increase in soil acidity, mineral imbalance and soil degradation (Ayoola and Makinde, 2008). Also, in Europe Petruzzelli (2012) has reported that soil contamination has been known as a vital point for action within the European Economic Community, because it affects a large expanse of the available land. The economy in China has experienced progressive growth within the past few years; this has led to increased environmental problems (Xi et al., 2011). Standard strategies are enforced to reduce major metals from contaminated water. However, most of the main strategies are ineffective and undesirable due to high costs, high sludge production, and incomplete removal (Lara et al., 2016). Several studies aiming at reducing operating costs and increasing efficiency in water treatment have been conducted. Some of the biomass already worked on include: shells of aquatic animals and egg, fruit peels, vegetable oil and its residues, nuts, zeolites and husks of some roots and tuber crops cocoa and corn cobs. The efficiency of the process is hinged on the nature of the biomass used (Tejada-Tovar et al., 2016). Biochar is produced by thermochemical breakdown of biomass under restricted environments (Cha et al., 2016; Gondim et al., 2018). Temperature, type of biomass and atmosphere (often slowly oxidizing) are the most critical variables considered in this process (Kim et al., 2012). Water correction Contamination of aquatic systems may be a serious environmental issue and so the event of associate economical and appropriate technology to get rid of significant metals from binary compound solutions is important. Many strategies are often employed to remove significant metals from contaminated water. They include chemical precipitation, action, adsorption, membrane filtration; reverse diffusion, solvent extraction, and chemistry treatment with several of those strategies suffering from high capital and operational prices (Khatri et al., 2017).
Soil correction was done by removal of significant metal by screening followed by Soil washing from Contaminated Soil. In this technique the contaminated soil is removed from contaminated sites (ex-situ) and washed, the limitation of this method; the operation cannot be performed for a really massive volume of soil. Benefits of excavation involve the entire removal of the contaminants and also the comparatively fast cleanup of a contaminated site. Disadvantages embrace the actual fact that the contaminants square measure merely affected to a special place, wherever they need to be monitored; the danger of spreading contaminated soil and dirt particles throughout removal and transport of contaminated soil; and also the comparatively high value. Excavation is often the foremost high-ticket choice once massive amounts of soil should be removed or disposal as risky or toxic industrial waste is needed (Khatri et al., 2017). Stabilizing metals within the soil, significant metals are often left on the site and treated during a means that reduces or eliminates their ability to adversely have an effect on human health and also the environment. This method is usually referred to as stabilization. Eliminating the bioavailability of significant metals on the site has several benefits over excavation. A technique of stabilising significant metals consists of adding chemicals to the soil that cause the chelation of minerals that contain the significant metals during a form that's not simply absorbed by plants, animals, or people. This technique is termed in-place fixation or stabilization. This method doesn't disrupt the setting or generate dangerous wastes. Instead, the significant metal combines with the additional chemical to make a less harmful compound. The significant metal remains within the soil, however in a form that is abundant and less harmful, the disadvantages is that it permits incomplete neutralization of metals (Khatri et al., 2017). Uses of plants growing plants will facilitate contain or scale back significant metal pollution often referred to as phytoremediation. It has the advantage of comparatively low value and wide public acceptance. It is often but 1 / 4 of the price of excavation or in-place fixation. Phytoremediation has the disadvantage of taking longer to accomplish than different treatments. Plants are often utilized in other ways. Generally, a contaminated site is just revegetated during a method referred to as phytostabilization (Liu et al., 2017).
EFFICIENT REMOVAL OF METALS FROM WATER BY BIOCHAR
BIOCHAR USED TO REMEDY METAL POLLUTED SOILS
Heavy metals stay for years and not easily biodegradable in soils that are polluted. For heavy metals to be removed from polluted soils it is expensive and takes a lot of time (Cui and Zhang, 2004). Heavy metals are stabilized in situ by amending the soil with organic additives; this is mostly done to decrease the mineralization of metals and reduce absorption by plants (Komárek et al., 2013). Heavy metals can be stabilized in polluted soils by biochar. Biochar can lead to the improvement of polluted soil (Ippolito et al., 2012) and can greatly reduce the adsorption of heavy metals by crops. Thus, biochar has the potential of providing a novel remedy for soils polluted with heavy metals. There could be possible a large number of mechanisms involved in stabilizing heavy metals in soils using biochar. Using Pb2+ for instance, the research suggested different mechanisms for Pb2+ sorption with sludge-derived biochar as follows:
MERITS OF APPLYING BIOCHAR TO REMEDY SOIL AND WATER
Cheap source and waste management
Biochars are normally produced from inexpensive and abundantly existing waste biomaterials. Precisely, biochar feedstocks are mainly manufactured from the biomasses and solid wastes of agricultural works. Agricultural remains are seen in large amounts and are often difficult to dispose. For example, producing biochar from invasive plant can solve disposal problems and waste management. Also, aquatic algae are normally many and can block waterways; thus, other uses like the synthesis of biochar can benefit local people.
Nutrient
Pyrolysis of feedstock, leads to concentration of elements such as P, K, Ca, and Mg in biochar. Soluble organic substances are also formed during the pyrolysis process. Currently, using chemical fertilizers to improve soil is costly and out of reach for small farmers. Some disadvantages of the widespread use of chemical fertilizers are the soil acidity increase, mineral imbalance, and soil degradation (Ayoola and Makinde, 2008; Onmonya and Umeobika, 2020). Research has also shown that soils and residues can be characterized by total deficiency or low levels of organic matter and macronutrients and usually have an acidic pH, although some soil residues can be alkaline (Krzaklewski and Pietrzykowski, 2002; Gbadebo and Ekwue, 2014). In addition, tailings should also be without a normal soil structure and support a highly stressed heterotrophic microbial community (Mendez et al., 2007; Southam and Beveridge, 1992). The addition of biochar can provide plants and microrganisms with bioavailable nutrients. However, the nature of the starting materials and the conditions of pyrolysis determines the quality of biochar produced. The levels of C and N differed significantly in biochar when made from chicken droppings, pine and groundnut shells at 400 against 500°C. In addition, pyrolysis at 500°C yielded higher level of P, K, Ca, and Mg in biochar than at 400°C. This was due to the higher pyrolysis temperature, which decreased the CEC but enhanced mineralization of the feedstocks. In this context the goal is to maintain high quality of biomass, as well as the biochar given the right conditions for the process. In general, the nutrient content of plant derived vegetable biochar is comparatively lower than that of manure (Woolf et al., 2010). In line with this, Onmonya and Umeobika (2020) recommended that researchers do more research on processed cow dung to improve soil quality.
Soil stability
Erosion effects in Nigeria is huge, especially gully erosion in the south and southeast of the country. The high water flow with the undulating topography creates a high water flow. The lack of drainage channels to control fluid flow and soil structure has contributed to the severe effects of erosion in this area (Hillili et al., 2011). Moreso, wind erosion predominates in some parts of the northern states. In 1995 it was estimated that over 700 million kg of metals were dumped in land mine debris each year (Warhurst, 2000). The global impact of such tailings dumps is enormous, since unused tailings piles typically lie fallow for several decades and exposed tailings piles can spread over several tens of hectares due to aeolian dispersion and water erosion (González and González-Chavez, 2006). This has the potential to contaminate nearby communities and ecologically sensitive areas (Gbadebo and Ekwue, 2014). It is therefore imperative to research for alternatives such as biochar for promotion of stable soils (Trazzi et al., 2018). Biochar acts as an isolated particle, distinct from other stable organic matter that trapped in aggregates, soil pores or adsorbed on mineral surfaces. Biochar makes it easier for carbon to be sequestrated in the soil, because it is highly stable in its organic form. Sun et al. (2018) reported a half-live of 102 – 107 years for carbon in biochar and stated that biochar mineralizes very slowly. Woolf et al. (2010) also reported that fine particles of biochar have been in soils in climates with low heat levels, like Amazon.
Effect of biochar on heavy metal mobility
Biochar can reduce the movement of heavy metals in polluted soils, resulting in a low risk uptake by plants. Research has shown that bamboo-derived biochar can absorb Cu, Hg, Ni and Cr from soil and water and Cd in contaminated soils. Cao et al. (2009) reported that biochar derived from dairy manure at 200 °C pyrolyzes Pb more effectively than biochar produced at 350°C due to the higher concentration of soluble phosphate in the biochar at 200°C. A remediation process can utilize different biochar and mechanism for multi-element polluted soils.Therefore, when using biochar to improve soils polluted with heavy metals, the types of heavy metals in the polluted soil and the temperature used in the production of biochar must be considered because their properties depend on the pyrolysis conditions such as the water content of the feedstock, highest treatment temperature, type of starting material used and residence time. The influence of biochar on the bioavailability of metals varies depending on the type of biochar products and heavy metals. The ratio of Cd and Zn in pore water of contaminated soil was reduced when biochar from hardwood was applied (Beesley et al., 2010). Similarly, addition of biochar to contaminated soil reduced the concentrations of Cd and Zn in the pore water by 300 and 45-fold in a column leaching experiment (Beesley et al., 2011). Namgay et al. (2010) showed that the ratios of As and Zn that can be extracted in soil became high with the application of biochar; extractable Pb reduced; Cu was unchanged and Cd was not constant. They also described that there was sorption of trace elements on biochar with initial loadings up to 200 mol at pH 7 in the order: Pb > Cu > Cd > Zn > As. Biochar can decrease the leakage of metals via its effect of redox reactions of metals (Choppala et al., 2012). The significant reduction in the leaching of Cr(III) is due to the uptake of Cr(III) onto cation exchange sites and precipitation as Cr(OH)3 resulting from the discharge of OH ions during the process of Cr(VI) reduction (Bolan et al., 2013) as illustrated in Figure 1.

Biochar production and identification of the main biomasses used in this process
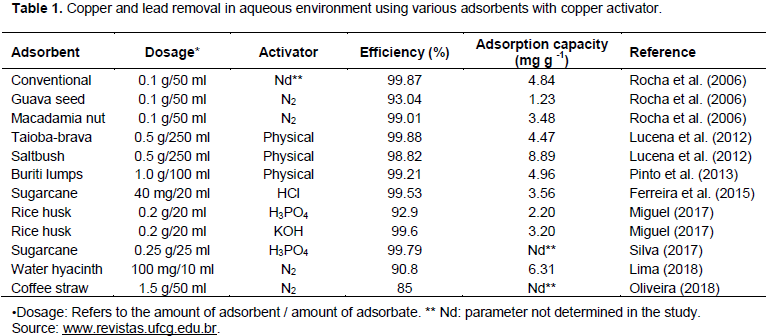
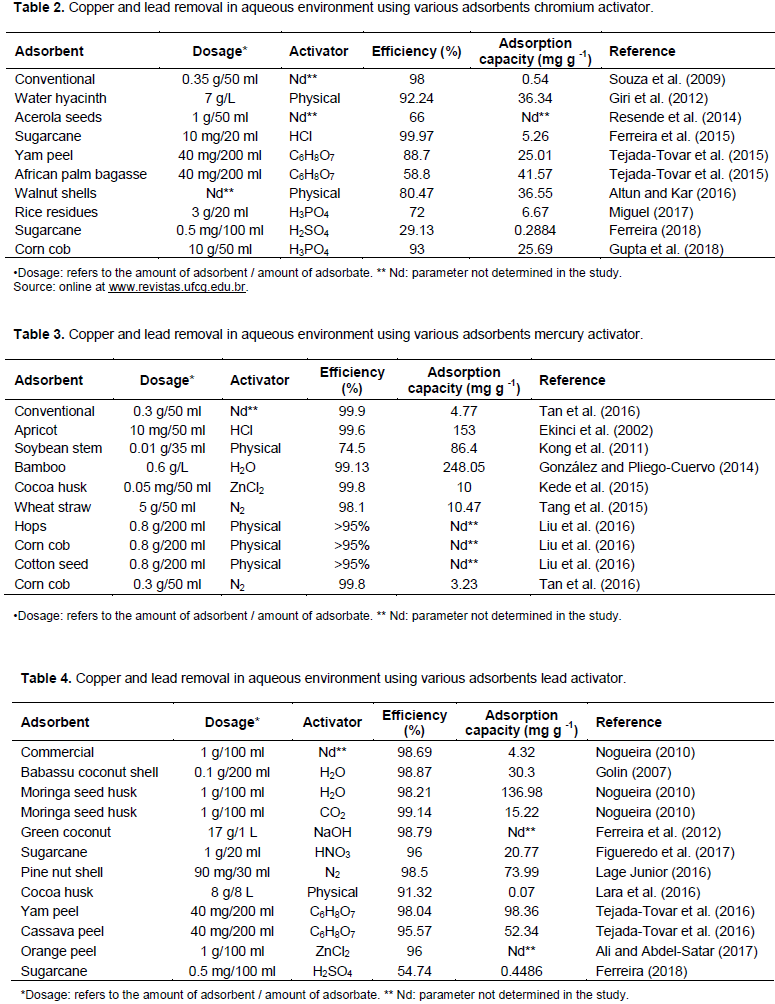
The two main techniques for converting biomass are: the use of enzymes and microorganisms (biochemical conversion) which is less expensive and environmentally friendly, although has a lower yield (Tripathi et al., 2016); and break down of biomass using heat (thermochemical conversion) (Kubilay et al., 2014). The thermochemical process includes conventional carbonization or pyrolysis, hydrothermal carbonization, incineration and gasification (Kubilay et al., 2014). The processes in this type of biochar production are mainly determined by the pyrolysis temperature, the residence time of the material in the reactor and the heating rate (Trazzi et al., 2018). Using biomass in combination with thermochemical synthesis has advantages in obtaining new carbonaceous materials with different applications, low cost, high availability in nature and fast regeneration (Santos, 2016). For (Novotny et al., 2015) various organic materials are suitable as raw materials in thermal processing, from agricultural and wood biomass to all available agricultural and industrial waste (husks, straw, seeds, bagasse, nut shells, wood chips, etc.) and even municipal waste (Table 2). The biochar produced in the pyrolysis process is high in energy comparable to the coal used in industry, owing to its microporous structure and high carbon content. In agriculture, it is used to improve soil quality and increase carbon storage. It slows down nutrient degradation and consequently improves soil quality. In the adsorption industry it is used to remove heavy metals such as Cr, Cd, Ni, Hg, Pb and organic compounds (Tripathi et al., 2016). Accordingly, low-cost alternative sources for the production of biochar are being researched into. Sofar agricultural residues such as rice husk (Doria et al., 2016), orange peel (Tejada-Tovar et al., 2015), corn cobs (Lopes et al., 2013), sugar cane bagasse (Ferreira et al., 2015) (Table 3) orange peel (Tejada-Tovar et al., 2015) (Table) 4 and the coir-chitosan composite (Costa et al., 2017) have shown great potentials for adsorption of pollutants.
HOW BIOCHAR AFFECT HEAVY METAL BIOAVAILABILITY
Heavy metals are generally found in small amounts in agricultural soils. However, due to their cumulative behavior and toxicity, they not only have a potentially harmful effect on crops but also on human health (Ekwue et al., 2012; Das et al., 1997). Heavy metal contamination of soil, water and crops and its health impact on local residents is an ongoing social problem, and several studies have identified health risks for local residents living near abandoned mines (Chung et al., 2005). Man-made pollutants can threaten human health and harm the natural ecosystem and environment (Hilli et al., 2021). The bioavailability of heavy metals determines toxicity in soil and potential risk upon entry into the environment. Fellet et al. (2011) reported that increased application of biochar resulted in increased pH, cation exchange capacity, and water-holding capacity and decreased bioavailability of some metals in mine tailings. In a study Zhou et al. (2008), used biochar derived from cotton stalks to improve Cd-contaminated soil, the biochar decreased the bioavailability of Cd in the soil. Mendez et al. (2012) also reported that biochar treatments reduced the plant availability of Ni, Zn, Cd and Pb compared to sewage sludge treatments in a Mediterranean agricultural soil. A reduced Cd, Cu and Pb uptake by Indian mustard was reported by Park et al. (2011) when they applied chicken manure and green waste derived biochar. The study also recorded reduced metal concentrations in plant except for Cu, with increased biochar application. Furthermore, biochar is highly effective in adsorption of many natural and anthropogenic sourced organic compounds (Sarmah et al., 2010). Owing to its highly aromatic nature, large surface area, micropore volume and abundant polar functional groups, biochar is effective in absorbing a wide range of organic chemicals including pesticides, PAHs and new emerging contaminants such as steroid hormones (Kookana et al., 2011). The level of aromaticity, type of biochar and organic carbon play a major role for effective removal of contaminants (irrespective of the other properties of the biochar) (Sarmah et al., 2010).
CONCLUSION
Bioremediation supplemented with biochar is one of the most important remediation technologies for the remediation of soil and water bodies contaminated with heavy metals. Biochar-enhanced remediation has great potential for immobilizing cationic heavy metals in mining tailings, tailings piles and water bodies, especially those with high acidity. Biochar can reduce the bioavailability and leachability of cationic heavy metals in soil and water, improve soil fertility and greening, and create a suitable environment for soil microbial diversity. To reduce the bioavailability of the organic pollutants and the risk of the pollutants entering the human food chain or leaching into groundwater, biochar could be of immense benefit. However, the long-term environmental fate of the deposited pollutants is still unknown and further research is warranted to fill this gap, especially under realistic field conditions through biochar-mediated remediation trials. Furthermore, it is important to select appropriate biochar to develop an effective strategy to immobilize anionic metals in situ. Future research should focus on: biochar stability and its impact on the fate and transport of metals in mining tailings and large-scale soils and waters; and understand the mechanisms of biochar-assisted bioremediation.
CONFLICT OF INTEREST
The authors have not declared any conflict of interest.
REFERENCES
|
Ali MH, Abdel-Satar AM (2017). Removal of some heavy metals from aqueous solutions using natural wastes orange peel activated carbon. IJRDO-Journal of Applied Science 3(3):13-30. |
|
|
Altun T, Kar Y (2016). Removal of Cr (VI) from aqueous solution by pyrolytic charcoals. New Carbon Materials 31(5):501-509. Annual Conference of Soil Science Society of Nigeria. Colloquia 44:457-463. |
|
|
Annual Conference of Soil Science Society of Nigeria. Colloquia 44:457-463. Available online at |
|
|
Ayoola OT, Makinde EA (2008). Performance of green maize and soil nutrient changes in fortified cow dump. African Journal of Plant Science 2:19-22. |
|
|
Beesley L, Marmiroli M (2011) The immobilization and retention of soluble arsenic, cadmium and zinc by biochar. Environmental Pollution 159:474-480. |
|
|
Beesley L, Moreno-Jimenez E, Clemente R, Lepp N, Dickinson N (2010). Mobility of arsenic, cadmium and zinc in a multi-element contaminated soil profile assessed by in-situ soil pore water sampling, column leaching and sequential extraction. Environmental Pollution 158(1):155-160. |
|
|
Bolan N, Kunhikrishnan A, Gibbs J (2013). Rhizoreduction of arsenate and chromate in Australian native grass, shrub and tree vegetation. Plant and Soil 367(1):615-625. |
|
|
Cao X, Ma L, Gao B, Harris W (2009). Application of bio-char for removal of toxic metals. Environmental Science and Technology 43:3285-3291. |
|
|
Cha JS, Park SH, Jung SC, Ryu C, Jeon JK, Shin MC, Park YK (2016). Production and utilization of biochar: A review. Journal of Industrial and Engineering Chemistry 40:1-15. |
|
|
Choppala GK, Bolan NS, Megharaj M, Chen Z, Naidu R (2012). The influence of biochar and black carbon on reduction and bioavailability of chromate in soils. Journal of Environmental Quality 41(4):1175-1184. |
|
|
Chung JH, Kang PS, Kim CY, Lee KS, Hwang TY, Kim GT, Park JS, Park SY, Kim DS, Lim OT, Sakong J (2005). Blood Pb, urine Cd and health assessment of residents in the vicinity of abandoned mines in Gyeongsangbuk-do. Korean Journal of Occupational and Environmental Medicine 17:225-237. |
|
|
Costa DA, Mendonça RH, Wysard Junior MM (2017). Avaliação da remoção de cromo (III) por materiais compósitos porosos adsorventes de PE-g-MA, fibra de coco e quitosana, usando planejamento experimental. Engenharia Sanitária and Ambiental, Rio de Janeiro 22(6):1203-1213. |
|
|
Cui DJ, Zhang YL (2004) Current situation of soil contamination by heavy metals and research advances on the remediation techniques. Chinese Journal of Soil Science 35:366-370. |
|
|
Das P, Samantary S, Rout GR (1997). Studies on cadmium toxicity in plants: A review. Environmental Pollution 98:29-36. |
|
|
Doria HGM, Uribe GCV, Anaguano AH, Suarez DG (2016). Preliminary study of modified rice husk and its effect on the adsorption of Cr(VI) in solution. Producción + Limpia, Caldas 11(1):103-116. |
|
|
Ekinci E, Budinova T, Yardim F, Petrov N, Razvigorova M, Minkova V (2002). Removal of mercury ion from aqueous solution by activated carbons obtained from biomass and coals. Fuel Processing Technology 77:437-443. |
|
|
Ekwue YA, Gbadebo AM, Arowolo TA, Adesodun JK (2012). Assessment of metal contamination in soil and plants from abandoned secondary and primary goldmines in Osun State, Nigeria. Journal of Soil Science and Environmental Management 3(11):262-274. |
|
|
Fellet G, Delle MG, Peressotti VA (2011). Application of Biochar on Mine Tailings: Effects and perspectives for land reclamation. Chemosphere 83(9):1262-1267. |
|
|
Ferreira DC, Da Silva NA, Lima AF, Begnini ML (2012). Biosorption of lead and nickel by the fibers of Cocos nucifera L. FAZU in Revista, Uberaba 9:64-68. |
|
|
Ferreira LA (2018). Utilização do bagaço de cana-de-açúcar como adsorvente dos íons Cd (II), Cr (III) e Pb(II). Fundação Educacional do Município de Assis - FEMA - Assis, Instituto Municipal de Ensino Superior de Assis. p.54. |
|
|
Ferreira PPL, Braga RM, Teodoro NMA, Melo VRM, Melo DMA, Melo M (2015). Cu 2+ and Cr 3+ adsorption in liquid effluents using sugarcane bagasse ash Cu 2+ adsorption adsorption and Cr 3+ in liquid effluents using sugarcane bagasse ash Brazil. |
|
|
Figueredo NA, Costa LM, Melo LCA, Siebeneichlerd EA, Tronto J (2017). Characterization of biochars from different sources and evaluation of release of nutrients and contaminants. Revista Ciência Agronoômica, Fortaleza 48(3):3-403. |
|
|
Gbadebo AM, Ekwue YA (2014). Heavy metal contamination in tailings and rock samples from an abandoned goldmine in Southwestern Nigeria. Environmental Monitoring and Assessment 186(1):165-174. |
|
|
Giri AK, Patel R, Mandal S (2012). Removal of Cr (VI) from aqueous solution by Eichhornia crassipes root biomass-derived activated carbon. Chemical Engineering Journal 185:71-81. |
|
|
Golin DM (2007). Removal of lead from liquid media through adsorption using activated charcoal of plant origin and plant residues. Dissertation: Graduate Program in Water and Mineral Resources Engineering. 111f. Federal University of Paraná - Curitiba, 2007. |
|
|
Gondim RS, Muniz CR, Lima CEP, Santos CLA (2018). Explaining the water-holding capacity of biochar by scanning electron microscope images. Revista Caatinga 31:972-979. |
|
|
González PG, Pliego-Cuervo YB (2014). Adsorption of Cd(II), Hg(II) and Zn(II) from aqueous solution using mesoporous activated carbon produced from Bambusa vulgaris striata. Chemical Engineering Research and Design 92(11):2715-2724. |
|
|
González RC, González-Chávez M C A (2006). Metal accumulation in wild plants surrounding mining wastes. Environmental Pollution 144(1):84-92. |
|
|
Gupta GK, Ram M, Bala R, Kapur M, Mondal MK (2018). Pyrolysis of chemically treated corncob for biochar production and its application in Cr (VI) removal. Environmental Progress and Sustainable Energy 37(5):1606-1617. |
|
|
Hilli JM, Gidado R, Badiru EO, Ugar SI, Onmonya YA, Yusuf HO, Onuora, DI, Hilili RU, Arowosegbe FA, Babade O, Eno E, Ojiego B, Oluyemi EO, Hilili MH (2021). Effects of Man's Activities On The Natural Ecosystem And Biotechnological Solutions: A Case Study Of Udeshi Village, Obanliku Local Government Area (L.G.A), Cross River State - Nigeria. International Journal of Science Academic Research 2(10):3068-3075, October, 2021 Available online at |
|
|
Hillili JM, Onyia OC, Ekwue YA, Yusuf HO (2011). Biotechnology and Environmental Sustainability International Journal of Tropical Medicine and Public Health Volume1, Issue1, 2011 Original Research Paper Cross house books. jes.2015.12.023 images. Revista Caatinga 31(4):972-979. |
|
|
Ippolito JA, Laird DA, Busscher WJ (2012). Environmental benefits of biochar. Journal of Environmental Quality 41(4):967-972. |
|
|
Kede CM, Ndibewu PP, Kalumba MM, Panichev NA, Ngomo HM, Ketcha JM (2015). Adsorption of Mercury(II) onto activated carbons derived from Theobroma cacao pod husk. South African Journal of Chemistry, Durban 68:226-235. |
|
|
Khatri N, Tyagi S, Rattan D (2017). Recent strategies for the removal of iron from water: A review. Journal of Water Process Engineering 19:291-304. |
|
|
Komárek M, Van?k A, Ettler V (2013). Chemical stabilization of metals and arsenic in contaminated soils using oxides-a review. Environmental Pollution 172:9-22. |
|
|
Kong H, He J, Gao Y, Wu H, Zhu X (2011). Cosorption of phenanthrene and mercury (II) from aqueous solution by soybean stalk-based biochar. Journal of Agricultural and Food Chemistry 59(22):12116-12123. |
|
|
Kookana RS, Sarmah AK, Van Zwieten L, Krull E, Singh B (2011). Biochar application to soil: agronomic and environmental benefits and unintended consequences. In Advances in agronomy (Vol. 112, pp. 103-143). Academic Press. |
|
|
Krzaklewski W, Pietrzykowski M (2002). Selected physicochemical properties of zinc and lead ore tailings and their biological stabilization. Water, Air, and Soil Pollution 141:125-142. |
|
|
Kubilay T, Selhan K, Sema B (2014). A review of hydrothermal biomass processing. Renewable and Sustainable Energy Reviews 40:673-687. |
|
|
Lage Junior NC (2016). Influence of pyrolysis conditions on the adsorption capacity of Pb(II) ions by biochar obtained from pine nut bark (Araucaria angustifolia). Dissertation: Graduate Program in Chemical Engineering, 87f, UFSC, Florianópolis - SC. Accessed from |
|
|
Lara PA, Rodríguez DC, Peñuela GA (2016). Application of coagulation by sweep for removal of metals in natural water used in dairy cattle. Afinidad 73(576). |
|
|
Lima JRA (2018). Removal of metals in water using Eichhornia crassipes in natura, biocoal and magnetic hybrid. Universidade Federal de Sergipe, São Cristóvão. |
|
|
Liu J, Mwamulima T, Wang Y, Fang Y, Song S, Peng C (2017). Removal of Pb(II) and Cr(VI) from aqueous solutions using the fly ash-based adsorbent material-supported zero-valent iron. Journal of Molecular Liquids 243:205-211. |
|
|
Liu P, Ptacek CJ, Blowes DW, Landis RC (2016). Mechanisms of mercury removal by biochars produced from different feedstocks determined using X-ray absorption spectroscopy. Journal of Hazardous Materials 308:233-242. |
|
|
Lu H, Zhang YY, Huang X, Wang S, Qiu R (2012) Relative distribution of Pb2+ sorption mechanisms by sludge-derived biochar. Water Research 46:854-862. |
|
|
Lucena GL, Silva AG, Honório LMC, Santos VD (2012). Copper adsorption kinetics (II) using bioadsorbents. Scientia Plena 8(9). |
|
|
Mench M, Lepp N, Bert V, Schwitzguébel JP, Gawronski SW, Schröder P, Vangronsveld J (2010). Successes and limitations of phytotechnologies at field scale: outcomes, assessment and outlook from COST Action 859. Journal of Soils and Sediments 10(6):1039-1070. |
|
|
Mendez MO, Glenn EP, Maier RM (2007). Phytostabilization potential of quailbush for mine tailings. Growth, metal accumulation, and microbial community changes. Journal of Environmental Quality 36:245-253. |
|
|
Miguel MFB (2017). Study of activated carbons from pyrolysis of rice production and processing residues Removal of Cr3+ in liquid medium by adsorption. lease use this identifier to cite or link to this item: |
|
|
Namgay T, Singh B, Singh BP (2010). Influence of biochar application to soil on the availability of As, Cd, Cu, Pb, and Zn to maize (Zea mays L.). Soil Research 48(7):638-647. |
|
|
Nogueira MW (2010). The use of activated carbon produced from the bark of Moringa oleifera as adsorbent in the removal of heavy metals in the presence of water. Dissertation: Federal University of Ouro Preto, Institute of Exact and Biological Sciences. Master's program in Environmental Engineering. 88f, Ouro Preto - MG, 2010. Accessed from |
|
|
Novotny EH, Maia CMBF, Carvalho MTM, Madari BE (2015). Biochar: pyrogenic carbon for agricultural use - a critical review. Revista Brasileira de Ciência do Solo, Viçosa 39(2):321-344. |
|
|
Oliveira GA, Gevaerd A, Mangrich AS, Marcolino-Junior LH, Bergamini MF (2021). Biochar obtained from spent coffee grounds: Evaluation of adsorption properties and its application in a voltammetric sensor for lead (II) ions. Microchemical Journal 165:106114. |
|
|
Onmonya YA, Umeobika VC, Oderinde OL (2020). Effect of Artisanal Mining on Sediment Quality in Southwest Nigeria. Proceedings of Annual Conference of Soil Science Society of Nigeria. Colloquia 44:457-463. Available online at |
|
|
Park JH, Choppala GK, Bolan NS, Chung JW, Chuasavathi T (2011). Biochar reduces the bioavailability and phytotoxicity of heavy metals. Plant Soil 348:439-451. |
|
|
Petruzzelli G (2012). Soil contamination and remediation strategies. Current research and future challenge. In EGU General Assembly Conference Abstracts (p. 7963). |
|
|
Pinto MVS, Silva DL, Saraiva ACF (2013). Obtaining and characterizing buriti pit activated charcoal (Mauritia flexuosa L. f.) for the evaluation of the copper adsorption process (II) Production and characterization of the activated carbon from buriti stone (Mauritia flexuosa L. f.) to evaluate the adsorption's process of copper (II) Acta Amazonica, 43(1). |
|
|
Resende JCT, Nunes DDA, Reis EN, Santos EJ, Ferraz C, Pagano RL, Silva DC (2014). Cr adsorption kinetics (VI) of aqueous solutions using acerola seeds. Scientia Plena 10(10). |
|
|
Rocha WD, Luz JAM, Lena JC, Bruña-Romero O (2006). Adsorção de cobre por carvões ativados de endocarpo de noz de macadâmia e semente de goiaba. Revista Escola de Minas 59(4):409-414. |
|
|
Santhosh C, Daneshvar E, Tripathi KM, Baltr?nas P, Kim T, Baltr?nait? E, Bhatnagar A (2020). Synthesis and characterization of magnetic biochar adsorbents for the removal of Cr (VI) and Acid orange 7 dye from aqueous solution. Environmental Science and Pollution Research 27(26):32874-32887. |
|
|
Santos AMS (2016). Production of carbon adsorbent prepared from the chemical and physical activation of banana peel residues Dissertation (master)-Federal University of Santa Catarina, Technological Center, Graduate Program in Chemical Engineering, Florianópolis, 2017 accessed online from |
|
|
Sarmah AK, Srinivasan P, Smernik RJ, Manley-Harris M, Antal MJ, Downie A, van Zwieten L (2010). Retention capacity of biochar- amended New Zealand dairy farm soil for an estrogenic steroid hormone and its primary metabolite. Soil Research 48(7):648-658. |
|
|
Silva IAA (2017). Synthesis and evaluation of adsorptive capacity of a magnetic hybrid adsorbent matrix using aguapé and its biocoal in cadmium and lead remediation. Relatório: Universidade Federal de Sergipe. |
|
|
Southam G, Beveridge TJ (1992). Enumeration of Thio bacilli within pH-neutral and acidic mine tailings and their role in the development of secondary mineral soil. Applied and Environmental Microbiology 58(6):1904-1912. |
|
|
Souza RS, Carvalho SML, Garcia Júnior MRL, Sena RSF (2009). Chromium (IV) adsorption by granular activated carbon by diluted solutions using a batch system under controlled pH. Acta Amazonica 39(3):661-668. |
|
|
Sun W, Zhang S, Su Chunming (2018). Impact of Biochar on the Bioremediation and Phytoremediation of Heavy Metal (loid)s in soil. Advances in Bioremediation and Phytoremediation. |
|
|
Tang J, LV H, Gong Y, Huang Y (2015). Preparation and characterization of a novel graphene/biochar composite for aqueous phenanthrene and mercury removal. Bioresource Technology 196:355-363. |
|
|
Tejada-Tovar C, Montiel Z, Acevedo D (2016). Utilization of Cassava and Yam Peels for the Treatment of Wastewater Contaminated with Pb (II). Información Tecnológica 27(1):9-20. |
|
|
Tejada-Tovar C, Ortiz AV, Patenina ER (2015). Adsorption kinetics of Cr (VI) using chemically modified residual biomass in batch and continuous systems. Revista Ion, Bucaramanga 28(1):29-41. |
|
|
Trazzi PA, Higa AR, Dieckow J, Mangrich AS, Higa RCV (2018). biocarvão: reality and potential for use in the forest environment biochar: reality and potential use in forestry Ciência Florestal, Santa Maria 28(2):875-887. |
|
|
Tripathi M, Sahu JN, Ganesan P (2016). Effect of process parameters on production of biochar from biomass waste through pyrolysis: A review. Renewable and Sustainable Energy Reviews 55:467-481. |
|
|
Umeobika VC, Onmonya YA (2020). Comparative Analysis of Handling Methods and Exposure Times on the chemical Properties of Cow Dung. NAMODA Techscope 12(1and2):1118-1672. |
|
|
Warhurst A (2000). Mining, mineral processing, and extractive metallurgy: An overview of the technologies and their impact on the physical environment. In A. Warhurst & L. Noronha (Eds.), Environmental Policy in Mining: Corporate Strategy and Planning for Closure. Boca Raton, FL: CRC Press LLC. |
|
|
Woolf D, Amonette J E, Street-Perrott FA, Lehmann J, Joseph S (2010). Sustainable biochar to mitigate global climate change. Nature Communications 1(1):1-9. |
|
|
Xi JF, Yu XZ, Zhou LX, Li DC, Zhang GL (2011) Comparison of soil heavy metal pollution in suburb fields of different regions. Soils 43:769-775. |
|
|
Zhang Z, Wang Z, Zhang Z, Zhang J, Guo J, LiE, Wang X, Liu H, Yan S (2016). Effects of engineered application of Eichhornia crassipes on the benthic macroinvertebrate diversity in Lake Dianchi, an ultra-eutrophic lake in China. Environmental Science and Pollution Research 23(9):8388-8397. |
|
|
Zhou JB, Deng CJ, Chen JL, Zhang QS (2008). Remediation effects of cotton stalk carbon on cadmium (Cd) contaminated soil. Ecology and Environment 17:1857-1860. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0